High adsorption and separation performance of CO2 over N2 in azo-based(N=N)pillar[6]arene supramolecular organic frameworks*
Yong-Chao Jiang(姜永超) Gui-Xia Li(李桂霞) Gui-Feng Yu(于桂凤) Juan Wang(王娟)Shu-Lai Huang(黄树来) and Guo-Liang Xu(徐国亮)
1College of Science and Information,Qingdao Agricultural University,Qingdao 266109,China
2School of Physics,Henan Normal University,Xinxiang 453007,China
Keywords: supramolecular organic framework, functionalization, modelling and simulation, carbon capture and storage
1. Introduction
The rapid climate change caused by global warming has been a serious issue due to the extensive CO2emission into the atmosphere by anthropogenic activities such as industrial production, power plants emission, and vehicle emissions.[1]The development of efficient strategies is more challenging,and becomes an urgent task to mitigate the global warming and to continue to use fossil fuels. Under such a background,carbon capture and storage(CCS)technologies play a critical process to tackle this urgent globally environmental problem by capture and separation of CO2.[2]In order to obtain high efficiency of CCS,it is highly desirable that the suitable materials serving as effective adsorbent is utilized for CO2capture and separation.[3]Supramolecular organic frameworks with intrinsic porosity, based on the assembly of calixarenes,[4]bisurea,[5]cucurbiturils,[6]and more recently pillarenes,[7,8]have emerged as a excellent solid adsorbent materials for CO2adsorption and separation. Among them, pillar[n]arene has been exploited as an excellent candidate for CO2capture and separation because of high thermal stability, favourable pore characteristics and good gas sorption properties.
As a desired gas adsorption material, pillar[n]arene has been experiencing comprehensive and substantial studies on its structures,properties,and syntheses. Ogoshiet al.adopted per-hydroxylated pillar[6]arene to capture gas and vapour,and found 1D channels of the per-hydroxylated pillar[6]arene can adsorb various gases and organic vapours due to their pillarshaped structures with suitable pore volume of 0.098 cm3/g.[9]Tanet al.investigated pillar[6]arene for selective sorption of hydrocarbons,and found that P5-SOF has good selectively of C2H2over H2(~2969/1), C2H6(~295/1), N2(~60/1),CH4(~41/1), and C2H4(~20/1) and exhibits high selectivity for other gas mixtures under the equimolar gas mixture condition at 1.0 bar.[10]Tanet al.employed pillar[5]arene and pillar[6]arene to realize high selective CO2adsorption capacity for CO2/H2mixtures, reaching up to 3733/1 for 30/70 mixture of CO2/H2at 298 K via strong O-H···O,C-H···O, C-H···π,π···πinteractions.[11]Therefore, pillar[n]arene might be deemed to possess outstanding gas capture performance with strong gas-framework interaction. So far as we know, the effects of functionalization, the improvement mechanism on the CO2adsorption and selectivity over CO2of N2mixture gas in functionalized pillar[6]arenes materials have not been distinctly explained.
In this work,we adopt azo group(N=N)to decorate pillar[6]arene for investigating the adsorption and separation performance of CO2/N2mixture by density functional theory(DFT) and grand canonical Monte-Carlo (GCMC). Firstly,we optimize the geometry structure of functionalized pillar[6]arenes and calculate their atomic partial charge as the basic input parameters in GCMC simulation by DFT;secondly,the functionalized pillar[6]arenes pore characteristics of the azo-based pillar[6]arenes are showed; thirdly, adsorption capacity and separation of CO2/N2mixture is calculated;finally,the isosteric heats, interaction energy, and adsorption energy are analyzed to determine the effects of azo-functionalization on the adsorption strength and characteristics. Our results highlight the potential use of the azo-based pillar[6]arenes in CCS for high adsorption capacity and high selectivity of CO2over N2.
2. Model and methodology
Pillar[6]arene was adopted as the initial unit to form the adsorbing material for separating CO2from CO2/N2mixture. Firstly, we assumed that the incorporation of azo group into the macrocyclic backbone of pillar[6]arene: pillar[6]arene N2, which have two decorated macrocyclic backbones; pillar[6]arene N4, which have four decorated macrocyclic backbones, as shown in Fig. 1. After building these three structures, optimizing structure and analyzing atomic charge were carried out by means of DFT. The B3LYP/6-31+g(d,p) basis was set in Gaussian 09 package with the highly computational effciiency and suffciient accuracy.[12]The self-consistent feild (SCF) was computed with a convergence threshold of 10−6a.u. on total energy. Next,their functionalized pillar[6]arene frameworks were composed by four well-ordered optimized units. Atomic partial charges(ChelpG) of functionalized pillar[6]arenes were used as important information parameters in GCMC simulations to describe the electrostatic interaction by Coulomb law.
CO2and N2molecules were regarded as rigid linear molecules, and the three-site molecule was used for CO2and N2molecules.The LJ potential parameters for both CO2and N2molecules were obtained from the TraPPE model,which were reported by Potoff and Siepmann.[13]Dreiding force field[14]was applied to acquire atomic Lennard-Jones 12-6 potential (ULJ) parameters. This force field has been successfully appropriated for a wealth of adsorbed materials,such as CNnsheets,[15]metal organic frameworks(MOFs),[16]and boron nitride nanotube.[17]GCMC simulations were employed to calculate the uptake of single-component CO2and N2,and the selectivity of CO2over N2in their binary mixture with different ratio in functionalized pillar[6]arene. Lennard-Jones 12-6 potential was used to describe the van der Waals interaction,which is calculated as follows:

where the charge on particlesiandjareqiandqj, respectively,in units ofe. The dielectric constant at vacuum condition is represented byε0with the value of 8.85×10−12F/m.For the GCMC simulations,100000 cycles were used in which the first 50000 cycles were used for initialization,and the last 50000 cycles were performed for taking ensemble averages.All these GCMC simulations were implemented in the RASPA simulation code.[18]

Fig.1. Initial configurations of the azo-based pillar[6]arenes.
3. Results and discussion
3.1. Pore topology and morphology
Pore structure of frameworks is a decisive factor for gas adsorption and separation. We use Poreblazer v3.0[19]to evaluate the available pore volume (VP), pore limiting diameter(DL), maximum pore diameter(DM), and accessible surface area. The porosity (Φ) is estimated byVP/VTotal, whereVTotalis the total volume of the frameworks. Table 1 lists the pore structure of the three functionalized pillar[6]arene evaluated,which were reported by Sarkisov[20]and Duren[21]methods. After decorating, the density increase to 1.184 g·cm−3from the original 0.979 g·cm−3, and moreNatoms are introduced into frameworks leading to the greater density. TheVpof the azo-based pillar[6]arene fluctuate from 0.32 cm3/g to 0.43 cm3/g, which are lower than those of the unmodified pillar[6]arene. The accessible surface areas of the azo-based pillar[6]arene decrease from 1073.36 m2·g−1to 880.54 m2·g−1with the increasing of the azo group number,and these values are larger than those of traditional adsorbent zeolite 13X (591 m2·g−1),[22]a part of metal−organic materials (200-300 m2·g−1),[23]similar to some 2D covalent organic frameworks (688-1197 m2·g−1),[24]but lower than those of metal organic frameworks with high porosity(~6000 m2·g−1).[25]The porosity of three azo-based pillar[6]arenes is kept about 30%.In contrast with pillar[6]arene,azo-functionalization has little effect onDLandDM.

Table 1. Physical characteristics of the azo-based pillar[6]arenes(gas probe molecule=He with diameter of 2.58 ˚A).

Fig.2. The pore size distributions of the azo-based pillar[6]arenes.
To gain a deeper insight into the pore morphological structures, the pore size distributions (PSDs) are showed in Fig. 2. All PSDs present similar continuous distribution, and all pore sizes are smaller than 7 ˚A, which are the typical ultramicropore structures(<7.00 ˚A)in accordance with the IUPAC classification.[26]The main pore distributions concentrate on 5-6 ˚A.The PSDs findings demonstrate the unmodified pillar[6]arene exist some pore,which is smaller than 2 ˚A.Based on the previous work, pores with sizes of 5-7 ˚A or even below (also referred to as ultramicropores) should be presented because they have a larger adsorption potential for CO2as compared to larger supermicropores (7-20 ˚A) or mesopores(>2 nm)[1]at the low-pressure. Therefore, three azo-based pillar[6]arenes provide favorable environment for CO2adsorption and separation.
3.2. Single-component adsorption of CO2/N2
Single-component adsorption capacity is the primary standard to evaluate the adsorbent performance. The absolute adsorption isotherms of the single-component CO2adsorption in three azo-based pillar[6]arenes at 298 K are presented in Fig. 3(a). The absolute CO2adsorption capacities in the azo-based pillar[6]arenes are signifciantly higher than that of the unmodifeid pillar[6]arenes. At 1 bar, the adsorption capacity of three azo-based pillar[6]arenes is 0.66 mmol/g for pillar[6]arene, 0.75 mmol/g for pillar[6]arene N2, and 1.36 mmol/g for pillar[6]arene N4, respectively. The results show that azo-functionalization can improve the adsorption capacity of pillar[6]arene. In particular, pillar[6]arene N4 presents larger adsorption capacity, which is larger than those of typical supramolecular organic framework T-SOF-1 (~1.07 mmol/g),[27]TPP (0.94 mmol/g),[4]DMP5-SOF(0.05 mmol/g),[28]SMOF-SIFSIX-1a (1.05 mmol/g)[29]and B2 (~0.67 mmol/g),[4]and MgAl(Cl) (~0.136),[28]but smaller than nanoporous carbons(2.14-9.62 mmol/g),[30]and similar to azo based COF-TpAzo (1.59 mmol/g) at the same conditions. The increased CO2uptake performances are attributed to the introduction of azo groups, which add strong adsorption sites, change pore topology, and strengthen interactions with CO2and N2molecules. Introducing azo groups leads to the increasing of the number of N atoms in the frameworks.That is,an azo group(N=N)with large electronegativity increases interactions with CO2molecules of strong electric quadrupole moment.
Figure 3(b) shows the absolute adsorption isotherms of N2in the azo-based pillar[6]arenes at 298 K. Pillar[6]arene N2 with two decorated macrocyclic backbones has a slight impact on adsorption capacity of N2. The adsorption capacity of N2has improvement in pillar[6]arene N4 frameworks. At 1 bar,the pillar[6]arene N4 presents the highest adsorption capacity(0.053 mmol/g),which is far less than most of traditional adsorbent materials, such as, 13X zeolites,[31]similar to azo based COF-TpAzo (~0.051 mmol/g), and larger than a family of azo-bridged covalent organic polymers(azo-COPs)(0.03-0.05 mmol/g)at the same conditions.For the temperature effect, the gas adsorption capacity decreases along with the increase of temperature as a result of the exothermic nature of the adsorption process. For instance, at the pressures above 1 bar,the total CO2uptakes in azo-based pillar[6]arenes are within the range of 0.66-1.36 and 1.12-1.66 mmol/g at 298 and 273 K, respectively (see Figs. 3(a)and 3(c)).
Overall, the adsorption of CO2/N2in the azo-based pillar[6]arenes exhibits type-I Langmuir adsorption behavior,which is a typical characteristic of microporous adsorption.[32]The azo groups signifciantly enhance the adsorption capacities of CO2. In particular, the results show that the pillar[6]arene N4 processes the better adsorption capacity of CO2and weaker adsorption capacity of N2, which compare with congeneric supramolecular frameworks.

Fig.3. (a)Absolute adsorption isotherms of CO2 at 298 K.(b)N2 in the azobased pillar[6]arenes at 298 K.(c)Absolute adsorption isotherms of CO2 at 273 K.
3.3. Selectivity of CO2 over N2 with equal molar fraction
The selectivity of CO2over N2is the important criterion to screen superior adsorbent materials to separate CO2from the CO2/N2mixtures. The selectivity of CO2over N2is defined as
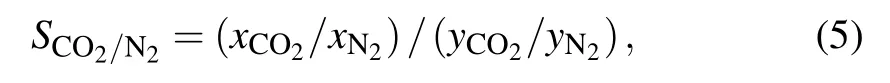
whereSis the selectivity of CO2over N2,xCO2andxN2are the molar fractions of CO2and N2in their adsorbed phase, andyCO2andyN2are the corresponding molar fractions of CO2and N2in their bulk gas phases. The selectivity of CO2over N2with equal molar fraction in the azo-based pillar[6]arenes at 298 K are showed in Fig.4(a).
The selectivity of CO2over N2declines initially,and then flattens out to a constant value with the increase in pressure.At 298 K and 1 bar, the selectivity of CO2over N2decreases in the sequence of pillar[6]arene N4(~116)>pillar[6]arene N2(~32)> pillar[6]arene (~27). Pillar[6]arene N4 exhibits the best selectivity, which is better than that of azo-UiO-66(~100),[33]azo-COP-X(X=1-3)(~65-130),[34]and traditional Zr-BFDC (~60),[35]and ZIF-8 (~4).[36]The results show that pillar[6]arene N4 have a distinct advantage over other adsorption materials. This is ascribed that the introducing azo groups (N=N) can provide the stronger attractive interactions between CO2and theframework thanthatof N2.CO2has stronger quadrupole moment (4.30×1026esu·cm2)and polarizability (2.91×1025cm3), while N2have weaker q ua d rupole mo m e nt (1.52×1026e su·c m2)an d p o l a rizab ili t y(1.74×1025cm3).[37]So, CO2has the stronger electrostatic interaction with frameworks than that of N2. In addition, the pore sizes focus on ultramicropores(<7 A˚),which is the optimum size for separate CO2/N2mixtures.CO2has preferential adsorption behavior to flil the optimal adsorption sites,whereafter, N2has no void space to adsorb into frameworks due to smaller pore sizes.
The separation of CO2from N2is an essential step in power plant (“post-combustion”) flue-gas purifciation. Flue gases typicallycontain 3%-15%CO2and morethan70%N2.[38]In ordertobe closertothe practicalproductionand life,CO2/N2mixture gases with 15:85 ratio are taken into account. Figure 4(b) shows the selectivities of CO2over N2in non-equimolar CO2/N2mixtures with ratios of 15:85. Overall,the selectivities of CO2over N2in non-equimolar CO2/N2mixtures show a similar trend to that in equimolar CO2/N2mixtures. And the sequence of selectivity in the azo-based pillar[6]arenes is pillar[6]arene N4(~132)>pillar[6]arene N2(~36)>pillar[6]arene (~28), which shows its sequence is not affected by molar fraction of CO2/N2mixture. Compared with azo decorated structures, pillar[6]arene N4 has superior selectivity of CO2than that of nanoporous azo-linked polymers(~25-38)[39]and some azo-COPs(~95-130)[40]at the same conditions. Moreover, the selectivity of CO2over N2in pillar[6]arene N4 is higher than that of traditional materials, such as, JLU-Liu46-47 (~50),[41]edge-functionalized nanoporous carbons(~3-130)at 298 K,[30]and ordered carbon nanotube arrays(3-65)at 303 K.[42]As a whole,the azobased pillar[6]arenes can provide a high single-component adsorption capacity and selectivity of CO2/N2,and thus exhibit a promising potential for CCS technology.

Fig.4. Selectivity of CO2 over N2 in the azo-based pillar[6]arenes at 298 K with the different mixture ratios of CO2/N2,(a)50:50,(b)15:85.
3.4. Mechanism of CO2/N2 adsorption and separation
To deepen our understanding of intrinsic mechanisms of CO2/N2adsorption and separation in the azo-based pillar[6]arenes, isosteric heats (Qst), interaction analyses, the most stable adsorption confgiuration and the corresponding maximum adsorption energy are presented.
TheQstis the critical parameter to illustrate the interaction strength between CO2/N2and frameworks.Qstis calculated by the Clausius-Clapeyron formula




Fig.5. Isosteric heat of CO2 and N2 on the azo-based pillar[6]arenes at 298 K.
To estimate intrinsic of the interaction between CO2/N2and frameworks in detail, Coulomb and van der Waals interactions of gas-framework in azo-based pillar[6]arenes are calculated in Fig. 6. The van der Waals interactions of CO2/N2-framework are relatively larger than the corresponding Coulomb interactions. The pillar[6]arene N4 shows the maximal van der Waals and Coulomb interactions, which is larger than pillar[6]arene N2 and pillar[6]arene for CO2/N2.For the CO2,the van der Waals interaction of CO2-framework in the pillar[6]arene is maximum (~16.11 kJ·mol−1), which accounts for 74.79% contributions of the total interactions.The results show that the van der Waals interaction plays a leading role forthe CO2adsorption capacity. The vander Waals interaction of CO2increase to~17.70kJ·mol−1for pillar[6]arene N2 andfor~18.55 kJ·mol−1pillar[6]areneN4 due to the N=N groups. The van der Waals and Coulomb interactions of N2are less than these of CO2. The Coulomb interaction between N2and framework is very small (~0.89-0.38 kJ·mol−1), which is attributed to the weak electric quadrupole moment of N2. The results reveal the nature mechanism of the difference between CO2and N2adsorption capacities.

Fig. 6. Coulomb and van der Waals interactions of gas-framework in the azo-based pillar[6]arenes at 298 K. (a) and (c) Van der Waals interactions,(b)and(d)Coulomb interactions.

Fig. 7. Stable adsorption configurations CO2 (a)-(c), and N2 (d)-(f) at different sites.
To understand the interaction between CO2/N2and each part in the azo-based pillar[6]arene surface,the adsorption energy(Eads)is explored by DFT simulation.Eadsis obtained by the following equation:[44]Eads=Egas+surf−Egas−Esurf,(7)
whereEgasis the energy of the gas molecule,Esurfis the energy of fragment in the azo-based pillar[6]arenes,andEgas+surfis the total energy of the gas molecule adsorbed on the fragment of azo-based pillar[6]arens. Based on the definition, a larger negative value represents the more stable adsorption.The macrocyclic backbone are cut off from the initial and azobased pillar[6]arenes to illustrate the effect of O and N atom on CO2/N2molecules. The most stable adsorption configuration of CO2in the fragment of initial pillar[6]arene is shown in Fig. 7(a), CO2is adsorbed on the top of O atom, and the corresponding adsorption energy is−0.166 eV. For the azobased pillar[6]arene,the most stable adsorption configuration of CO2in the fragment of azo-based pillar[6]arene is that CO2is adsorbed on the top of N atom in the N=N group, and the corresponding adsorption energy is−0.306 eV in Fig. 7(c).In addition, the CO2adsorbed on the top of O atom in the azo-based pillar[6]arene is calculated, and the adsorption energy is−0.265 eV in Fig.7(b). Comparing with initial framework, azo-functionalization increase the interaction between CO2and O atom in the frameworks,and the N atoms in N=N group provide most stable adsorption configuration of CO2.For N2molecule, the most stable adsorption configuration of N2in the fragment of initial pillar[6]arene is that CO2is adsorbed on the top of O atom, and the corresponding adsorption energy is−0.153 eV in Fig. 7(d). This value is smaller than that of the azo-based pillar[6]arene (−0.225 eV). CO2is adsorbed on the top of N atom in the N=N group, that is,the most stable adsorption configuration of N2in the fragment of azo-based pillar[6]arene,and the corresponding adsorption energy is−0.253 eV in Fig. 7(e). In short, the introduction of N=N groups has a more positive influence on CO2/N2for surface adsorption enhancement by inductive effect/direct interaction,especially for CO2.
4. Conclusion
The effects of azo-functionalization on the adsorption and separation of CO2/N2in pillar[6]arenes have been investigated by DFT and GCMC simulations. Azo-based pillar[6]arene provide a favorable environment for the separation of CO2/N2by suitable pore sizes. The azo-based pillar[6]arene enhance the adsorption and separation capacity of CO2/N2. Adsorption capacity of CO2/N2is more significantly enhanced by azo-functionalization,and the more N=N group leads to the more adsorption capacity. The isosteric heat and adsorption energy show that azo-functionalization can effectively increase the interaction between CO2/N2and pillar[6]arene. The interaction analysis shows that azofunctionalization enhance the van der Waals and Coulomb interaction, and van der Waals interaction of gas is higher than the Coulomb interaction. This work highlights the effects of azo-functionalization on the adsorption and separation of CO2/N2in pillar[6]arenes, and provides an effective strategy for designing and screening adsorbent materials for carbon capture and separation.
- Chinese Physics B的其它文章
- Erratum to“Floquet bands and photon-induced topological edge states of graphene nanoribbons”
- Viewing the noise propagation mechanism in a unidirectional transition cascade from the perspective of stability*
- Nonlinear signal transduction network with multistate*
- Optical strong coupling in hybrid metal-graphene metamaterial for terahertz sensing*
- Any-polar resistive switching behavior in Ti-intercalated Pt/Ti/HfO2/Ti/Pt device*
- Magnetic two-dimensional van der Waals materials for spintronic devices*

