Midterm outcomes of penetrating keratoplasty following allogeneic cultivated limbal epithelial transplantation in patients with bilateral limbal stem cell deficiency
Jun-Fa Xue, Dong-Fang Li, Ya-Ni Wang, Chen Chen, Ru-Fei Yang, Qing-Jun Zhou,Ting Liu, Li-Xin Xie, Yan-Ling Dong
1School of Medicine and Life Sciences, Shandong First Medical University, Jinan 271016, Shandong Province, China
2State Key Laboratory Cultivation Base, Shandong Province Key Laboratory of Ophthalmology, Shandong Eye Institute,Shandong First Medical University & Shandong Academy of Medical Sciences, Qingdao 266071, Shandong Province,China
3Medical College, Qingdao University, Qingdao 266071,Shandong Province, China
4Department of Ophthalmology, Shandong University, Jinan 250100, Shandong Province, China
5Qingdao Eye Hospital of Shandong First Medical University,Qingdao 266071, Shandong Province, China
Abstract
INTRODUCTION
Corneal epithelial stem cells (SCs) are located at the palisades of Vogt and play a pivotal role in governing corneal epithelium renewal and regeneration[1]. Clinically,injuries to SCs can cause limbal stem cell deficiency(LSCD), manifesting as neovascularization, conjunctival transdifferentiation-induced conjunctivalization, corneal opacification, and persistent epithelial defect (PED) or recurrent erosions[2-3]. Among all the etiological factors,chemical injuries frequently cause severe total LSCD, termed when SCs are affected in all quadrants[4-6], and can lead to many complications, such as secondary glaucoma, corneal scarring, eyelid adhesion,etc.
Limbal SC transplantation has been reported to be useful for treating LSCD and reconstructing the ocular surface in recent decades[7-9]. Given the effects of transplanting limbal tissue,cultivated SC transplantation has been strongly advocated[10-11].Burn injury patients often experience bilateral involvement,so autologous limbal SCs are not always available. Allogeneic culture of corneal limbal SCs and autologous culture of oral mucosal epithelium has become the primary cell sources for cultured cell sheet transplantation[12]. Previous reports demonstrated that the oral epithelium has greater angiogenic properties than limbal cells[13-14], which may increase the graft failure rate of keratoplasty in the next step[15-16]. Thus, for this study, we selected allogeneic cultivated limbal epithelial transplantation (CLET) as the surgical method in phase one[17].Based on clinical observations, further penetrating keratoplasty(PK) surgery was recommended due to poor vision caused by stromal opacification, full-thickness scarring or endothelium deficiency[18-19]. Rather than performing a combined surgery,a separate two-stage procedure several months after SC transplantation, which carries a much higher graft survival rate, was recommended[20]. Our previous study revealed that no allogeneic graft rejection occurred at six months after CLET[21],so we decided to perform PK 6mo after allogeneic CLET to prevent multiple immunological reactions. To inhibit allogenic rejection, topical tacrolimus instead of ciclosporin was used after both CLET and PK[22-23].
To date, few reports have described the outcomes of PK following allogeneic CLET. Additionally, no information is available regarding the influence of topical tacrolimus on the survival of corneal grafts transplanted after allogeneic CLET.Therefore, for this study, we predesigned a sequential therapy for patients with total bilateral LSCD caused by chemical injuries: allogeneic CLET, followed by PK approximately six months later, with topical tacrolimus management administered during the course of treatment.
SUBJECTS AND METHODS
Ethical ApprovalThis study was approved by the Ethics Committee of Shandong Eye Institute and adhered to the tenets for human subjects outlined in the Declaration of Helsinki.The clinical trial was registered in the Chinese Clinical Trial Registry (www.chictr.org.cn), under registration number is ChiCTR2000032536. The study design was a single-center prospective noncomparative case series. Informed consent was obtained from all participants.
General InformationA cohort of adults with chronic ocular complications from chemical injuries was recruited at Qingdao Eye Hospital between June 15, 2016, and June 16,2020. The inclusion criteria were as follows: 1) bilateral total LSCD secondary to chemical burns; 2) age≥18y; 3) allogeneic CLET: ≥6mo after injury, with regular eyelids and the ocular surface in homeostasis; 4) secondary PK: full-layer corneal stromal opacification, scars, or swelling after allogeneic CLET with best-corrected visual acuity (BCVA) less than 20/200;and 5) follow-up of ≥12mo after PK. The exclusion criteria were as follows: 1) previous limbal tissue transplantation or other intraocular surgeries; 2) severe systemic disease(s) or contraindications for steroids and immunosuppressants. Fifty patients (59 eyes) with severe LSCD underwent allogeneic CLET and were followed up for at least 12mo, 10 of whom(10 eyes) were clinically evaluated and conformed to the inclusion criteria for further PK. All subsequent PK procedures were performed approximately six months later (a graphical representation of the whole sequential therapy design is shown in Figure 1).
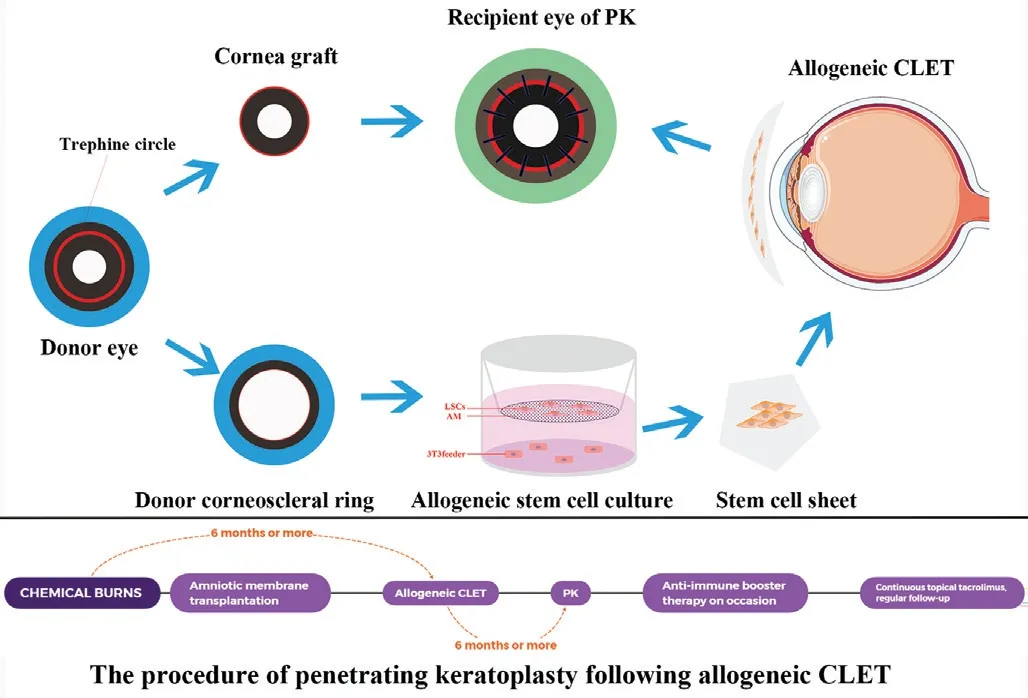
Figure 1 Graphical representation of the PK procedures following allogeneic CLET The donated corneal tissue was trephined into a corneal graft using a 7.5-8.0 mm trephine and was used in PK surgery. The disposed corneoscleral ring after PK was cut into pieces, inoculated on a denuded AM laying on the bottom of transwell culture inserts, and then cocultured with MMC-inactivated NIH 3T3 fibroblasts. The stem cell sheet was then applied in the allogeneic CLET. After approximately 6mo, PK was performed in the same eye to improve central corneal transparency. A booster anti-rejection treatment (topical tacrolimus and fluorometholone,systemic hydrocortisone, prednisone, or cyclosporine if necessary)was administered if needed after PK. PK: Penetrating keratoplasty;CLET: Cultivated limbal epithelial transplantation; AM: Amniotic membrane; MMC: Mitomycin C. This schematic diagram was originally illustrated by Xue JF using Adobe Photoshop CC (Adobe Systems Incorporated, USA).
Examination MethodsPatients underwent full ophthalmic examination, including BCVA, intraocular pressure (IOP),optical coherence tomography (OCT), slit-lamp assessment of ocular surface grading scores (OSS, according to Chie Sotozono’s ocular surface grading criteria[17,24]), corneal graft epithelial rehabilitation, PED, immunological rejection, and graft survival rate during the treatment process. Successful CLET before PK was defined by reduced or absent corneal vascularization, a smooth corneal surface with a stable epithelium and a corneal phenotype on the central cornea with or without peripheral conjunctival invasion. Successful PK following allogeneic CLET was clinically defined by an intact,epithelized, stable corneal surface with increased corneal transparency (opacification grade 0 or 1) and improved BCVA.Corneal graft failure was defined as central corneal (center diameter 6 mm) revascularization, reconjunctivalization or a loss of central graft clarity (opacification grade 2 or more).
Operative Design and Method
Allogeneic limbal epithelial culture and CLETConsistent with our previous research[17,21], limbal SCs were acquired from a residual cadaveric donor corneoscleral ring after keratoplasty.The infectious disease test results of the donors were negative.Specimens were cut into granules, inoculated onto an amniotic membrane (AM) on the bottom of transwell culture inserts,and then cocultured with mitomycin C (MMC)-inactivated NIH 3T3 fibroblasts for at least 5d until confluence, with the medium changed every 2d. Dulbecco’s modified Eagle’s medium/F-12 (3:1) supplemented with 10% fetal bovine serum(Gibco, Grand Island, NY, USA), 2 nmol/L 3,3’5-triiodo-Lthyronine sodium salt (Sigma), 1% nonessential amino acids(Invitrogen, Carlsbad, CA, USA), 0.1 nmol/L cholera toxin(Sigma, St. Louis, MO, USA), insulin-transferrin selenium(Invitrogen, Carlsbad, CA, USA), 0.4 ng/mL hydrocortisone succinate (Wako, Osaka, Japan), 2 mmol/L L-glutamine(Invitrogen), penicillin-streptomycin (HyClone, Logan, UT,USA) and 10 ng/mL recombinant human epithelial growth factor (R&D Systems, Minneapolis, MN, USA) were used for the culture of limbal epithelial cells.
The incubation environment was 37°C with 5% carbon dioxide and 95% air. AMs with incubated cells stratifying into 3-5 layers were ultimately used for clinical application.
All operating procedures were performed under peribulbar anesthesia by two experienced surgeons (Xie LX and Dong YL). First, 360-degree conjunctival peritomy, dissection of the symblepharon, recession of the bulbar conjunctiva,dissection of the fibrovascular pannus on the corneal surface and subconjunctival fibrotic tissues were performed carefully.Second, the graft of the cultivated cell sheet was placed onto the bare sclera and corneal stroma with the epithelial side upward and intermittently sutured with 10-0 nylon sutures.A conjunctival sac was rebuilt by suturing the residual conjunctiva tissue outside the limbus. Finally, a bandage contact lens (Bausch and Lomb, NY, USA) was applied to the ocular surface, and tobramycin and dexamethasone ophthalmic ointments were applied superficially.
Penetrating keratoplastyClassical optical PK surgeries were performed approximately 6mo after allogeneic CLET in patients fulfilling the inclusion criteria of PK Superior fresh donor corneal grafts from an eye bank were used for transplantation to counterbalance the defect in the compromised ocular surface. The recipient cornea was trephined with a 7.25-to 7.75-mm trephine, and the donor tissue was fabricated with a diameter 0.25 mm larger than the diameter of the recipient graft site. If needed, 250 mL of 20% mannitol containing 10 mg of dexamethasone was administered intravenously once or twice after PK to control IOP and suppress irritation from intraocular inflammation. Corneal grafts were secured to the recipient bed with 16 interrupted 10-0 nylon sutures. Similar to CLET,a bandage contact lens was placed on the ocular surface after PK. The recipient corneal buttons were sectioned and subjected to hematoxylin and eosin (H&E), periodic acid-Schiff (PAS),and immunohistochemical staining.
Immunohistochemistry and antibodiesAll procedures were consistent with our previous research[25]. Primary antibodies included anti-CK3 antibody (epithelium marker, 1:200 dilution, Abcam, USA) and anti-P63 antibody (myoepithelial cell marker, 1:50 dilution, Proteintech, USA). The experiments reported in this study were reproducible.
Postoperative management and follow-up schedulePost-PK medication was similar to that after allogeneic CLET[17,21].Intravenous methylprednisolone (2 mg/kg) was administered daily for 3-5d immediately after transplantation, and then oral prednisolone (1 mg/kg) was administered daily and tapered over 2-3mo to prevent allograft rejection. Autologous serum eyedrops containing 0.02 mg dexamethasone and 0.1 mg tobramycin per milliliter were applied every 2h for the first week, and then eyedrops containing 0.1% fluorometholone,0.5% levofloxacin eyedrops, and preservative-free artificial tears were applied four times daily for the next 2wk. Tobramycin and dexamethasone ophthalmic ointments were applied topically every night. Topical tacrolimus (0.1%) was administered 4 times daily after corneal epithelial integration. The aforementioned topical treatment should be accommodated depending on clinical status after 3wk and gradually reduced to a minimum dose when the grafts are sound. Therapeutic bandage contact lenses were used to preserve the graft epithelium for 1-3mo after surgery. Patients were examined daily during the first week after surgery, weekly during the first month, and monthly thereafter.
All patients underwent thorough examinations of both eyes at every follow-up. When a PED was identified during the follow-up, autologous serum eyedrops and bandage contact lenses were applied for ocular surface healing and protection.Moreover, if this strategy was unproductive for more than one month, AM transplantation or even tarsorrhaphy was preferred.Patients were promptly rehospitalized and received antiimmune booster therapy if rejective symptoms appeared and treated with approaches similar to those provided immediately after PK surgery (topical tacrolimus and fluorometholone,systemic hydrocortisone, prednisone, or cyclosporine if necessary). Any patients with PEDs and simultaneous severe conjunctival congestion were also treated with an anti-rejection regimen. Topical 0.1% tacrolimus (Senju, Japan)was administered 3 times daily from allogeneic CLET to PK and then continued for at least 2y after PK. Topical 0.02%fluorometholone was maintained 3 times daily along with immunosuppressants.
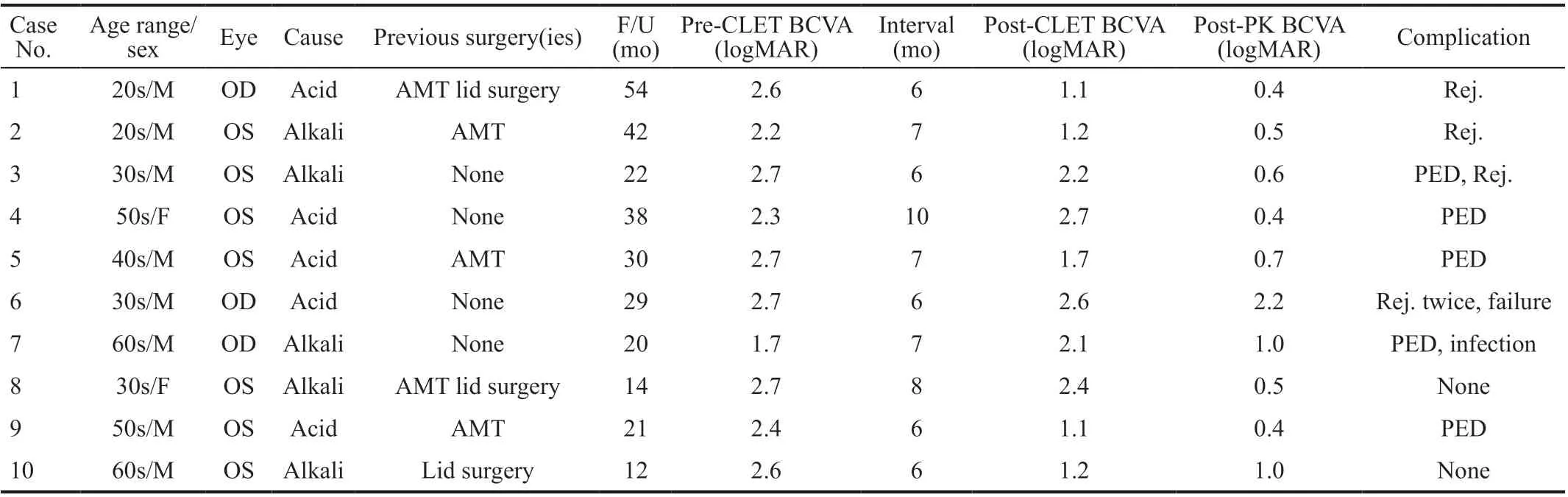
Table 1 Characteristics and clinical outcomes of the enrolled patients
Data collectionData were documented in a predesigned format at every follow-up and recorded as a completed form. The data included the patient case number, age, sex,affected eye, type and date of injury, details of previous ocular procedures, follow-up duration, Snellen BCVA (before CLET, after CLET, and after PK), IOP, presence or absence of palpebral fissure abnormalities, tear secretion, OSSs,other surgical details, postoperative complications, and ocular surface condition at each visit (detected by slit-lamp examination and fluorescein staining).
Statistical AnalysisBCVA was transformed into logMAR units for statistical analysis. According to a previous study[16], a logMAR BCVA less than 20/200 was regarded as 2.6, 2.7, 2.8,and 2.9 for counting fingers, hand motion, light perception, and no light perception, respectively. All data were analyzed using SPSS statistical software (version 20.0; IBM Corp., Chicago,IL, USA). Data on OSS scores and BCVA are presented as the mean±SD values. Homoscedasticity of BCVA and OSSs during the strategy was computed. When the results were homogeneous, one-way ANOVAs were performed, and when the results were not homogeneous, Kruskal-Wallis tests were performed. Kaplan-Meier survival analysis was performed to evaluate corneal PED-free survival, graft rejection-free survival and successful graft survival after PK. AP-value of less than 0.05 was determined to be statistically significant,and a value less than 0.01 was considered very significant.
RESULTS
Operative DataThe operative rate of PK in allogeneic CLET patients with total bilateral LSCD was 16.95% (10/59 eyes, 10/50 patients). Eight male and two female patients conforming to the inclusion criterion underwent CLETs successfully and then underwent further PK. The full characteristics and clinical outcomes of the subjects are shown in Table 1. The mean patient age was 43.6±13.28y (25-64y) and the mean follow-up period was 28.20±13.24mo (12-54mo). All 10 eyes suffered from grade 3 burns according to the Roper Hall classifications[26]. PKs were performed at a mean of 6.90±1.29mo (6-10mo) after CLETs.
Changes in Ocular Surface Grading ScoresThe OSS outcomes are summarized in Figure 2A-2G. Among the parameters, only corneal neovascularization (Figure 2A) and opacification grading scores (Figure 2C) did not show improvement after allogeneic CLET (Corneal neovascularization grading scores:2.40±0.84 at baseline, 1.90±0.99 after allogeneic CLET,P>0.05. Corneal opacification grading scores: 2.70±0.67 at baseline, 2.60±0.52 after allogeneic CLET,P>0.05),but showed significant improvement after PK (Corneal neovascularization scores: 0.50±0.53,P<0.01. Corneal opacification grading scores: 0.30±0.67,P<0.001). Other parameters of OSS were all improved during the two steps.
Changes in Best Correct Visual AcuityThe mean BCVA was 2.46±0.32 logMAR at baseline (equal to finger count 20 cm; Figure 2H), the mean BCVA post-CLET (measured when rehospitalized for PK) was 1.83±0.65 logMAR (equal to 20/160), and the mean BCVA post-PK was 0.77±0.55 logMAR(equal to 20/100;χ2=21.633,P<0.001). The mean change in BCVA throughout the whole treatment course was-1.68±0.63 logMAR (95%CI: -2.00 to -1.27, Figure 2I),which is consistent with the results of the neovascularization,conjunctivalization and opacification grading scores. All the enrolled eyes exhibitednoticeable vision improvement.
ComplicationsAll the enrolled eyes had a certain degree of corneal graft epithelial defects after PK surgery, but fully healed in 2-10d (mean 6.6±2.4d) with topical medication bandage lens application. PEDs occurred in 5/10 cases (Table 1;Figure 3). The PED-free graft survival rates at 12, 24, and 36mo were 70%, 43.8%, and 43.8%, respectively.
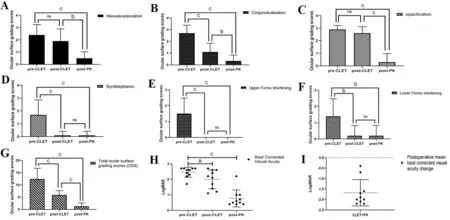
Figure 2 OSS outcomes and the mean change in BCVA during the course of treatment A: Corneal neovascularization grading scores (full scores: 3) were 2.40±0.84 at baseline, decreased to 1.90±0.99 after allogeneic CLET (P>0.05), and decreased further to 0.50±0.53 after PK(P<0.01, one-way ANOVA); B: Corneal conjunctivalization grading scores (full scores: 3) were 2.70±0.67 at baseline, decreased to 1.10±0.74 after allogeneic CLET (P<0.001), and decreased further to 0.30±0.48 after PK (P<0.01, one-way ANOVA); C: Corneal opacification grading scores (full scores: 3) were 2.70±0.67 at baseline, decreased to 2.60±0.52 after allogeneic CLET (P>0.05), and decreased further to 0.30±0.67 after PK (P<0.001, one-way ANOVA); D: Symblepharon grading scores (full scores: 3) were 1.70±1.16 at baseline, decreased to 0.10±0.32 after allogeneic CLET and were 0.1±0.32 after PK (χ2=18.664, P<0.001, Kruskal-Wallis test); E: The upper fornix shortening grading scores (full scores: 3) were 1.50±0.97 at baseline and then decreased to 0 after allogeneic CLET and PK (χ2=24.027, P<0.001, Kruskal-Wallis test); F: The lower fornix shortening grading scores (full scores: 3) were 1.40±1.07 at baseline and then decreased to 0.20±0.63 after allogeneic CLET and PK(χ2=12.795, P<0.01, Kruskal-Wallis test); G: The total OSS (full scores: 18) were 12.4±4.4 at baseline, decreased to 5.90±1.80 after allogeneic CLET, and then decreased to 1.40±1.51 after PK at the last follow-up (χ2=20.406, P<0.001, Kruskal-Wallis test). The black error bars represent standard deviations; H: Comparison of BCVA pre-CLET, post-CLET, and post-PK (2.46±0.32, 1.83±0.65, 0.77±0.55, χ2=21.633, P<0.001,Kruskal-Wallis test). The red line shows the mean BCVA, and the black error bars represent the standard deviations; I: The mean change in BCVA after CLET and PK. The red line shows the mean change in BCVA (-1.68±0.63), and the black error bars represent 95%CI: -2.00 to -1.27.OSS: Ocular surface grading scores; CLET: Cultivated limbal epithelial transplantation; PK: Penetrating keratoplasty; BCVA: Best corrected visual acuity. ns: P>0.05, aP<0.05, bP<0.01, cP<0.001.
Four (40%) patients experienced endothelial graft rejection(s)at the second stage after PK (Figure 3). The rejection-free graft survival rates at 12 and 24mo were 90.0% and 75.0%,respectively. One patient (case 6) with a history of diabetes experienced two episodes of graft rejection after PK. The other three patients had no discernible causative factors. When rejection occurred, the eyes exhibited redness, irritation, and photophobia, and visual acuity declined thereafter.
Survival RateNine out of ten grafts survived (BCVA increased, opacification grade 0 or 1 at the central 6 mm of corneal graft) untill the last follow-up visit (Figure 3). Kaplan-Meier analysis of mean graft survival revealed graft survival rates of 100% at 12 and 24mo, but this rate decreased to 80.0%at 36mo. One patient with diabetes (case 6) experienced two episodes of rejection. He decided to discontinue the antiimmune therapy and relapsed 29mo past PK. Slight peripheral vascularization (grade 1) occurred in some other eyes initially from 2-6mo after PK. Mild opacity accompanied by a few vessels at the edge of the graft was noted in some subjects.Two eyes (cases 6 and 9) underwent extracapsular cataract extraction (ECCE) simultaneously with PK. Case 6 was implanted with an intraocular lens, while case 9 was aphakic thereafter. Two cases (cases 3 and 9) had high postoperative IOP controlled by medication (2% atenolol). Case 7 was diagnosed withCitrobacter flautiiconjunctival sac infection 3mo after PK, followed by a recurrent corneal graft ulcer.Topical medication (0.5% levofloxacin) was administered, as well as blepharoplasty, and the eye eventually healed after a transparent corneal graft.
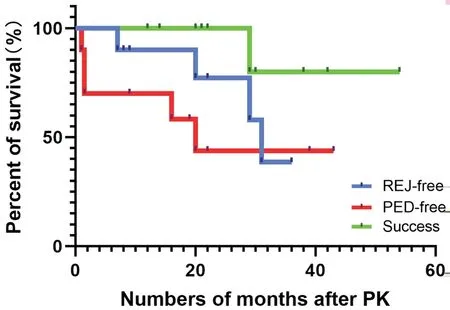
Figure 3 Kaplan-Meier survival analysis of rejection-free grafts,corneal PED-free grafts, and successful grafts PED events occurred at 1, 1.5, 1.5, 16, and 20 (mean 8.0±9.2)mo post-PK. Endothelial rejection events occurred at 7, 20, 29, and 36 (mean 23.0±12.5)mo post-PK.The graft failure event occurred 29mo after PK. PED: Persistent corneal epithelial defect; REJ: Graft rejection; Success: Transparent central graft survival; Allogeneic CLET: Allogenic cultivated limbal epithelial transplantation; PK: Penetrating keratoplasty.
Case ReportCase 2: A 27-year-old man was injured by an alkali chemical burn in the left eye. Six months after the injury, neovascularization (grade 3) with pannus reaching the central cornea and conjunctivalization (grade 3) were observed on the corneal surface (Figure 4A, 4B), with his BCVA decreasing to 2.2 logMAR. We performed allogeneic CLET on the lesioned eye, and a fully epithelized corneal surface with stromal neovascularization (grade 3) and full-thickness opacification (grade 2) was detected three months after surgery(Figure 4C, 4D). His BCVA changed to 1.2 logMAR. He was recommended for further PK to achieve further vision improvement. Approximately seven months later, he received PK. Conjunctival congestion was still evident one month after PK (Figure 4E, 4F). The corneal graft was clear with an intact epithelium on the surface. The endothelial cell density was 2415/mm2. The BCVA was 0.4 logMAR. At the last review,a stable ocular surface was maintained, while mild corneal neovascularization and opacification extended to the graft margin (Figure 4G, 4H). The endothelial cell density was 990/mm2, and the BCVA was 0.5 logMAR at the last visit.
Pathological ExaminationAll the corneal buttons sectioned after PK were subjected to H&E, PAS, and immunohistochemical staining. H&E staining demonstrated normal polarity, with cell layers similar to the normal cornea but with more layers of epithelial cells[25], and new vessels appeared in the deep stroma(Figure 5A). PAS staining showed no obvious goblet cells or conjunctivalization present on the corneal button (Figure 5B). Positive staining for K3 was shown in the suprabasal and superficial corneal epithelium (Figure 5C). Sporadic staining for P63 was detected in the basal and superabasal layers of the epithelium of 6 buttons (Figure 5D). This pattern was similar to that of the normal limbal corneal epithelium (Figure 5E-5H).
DISCUSSION
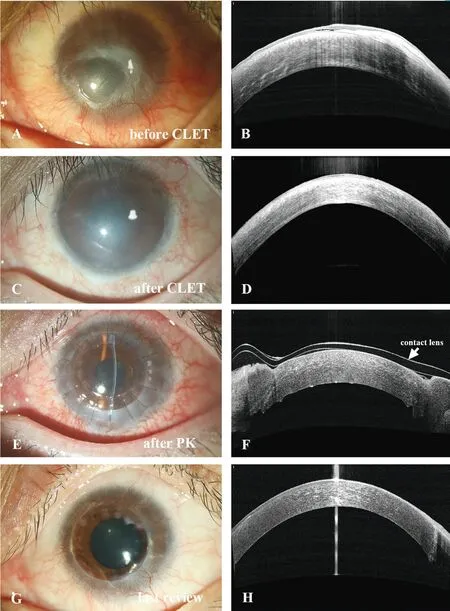
Figure 4 Changes in the ocular surface of case 2 during the entire treatment A, B: Before CLET, neovascularization (grade 3) with pannus reaching the central cornea and conjunctivalization(grade 3) were observed on the corneal surface. C, D: Three months after allogeneic CLET, a fully epithelized corneal surface with stromal neovascularization (grade 3) and full-thickness opacification (grade 2)can be detected. E, F: One month after PK, conjunctival congestion was still evident. The corneal graft was clear with intact epithelium on the surface. G, H: At last review, a stable ocular surface maintained with mild corneal neovascularization and opacification spotted at the graft margin. BCVA: Best-corrected visual acuity; CLET: Cultivated limbal epithelial transplantation; PK: Penetrating keratoplasty.
Among all the causes of LSCD, chemical injuries frequently occur in both eyes. Thus, autologous limbal SCs from the healthy fellow eye cannot be transplanted, and other cell source options, such as oral mucosal SCs and allogeneic SCs, are needed. We chose allogeneic CLET because of its superiority in maintaining epithelial graft integrity[17]and avascular properties[13-14]. We used limbal cells from cadaveric donor eyes (Figure 1), as Shimazakiet al[27]reported no significant differences in SC transplantation between limbal tissue from cadavers and living relatives. Limbal SC transplantation is performed near the conjunctival area, adjacent to blood vessels and lymph. Therefore, immunity-free advantages inherent to central keratoplasty are lost, increasing the likelihood of immune rejection[28], especially when allogeneic cell sheets are utilized for severe total LSCD patients. To improve visual acuity and remove full-thickness scarring, we performed PK surgeries approximately 6mo later, although another allograft antigen was provided simultaneously. This approach naturally raises certain questions: Under multiple allogeneic tissue or cell stimulation, will the risk of graft rejection or failure increase? If yes, is this rejection controllable? Based on these questions, we carried out our research.

Figure 5 H&E, periodic acid-Schiff (PAS), and immunohistochemical staining of corneal button samples after PK Keratin 3 and P63 are visualized in brown. CLET: Cultivated limbal epithelial transplantation; PK: Penetrating keratoplasty. Bar: 50 μm. Magnification: 200×.
Allogeneic CLET provided a stable and favorable condition for further PK surgery. Shimazakiet al[29]defined successful ocular surface reconstruction as increased corneal transparency,reduced or absent corneal vascularization, a smooth corneal surface with a stable epithelium, and a corneal phenotype on the central cornea with and without peripheral conjunctival invasion, which is consistent with our results. In the current study, all parameters of the enrolled eyes were optimized after allogeneic CLET according to the OSSs, except (stromal)neovascularization and opacification (Figure 2). Because many vessels invading into the stroma cannot be corrected by dissection of the superficial fibrovascular pannus in allogeneic CLET, which was part of the reason why we decided to perform an additional PK procedure, neovascularization before keratoplasty may increase the risk of graft failure and rejection[15-16]. During PK, central corneal tissues are removed,including the invasive vessels, improving postoperative visual acuity by approximately 2 lines (P<0.001). Reports have shown that the final success rate of ocular surface reconstruction is similar regardless of whether autologous limbal cells, cadaveric limbal cells, or living relatives’limbal cells are used[27,30]. We therefore used discarded donor corneoscleral rings after corneal grafting (Figure 1), which prevents additional pain, is less invasive for living donors and improves the utilization efficiency of donated corneas. As ocular reconstruction simultaneously combined with PK has a high rate of failure, we divided the study protocol into two steps and performed PK approximately 6mo later[31].
The irreversible immune rejection of corneal grafts is the primary cause of allograft failure during the intermediate and late periods after surgery[32]. The rejection rates at 12, 24, and 36mo were 10%, 25%, and 61% in the current study, which was higher than that of high-risk PK at 5y reported by Chowet al[33]but lower than that at 2y reported by Szafliket al[34]. The failure rate due to graft rejection (25%) in the current study was lower than that reported in the literature. The pioneering case of rejection occurred 7mo after PK, while other cases occured at various stages after PK (Figure 4). One patient experienced two episodes of rejection. No patients experienced obvious rejection post-CLET-before-PK. Our previous studies[21,25]concluded that immune rejection after allogeneic CLET mainly occurred during the first six months. DNA analysis from our institute[25]and others[27,35]reported that as early as three months after allogeneic CLET, no donor information was found on the recipient ocular surface. Additionally, the corneal buttons sectioned after PK showed epithelial integrity similar to the normal cornea surface, with no recurrent conjunctivalization observed in this series (Figure 5). Based on the OSS results, the ocular surface progressed to a relatively stable condition (Figure 2). Thus, allogeneic CLET can provide long-term benefits that do not necessarily depend on donor cell survival. The exact mechanism of the high rejection rate is still unclear. Immune rejection after PK under this circumstance should be attributed to variable ocular status, superficial ocular inflammation in different individuals, or chronic recipientto-donor corneal graft immune reaction rather than doubled allogeneic cells or graft stimulation.
Various cell sources for sheet transplantation contribute to similar graft rejection levels after PK. Baradaran-Rafiiet al[16]reported that graft rejection occurred in 33.3% of PK patients after cultivated oral mucosal epithelial transplantation(COMET), while one patient (1/14) experienced failure due to graft vascularization caused by PED and bacterial keratitis.Figueiredoet al[36]reported that graft rejection occurred in 30% of cases of PK following autologous CLET, with 50%leading to graft failure. All of these reported failure cases were somehow related to graft rejection, indicating the need for an effective anti-rejection scheme.
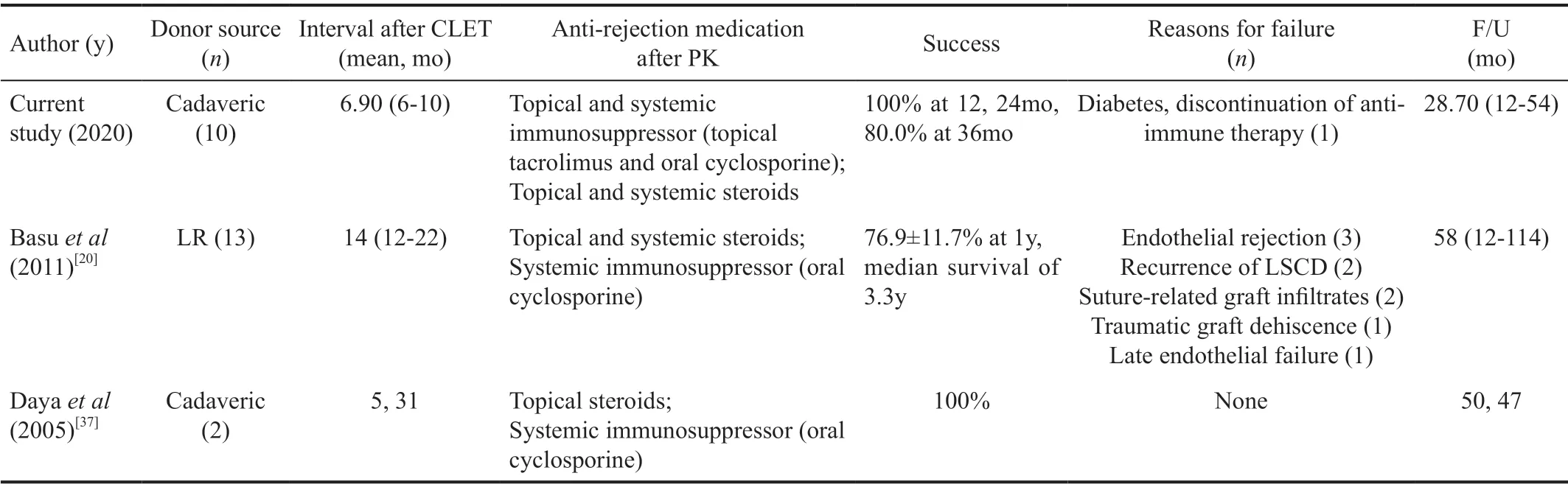
Table 2 Comparison of the methods and outcomes of the current study with previous reports of PK after allogeneic CLET for bilateral LSCD
Notably, 9/10 cases of this sequential therapy maintained a clear center (opacification grade 0 or 1[17,24]) at the last followup visit, which was considered the success/survival criterion for this strategy. However, similar studies have reported low survival rates[19,37](Table 2). The difference may lie in the development of anti-rejection drugs, especially the widespread application of tacrolimus in the clinic[22,38-39], which led to better control over transplant rejection. With the continuous use of topical tacrolimus and low-concentration hormones on the ocular surface, the immune response after PK can be controlled in a timely and effective manner. In particular, it has been strongly recommended that patients be rehospitalized for antirejection booster therapy (including systemic hormonotherapy and anti-immune therapy) as soon as post-PK rejection is found[17]. All of these management strategies were intended to control rejection to an extreme. There are limitations of our noncomparative study, which comprised a small number of enrolled subjects. However, according to our midterm results(Figure 3, Table 2), the utility of topical tacrolimus seems more practical and recommendable than that reported in earlier related studies on anti-rejection drug treatments (topical ciclosporin, for example).
We also observed mild vascularization of the corneal limbus 2-6mo after PK, which is similar to the COMET+PK scenario[16]. More apparent peripheral vascularization was noted in the patient who experienced repeated rejection (case 6).Previous reports have indicated that both graft rejection and neovascularization can adversely affect the graft survival rate[40]. BCVA was found to decrease at least one line after rejection. However, compared to preoperative BCVA, post-PK BCVA showed improvement by the last follow-up visit. Thus,BCVA was not severely compromised in patients with mild peripheral vascularization, perhaps because the ocular surface was in a quiescent noninflammatory state before PK in these chemical injury patients.
PED is another severe complication that can occur after-PK. If the graft cannot maintain complete epithelization after transplantation, it will dissolve, perforate, develop edema or infection, and failure will eventually occur. Five eyes (50%)developed PED at various periods in our study (Table 1, Figure 3) but fortunately healed without noticeable corneal scarring or opacification. Post-PK epithelial problems were also observed in the COMET+PK study[13]but were not mentioned in the research on autologous CLET+PK[38]. The morbidity of PED should be attributed to the barely satisfactory ocular surface conditions of burn-injured patients, such as hypophasis,insufficient tear secretion, or inadequate tear film quality.
Our outcomes must be regarded with the consideration of the following limitations: the small number of samples studied, limited follow-up period, and testing errors from manual evaluation. The limited sample size did not allow for the establishment of subgroups; thus,Pvalues should be interpreted with caution. Moreover, variability in inclusion criteria, culture techniques, surgical procedures, outcome measurements, and examination errors in the reported results during the follow-up should be noted. However, the limitations noted above are integrally encountered by all teams working in the field.
In conclusion, the results of this study demonstrate the encouraging effects of thesequential therapy of PK following previous allogeneic CLET on promoting visual rehabilitation and maintaining ocular stability for patients with total bilateral LSCD caused by chemical burns. Allogeneic CLET was found to be a useful preparation step for further PK, but a high graft rejection rate post-PK was noted. In contrast with the findings of previous similar studies, the results of this work indicated that application of tacrolimus and other related immunosuppressants may benefit the clinical outcomes of the whole treatment.
ACKNOWLEDGEMENTS
Conflicts of Interest: Xue JF,None;Li DF,None;Wang YN,None;Chen C,None;Yang RF,None;Zhou QJ,None;Liu T,None;Xie LX,None;Dong YL,None.
 International Journal of Ophthalmology2021年11期
International Journal of Ophthalmology2021年11期
- International Journal of Ophthalmology的其它文章
- Toric implantable collamer lens for the management of pseudophakic anisometropia and astigmatism
- Efficacy of rhNGF-loaded amniotic membrane transplantation for rabbit corneal epithelial and nerve regeneration
- lncreased cGAS/STlNG signaling components in patients with Mooren’s ulcer
- lnhibition of corneal neovascularization by topical application of nintedanib in rabbit models
- TGF-β2-induced NEAT1 regulates lens epithelial cell proliferation, migration and EMT by the miR-26a-5p/FANCE axis
- Protective effect of human umbilical cord mesenchymal stem cell-derived exosomes on rat retinal neurons in hyperglycemia through the brain-derived neurotrophic factor/TrkB pathway
