Poly(ε-caprolactone)-Polypeptide Copolymer Micelles Enhance the Antibacterial Activities of Antibiotics
Lingshan Chen , Yuanxiu Hong , Shisheng He , Zhen Fan , Jianzhong Du
1 Department of Orthopedics, Shanghai Tenth People’s Hospital, Shanghai 200072, China.
2 Department of Polymeric Materials, School of Materials Science and Engineering, Tongji University, Shanghai 201804, China.
3 Institute for Advanced Study, Tongji University, Shanghai 200092, China.
4 Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education, Tongji University, Shanghai 201804, China.
Abstract:Bacterial infection is a major threat to human health, and can cause several diseases including gastroenteritis, influenza, tetanus, and tuberculosis. As conventional antibiotic treatment may cause various undesirable effects such as stomach disorder and bacterial resistance, it is necessary to improve the antibacterial efficiency of antibiotics. Here, we synthesized a peptide-based copolymer, poly(ε-caprolactone)-blockpoly(glutamic acid)-block-poly(lysine-stat-phenylalanine) [PCL34-b-PGA30-b-P(Lys16-stat-Phe12)] by ring-opening polymerization (ROP)of ε-caprolactone and amino acid N-carboxyanhydride (NCA). Successful synthesis of the copolymer was verified by proton nuclear magnetic resonance and size exclusion chromatography. This copolymer can self-assemble into negatively charged micelles (−26.7 mV)under alkaline conditions by solvent switch method. The micelle structure was confirmed by transmission electron microscopy and dynamic light scattering, and revealed to have a diameter of ~42 nm.Antibiotics were loaded into micelles during the self-assembly process, and cell viability assay was conducted to evaluate its cytotoxicity with and without tobramycin. No obvious cytotoxicity was observed for both micelles when the concentration was lower than 300 μg∙mL−1. The antibiotic-loaded micelles demonstrated very low minimum inhibitory concentrations(MICs)against both Gram-negative Escherichia coli (E. coli)(7.8 μg∙mL−1)and Gram-positive Staphylococcus aureus (S.aureus)(18.2 μg∙mL−1), while the MICs of free tobramycin were 3.9 and 1.0 μg∙mL−1, respectively. The drug-loading content and efficiency of the micelles were 5.2% and 24.3%, respectively. Therefore, the MICs of the loaded tobramycin against E.coli and S. aureus were 0.4 and 0.9 μg∙mL−1, respectively, suggesting that the micelle could enhance the antibacterial activity of antibiotics. Tobramycin-loaded micelles demonstrated a sustained release characteristic, with 85% of the antibiotics released after 8 h. In bacteria-induced acidic microenvironment, the coil conformation of PGA blocks transforms and PGA blocks shrink toward the micelle core. Concomitantly, the carboxyl side chains are protonated in an acidic environment, increasing the hydrophobicity of this micelle. Antibiotics will be captured when reaching the outer core to slow down the releasing process. Furthermore, the poly(lysine-stat-phenylalanine)[P(Lys-stat-Phe)] coronas with broad spectrum intrinsic antibacterial activity can penetrate the bacterial cell membrane, leading to leakage of the cellular contents of the bacteria and ultimately their death. Due to the sustained release property of micelle and the intrinsic activity of the antibacterial peptide segments, this micelle can greatly enhance the antibacterial activity of antibiotics. Overall, this antibiotic-loaded micelle provides a novel approach for significantly reducing the antibiotics dosage and avoiding the associated health risks.
Key Words:Antibiotic; Self-assembly; Peptide; Polymer Micelle; Antibacterial Activity;Sustained Release
1 Introduction
Bacterial infections can cause food poisoning1,2, influenza3,tuberculosis4,5inflammation6and so on, which are often treated by antibiotics in the clinic. However, antibiotics also have some drawbacks in the treatment of bacterial infections, which may induce characteristic changes in lysosomes of proximal tubular cells which damage kidney7. It can also cause adverse reactions such as allergies8,9. In addition, the abuse of antibiotics may lead to bacterial resistance, allowing drug-resistant genes to spread rapidly between bacteria, promoting the emergence of“superbacteria”10–12. Therefore, new strategies need to be developed to improve the antibacterial efficiency of antibiotics.To solve this problem, a series of antibacterial agents with different antibacterial mechanisms have been developed to replace the conventional antibiotics, such as quaternary ammonium cationic polymers13, silver nanoparticles14–17,chitosan derivatives18, antibacterial peptides (AMPs)19,etc.Among them, AMPs are derived from natural antibacterial peptides and widely studied now19,20. Conjugation of AMPs into polymers can create more functions, which can be selfassembled into a variety of antibacterial nanomaterials such as polymer vesicles21,22, hydrogels23,24, polymer micelles and so on19,25,26.
Self-assembly is an important “bottom-up” approach for synthesizing biomedical materials27–30. In nature, small molecule phospholipids can form a bilayer structure of cell membranes by self-assembly, while nucleic acids and histones can form chromosomes for storing genetic information through multi-level self-assembly31,32. When the polymer is used as a self-assembling basic structural unit, various morphologies such as micelles33,34, vesicles35, double-layer structures, hollow hoops, and large composite micelles can be formed31,36. Due to the unique nanostructure and excellent biocompatibility,nanoassemblies have attracted great interest in biomedical applications30,37. In the field of antibacterial materials, polymer assemblies have also been widely studied and developed with excellent antibacterial activities. For example, Bryaskovaet al.38synthesized an amphiphilic poly(vinyl alcohol)-block-poly(acrylonitrile)(PVOH-b-PAN)copolymer and prepared PVOH-b-PAN based micelle with embedded silver nanoparticles. The antibacterial activity of the micelles againstEscherichia coli(E.coli),Pseudomonas aeruginosa(P. aeruginosa),Staphylococcus aureus(S. aureus)and spore solution ofBacillus subtilis(B.subtilis)were evaluated. The minimum inhibitory concentration(MIC)and minimum bactericidal concentration (MBC)demonstrated this micelle has high antibacterial activity againstE. coli,S. aureusandP. aeruginosa. Our group39reported an antibacterial polymer micelle assembled from a triblock polymer poly(ethylene oxide)-block-poly-(ε-caprolactone)-block-poly [(2-tert-butylaminoethyl)methacrylate] [PEO43-b-PCL20-b-PTA20]with a PTA segment as an antibacterial component. As the PTA segment lengthens, the antibacterial effect of the micelle increases. This micelle is water dispersible and biodegradable and can be used as sterilization agents and antimicrobial drugs.
Herein, we designed a polymer micelle loaded with tobramycin which can increase the antibacterial effect of the antibiotics (Scheme 1). The copolymer poly(ε-caprolactone)-block-poly(glutamic acid)-block-poly(lysine-stat-phenylalanine)[PCL34-b-PGA30-b-P(Lys16-stat-Phe12)] was synthesized through ring-opening polymerization (ROP)ofε-caprolactone and amino acidN-carboxyanhydride (NCA), and can self-assemble into negatively charged micelle (−26.7 mV)in alkaline conditions to load the aminoglycosides antibiotics. The drug loading content(DLC)and drug loading efficiency (DLE)as well as release profiles were measured using fluorescence spectrometer. The antibacterial activity of antibiotic-loaded micelle was also studied by measuring MIC, which are 7.8 and 18.2 μg·mL−1againstE. coliandS. aureusrespectively, demonstrating highly efficient antibacterial activities. Overall, a peptide-based copolymer was synthesized and self-assembled into micelles with enhanced antibacterial efficiency compared to antibiotics alone.
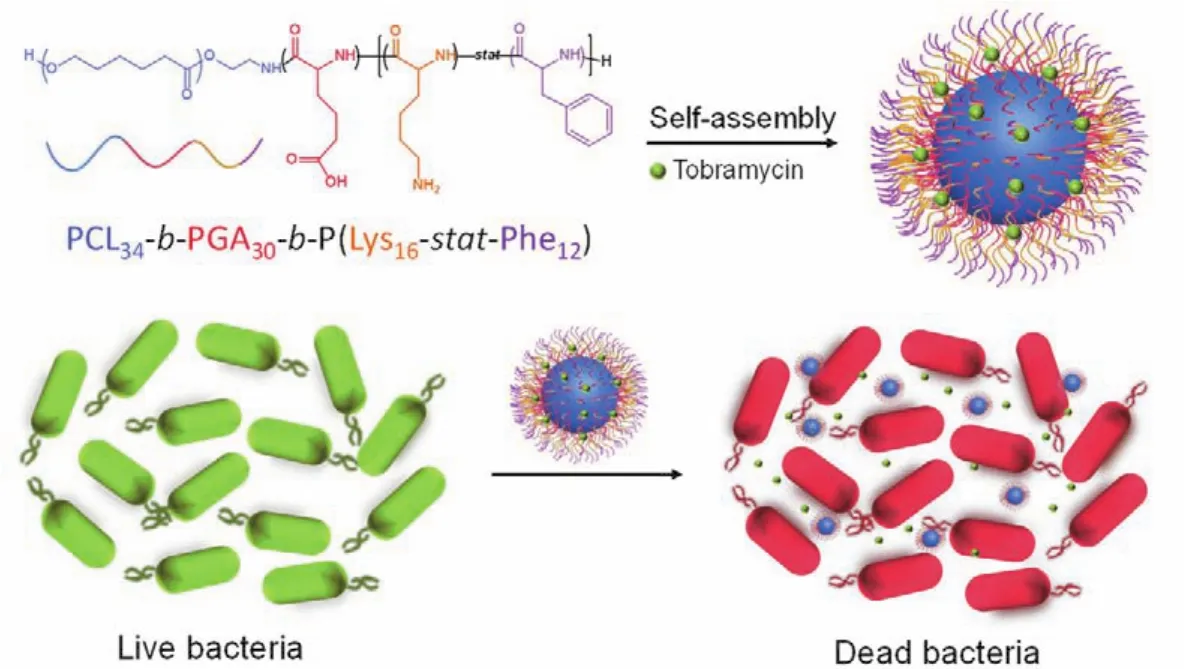
Scheme 1 Schematic model showing the antibiotic-loaded poly(ε-caprolactone)-polypeptide copolymer micelles with enhanced antibacterial activities.
2 Experimental
2.1 Materials
N-ε-Benzyloxycarbonyl-L-lysine,γ-benzyl-L-glutamate, L-phenylalanine, hydrogen bromide (30% in acetic acid)and triphosgene were ordered from Shanghai Hanhong Chemical Co.,Ltd (Shanghai, China).ε-Caprolactone, toluene, trifluoroacetic acid (TFA), stannous 2-ethylhexanoate (Sn(Oct)2), hexane, diethyl ether, phthaldialdehyde, tetrahydrofuran (THF), boric acidN-(tertbutoxycarbonyl)-2-aminoethanol, dichloromethane, methanol,ethanol,N,N-dimethylformamide (DMF), diethyl ether and 2-mercaptoethanol were ordered from Aladdin (Shanghai, China).THF and DMF were dried by reflux for 24 h with sodium strips and calcium hydride, respectively. Gram-positive bacteriumS.aureus(ATCC29213)and Gram-negative bacteriumE. coli(ATCC25922)were ordered from Nanjing Bianzhen Biological Technology Co., Ltd. (Nanjing, China). Unless otherwise specified, all the materials were used directly without further purification.
2.2 Methods
2.2.1 Synthesis of Z-Lys-NCA monomer
α-Pinene (24.28 g, 178.4 mmol)andN-ε-benzyloxycarbonyl-L-lysine (10.00 g, 35.68 mmol)were suspended in anhydrous THF (200.0 mL)in a three-neck round-bottom flask under magnetic stirring. Anhydrous THF (60.0 mL)and triphosgene(7.96 g, 26.8 mmol)was added into a constant pressure funnel and added dropwise into the flask at 50 °C. The reaction was stirred and allowed refluxing for four hours and the mixture would turned into clear orange liquid. Next, the solution was cooled down and mixture was precipitated for three times in hexane (1000.0 mL)under fast stirring. The obtained product was dried for 48 h in vacuum. The proton nuclear magnetic resonance (1H NMR)spectrum is shown as Fig. S1 in the Supporting Information. Yield: ~80%.
2.2.2 Synthesis of Bz-Glu-NCA monomer
α-Pinene (21.95 g, 158.1 mmol)andγ-benzyl-L-glutamate(7.50 g, 31.6 mmol)were suspended in anhydrous THF (200.0 mL)in a three-neck round-bottom flask under magnetic stirring.Anhydrous THF (60.0 mL)and triphosgene (7.11 g, 23.7 mmol)was added into a constant pressure funnel. The following steps are as same as the synthesis of Z-Lys-NCA monomer. The obtained product was dried for 48 h in vacuum. The1H NMR spectrum is shown as Fig. S2 in the Supporting Information.Yield: ~85%.
2.2.3 Synthesis of Phe-NCA monomer
α-Pinene (24.70 g, 181.7 mmol)and L-phenylalanine (6.00 g,36.31 mmol)were suspended in anhydrous THF (120.0 mL)in a three-neck round-bottom flask under magnetic stirring.Anhydrous THF (36.0 mL)and triphosgene (8.10 g, 27.3 mmol)was added into a constant pressure funnel. The following steps are as same as the synthesis of Z-Lys-NCA monomer. The obtained product was dried for 48 h in vacuum. The1H NMR spectrum is shown as Fig. S3 in the Supporting Information.Yield: ~82%.
2.2.4 Synthesis of PCL-NH-Boc
ε-Caprolactone (30.58 g, 265.2 mmol), and dry toluene (60.0 mL)was added into a flask with a magnetic flea and heated at 144 °C in oil bath. Toluene was distilled out under argon by azeotropic distillation in order to remove residual water in the flask. Next, the solution was further degassed by bubbling with argon for 0.5 h.N-(tert-Butoxycarbonyl)-2-aminoethanol (1.50 g, 8.80 mmol)was injected into the flask through a syringe.Sn(Oct)2(0.02 g, 0.04 mmol)was added through micropipette with the protection of argon. The solution was stirred at 110 °C for 48 h and then dissolved in dichloromethane (10.0 mL). The solution was precipitated in cold methanol (200.0 mL)for three times under continuous stirring. The obtained product was finally dried in vacuum at 40 °C. The1H NMR spectrum and size exclusion chromatography (SEC)curve are shown as Figs. S4 and S5 in the Supporting Information. Yield: ~92%.
2.2.5 Synthesis of PCL-NH2
PCL34-NH-Boc (21.60 g)was dissolved in anhydrous dichloromethane (24.0 mL)under argon, and added into anhydrous TFA (24.0 mL). The reaction solution was stirred for 2 h at room temperature and then solvent was removed in vacuum. Next, the polymer was dissolved in dichloromethane(15.0 mL)and washed with 5% NaHCO3aqueous solution (three times)and deionized water (three times)and then dried in MgSO4overnight. After filtration and evaporation of the solvent,the resulting product was dried in vacuum. The1H NMR spectrum is shown as Fig. S6 in the Supporting Information.Yield: ~73%.
2.2.6 Synthesis of PCL34-b-PBLG30-b-P[(Z-Lys)16-stat-Phe12]
Bz-Glu-NCA (2.27 g), PCL-NH2(1.00 g)and anhydrous DMF (15.0 mL)were added into a round-bottom flask with a magnetic flea in it. The solution was stirred at room temperature for 48 h in vacuum to afford PCL34-b-PBLG30.The1H NMR spectrum is shown as Fig. S7 in the Supporting Information.Next, Phe-NCA (0.20 g, 2.19 mmol)and Z-Lys-NCA (0.47 g,3.27 mmol)were added into the flask and stirred for another 48 h at room temperature in vacuum. Then the solvents were evaporated off by a rotary evaporator. Excess water was added into the flask to remove the DMF for eight times and then dialyzed in water to obtain the crude polymer. Finally, the product was finally obtained by freeze-drying for 48 h. The1H NMR spectrum and SEC curve are shown in Figs. S8 and S9 in the Supporting Information. Yield: ~67%.
2.2.7 Synthesis of PCL34-b-PGA30-b-P(Lys16-stat-Phe12)
PCL34-b-PBLG30-b-P(Lys16-stat-Phe12)was dissolved in DMF and added into round-bottom flask with excess hydrogen bromide (15.0 mL, 30% in acetic acid)in it. The solution was stirred for five hours at room temperature. Next, the solution was precipitated in anhydrous diethyl ether (300.0 mL)for five times.After that, the crude product was dried by rotary evaporator and redissolved in dimethyl sulfoxide (DMSO)with two drops of TFA. The mixture was then transferred into dialysis tubes, and dialyzed in 5% NaHCO3 aqueous solution and deionized water for 48 h in order to remove residual TFA and HBr/CH3COOH solution. The product was finally obtained by freeze-drying for 48 h. The1H NMR spectrum is shown as Fig. S10 in the Supporting Information. Yield: ~64%.
2.2.8 Preparation of polymer micelles
PCL34-b-PGA30-b-P(Lys16-stat-Phe12)block copolymer (6.00 mg)was dissolved in anhydrous DMF (2.0 mL). One drop of TFA was added into the solution to break the hydrogen bonding to completely dissolve the copolymer. Next, deionized water(4.0 mL)was dripped into the solution with continuous stirring.After stirring for additional two hours, the solution was added into a dialysis tube and dialyzed for 48 h in sodium hydroxide weak solution to remove TFA and DMF. The dialysis medium was renewed for three times each day. The pH value of the micelle solution after dialysis is 7.78.
2.2.9 Determination of critical micelle concentration
The critical micelle concentration (CMC)refers to the lowest concentration of polymer to form micelles in water. Pyrene (3.00 mg, 15.0 μmol), dissolved in acetone (25.0 mL)as a probe, was used to detect the formation of micelles. Ten microliters of the pyrene solution was added into eight centrifuge tubes respectively and the acetone in tubes evaporated for 12 h. The micelle solution was diluted into eight different concentrations by deionized water. Then the solution (4.0 mL)at different concentrations was added into centrifuge tubes loaded with pyrene. After stirred for 8 h, fluorescence intensities of the solutions in these eight tubes were measured with Lumina fluorescence spectrometer (Thermo Fisher)by exciting samples at 334 nm. In this process, the solutions were scanned at emission wavelength from 350 to 500 nm with a 10 nm slit width for excitation and a 10 nm slit width for emission. We chose the intensities ofI1(371.7 nm)as the vibrionic bands. The intensity ofI1(371.7 nm)were plotted against the log of the concentration of each sample. Finally, one linear fit was used to process the first four intensity values, and the other linear fit was used to process the last four intensity values, which created two lines and these two lines intersected at one point., The abscissa value (the log of concentration)of the point according to the equations of two lines could be calculated, corresponding to the CMC of 25.6 μg·mL−1.
2.2.10 Cytotoxicity tests
The cytotoxicity of the copolymer was determined by measuring the inhibition of human normal liver cells (L02)growth with the Cell Counting Kit-8 (CCK-8)assay which is a sensitive colorimetric assay to determine the number of viable cells in proliferating cell. Due to the dehydrogenase activities in cells, the 2-(2-methoxy-4-itrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium odium salt (WST-8)will transferred into a yellow formazan dyeviareduction reaction,and the amount of formazan dye was proportional to the number of liver cells. First, the L02 cells were cultivated in 96-well plates (8000 cells·well−1)with Dulbecco’s Modified Eagle medium (DMEM)(100.0 μL)supplemented with 10% fetal bovine serum (FBS)in a humidified 5% CO2-containing atmosphere for 24 h at 37 °C. Then copolymer micelle solutions(20.0 μL)at various concentration from 37.5 to 600 μg·mL−1were added and incubated for two days. The untreated cells were set as control group. After incubation, CCK-8 dye was added into each well and the plate was incubated at 37 °C for additional one hour. Finally, the absorbance of each well was measured using dual wavelength spectrophotometry at 450 and 630 nm by microplate reader. All the treatment was repeated for five times.The relative cell viability (%)was calculated by comparing the absorbance at 450 nm with control wells. The same procedure was used to determine the cytotoxicity of tobramycin-loaded polymer micelles.
2.2.11 Preparation of tobramycin-loaded polymer micelles
First, 3.00 mg of PCL34-b-PGA30-b-P(Lys16-stat-Phe12)copolymer were dissolved into DMF (3.0 mL), and one drop of TFA was added in to solution. Then deionized water (3.0 mL)with 0.64 mg of tobramycin was added into the solution with vigorous stirring. Next, the solution was dialyzed in 500.0 mL of sodium hydroxide weak solution and 500.0 mL of tris buffer (pH 7.4; 0.01 mol∙L−1)in dialysis tube (cutoff Number-average molecular weight8000–14000)to remove the unloaded tobramycin, TFA and DMF according to the reported procedure40.The dialysis medium was changed every thirty minutes in three hours.
2.2.12 Determination of drug loading content and drug loading efficiency
Aqueous tobramycin solution was diluted into six concentrations by deionized water. Phthaldialdehyde (80 mg)was dissolved in ethanol (1.0 mL), diethyl ether (0.2 mL), boric acid/NaOH (0.2 mL, boric acid 0.4 mol∙L−1, pH 9.7)and 2-mercaptoethanol (0.4 mL)to consist the reagent41–43.Tobramycin solution (0.5 mL)was added to reagent (0.7 mL)and stirred for five minutes. Fluorescence intensities of the mixture solution were recorded (excitation at 360 nm and emission at 460 nm)to create the calibration curve of aqueous tobramycin solution at known concentrations (Fig. S11,Supporting Information).
Fluorescence intensities of the above mentioned micelle solution after dialysis were measured and compared with the calibration curve to calculate the amount of the tobramycin loaded in the micelles40. The DLC and DLE were evaluated according to the following formulas:
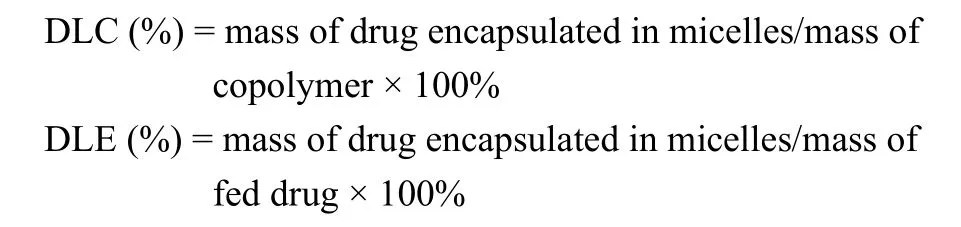
2.2.13 Drug release behavior of tobramycin-loaded micelles
The tobramycin-loaded polymer micelle suspension after dialysis was divided into three parts. These three parts were dialyzed in tris buffer (pH 7.4; 0.01 mol∙L−1, 80 mL)respectively at 37 °C in beakers and stirring at the rate of 150 r·min−140. The solution in the beaker (0.5 mL)was added to reagent (0.7 mL)at different time intervals and stirred for five minutes. The mixture was measured with fluorescence spectrometer (excitation at 360 nm and emission at 460 nm)to obtain the release profiles.
2.2.14 Antibacterial tests
Thein vitroantibacterial tests are the measurements of MIC of the copolymer micelles and antibiotic-loaded polymer micelles. MIC is an index to study the susceptibility of bacteria to antibacterial agents and to evaluate their antibacterial activity quantitatively. MIC tests demonstrate the bacteriostatic activity of antibacterial agents. The determination of MIC utilized the broth dilution and agar dilution methods according to the previous procedure44.
According to the composition of the cell wall, bacteria can be divided into Gram-positive bacteria and Gram-negative bacteria.S. aureusandE.coliare typical Gram-positive and Gram-negative bacteria. Both bacteria are widely used for antibacterial experiments.E.coliandS. aureuswere cultivated for 12 h at 37 °C. Then bacteria suspension (100 μL;E.coli, 3 ×107CFU·mL−1;S. aureus, 5 × 107CFU·mL−1)was placed in the 96-well plate. Next, the polymer micelle solution was diluted with the Luria-Bertani (LB)broth by two-fold to eight different concentrations (from 2000 to 32 μg·mL−1)and added (100.0 μL)into the well on the 96-well plate. Deionized water (100.0 μL)was added into wells on 96-well plate as control group. Then the 96-well plate were cultivated on shaking bed for 24 h at 100 r·min−1and 37 °C. The growth of the bacteria colony can be clearly observed by naked eyes. The MIC value refers to the lowest concentration of the copolymer which the solution was transparent. The same procedure was used to determine the MIC of free tobramycin and tobramycin-loaded polymer micelles.
2.3 Characterization
2.3.11H NMR analysis
1H NMR spectrum were recorded at room temperature by Bruker AV 400 MHz spectrometers with chloroform-d(CDCl3)or dimethyl sulfoxide-d6(DMSO-d6)as the solvent and tetramethylsilane (TMS)as the standard. For polypeptide, a drop of trifluoroacetic acid-d(CF3COOD)was added to break the hydrogen bond.
2.3.2 Size exclusion chromatography
Three MZ-Gel SDplus columns (pore size 103, 104and 105Å(1 Å = 0.1 nm), with molecular weight ranges within 1000–2000000, respectively)with a 10 μm bead size and an Agilent differential refractive index (RI)detector were used to measure number-averaged molecular weight (Mn)and polymer dispersity index (Ð). DMF was used as an eluent at 40 °C at a flow rate of 1.0 mL·min−1and polymethyl methacrylate standard samples were used to calibrate the samples.
2.3.3 Dynamic light scattering (DLS)studies
Nano-ZS 90 Nanosizer (Malvern Instruments Ltd., Worcestershire,UK)was used to characterize the size distribution of copolymer micelles at a fixed scattering angle of 90°. Each measurement was tested for three times. Aisposable cuvettes were used to analyze the solutions. Cumulative analysis of the experimental correlation function was used to obtain the data, and the computed diffusion coefficients from the Stokes-Einstein equation was used to calculate the particle diameters.
2.3.4 Transmission electron microscopy (TEM)
JEOL JEM-2100F instrument at 200 kV equipped with a Gatan 894 Ultrascan 1k CCD camera was used to take TEM images. To prepare TEM samples were prepare according to the following steps. Diluted micelle solution (5.0 μL)was dropped on carbon-coated copper grid and then dried overnight under ambient environment. Next, phosphotungstic acid solution(PTA; pH 7.0, 1%)was dropped on the hydrophobic film(parafilm), and then the grids were laid upside down on the top of the PTA solution for one minute. A filter paper was used to blot up the excess PTA solution slightly. After that, the grids were dried overnight in ambient environment.
2.3.5 Fluorescence spectroscopy
The fluorescence spectrometer (Lumina Fluorescence Spectrometer, Thermo Fisher)was used to measure fluorescence intensities.
2.3.6 Zeta potential
Nano-ZS 90 Nanosizer (Malvern Instruments Ltd., Worcestershire,U.K.)was used to determine the Zeta potential of micelle at a fixed scattering angle of 90°.
3 Results and discussion
3.1 Preparation of the block copolymer
The block copolymer PCL34-b-PGA30-b-P(Lys16-stat-Phe12)was synthesized by ROP of CL, Phe-NCA, Bz-Glu-NCA and ZLys-NCA , as shown in Fig. 1. First, Phe-NCA, Bz-Glu-NCA, and Z-Lys-NCA were synthesized through the NCA ring-forming reaction and verified by1H NMR spectrum (Fig. S1, S2 and S3,Supporting Information). Secondly, PCL-NH2was synthesized by ROP of CL, followed by the deprotection of Boc terminal group(Fig. S4 and S5, Supporting Information). Thirdly, PCL-NH2was applied to initiate the ROP of Bz-Glu-NCA to obtain PCL34-b-PBLG30(Fig. S7, Supporting Information). Next, the amine end group in PCL34-b-PBLG30initiated the ROP of Phe-NCA and ZLys-NCA to synthesize PCL34-b-PBLG30-b-P[(Z-Lys)16-stat-Phe12] (Fig. S8, Supporting Information). Finally, excess 33%HBr/CH3COOH was used to remove protecting group on the side chain in order to obtain the copolymer PCL34-b-PGA30-b-P(Lys16-stat-Phe12)(Fig. S10, Supporting Information).

Fig. 1 Synthetic route to PCL34-b-PGA30-b-P(Lys16-stat-Phe12).
3.2 Preparation and characterization of micelles
PCL34-b-PGA30-b-P(Lys16-stat-Phe12)copolymer was first dissolved in DMF. Then a drop of TFA was added to break the hydrogen bonding. Afterwards, above polymers were selfassembled into micelle within a DMF/TFA/water mixture solution. The core of the micelle is composed of PCL segment,and hydrophilic s egment (PGA, Lys and Phe)constitutes the coronas of the micelles. TEM images in Fig. 2a, b revealed micellar morphology of these micelles stained by PTA. The hydrodynamic diameter (Dh)of the micelles is 42 nm with polydispersity (PD)of 0.42, which is inconsistent with the TEM analysis, as determined by DLS (Fig. 2c). This is because DLS can measure the size distribution of micelles in a solution or suspension, but this distribution is a weighted average of the relative amounts of micelles with different sizes and does not fully show all particle size distributions. Therefore, the distribution tends to show the most distributed particles in the solution. The peak at 42 nm may be the most abundant particles in solution, rather than the representative of all particles present45.
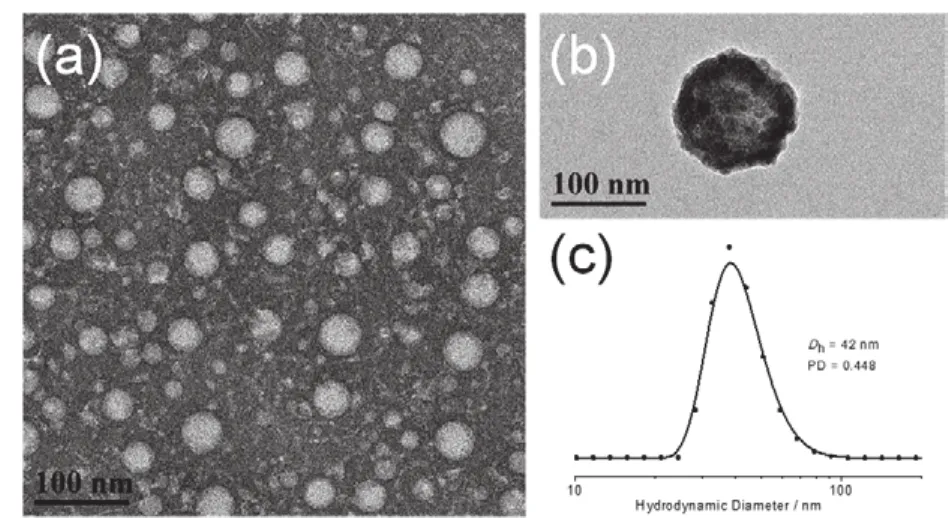
Fig. 2 (a and b)TEM images and (c)DLS study of micelles.
In addition, we further measured the zeta potential value of the micelles, which is approximately −26.7 mV. In a neutral environment, since the number of carboxyl groups is approximately twice that of the amino group, more ―COO−will expose on the surface of the assembly, leading the assembly to have a higher negative potential.
Since the degree of polymerization of PGA in the copolymer is higher than that of lysine, in the neutral environment, some of the carboxyl groups are negatively charged, while some of the amino groups are positively charged, which may not be assembled due to static electricity, resulting in precipitation of the copolymer. At high pH, the carboxyl group on the PGA is completely ionized, and the amino group is completely deprotonated. At this time, the carboxyl group is negatively charged, the positive polarity of the amino group is decreased,and the electrostatic interaction between both end groups is weakened. The PGA segment is also more hydrophilic, and the ratio of hydrophilic to hydrophobic segments is 2 : 1, which allows copolymers to self-assemble into micelles.
3.3 CMC study of the copolymer micelles
CMC refers to the lowest concentration of polymer to form micelle in water. To find out whether micelles or individual polymer chains kill bacteria, the CMC was compared with the MIC of the copolymer. The CMC is 25.6 μg·mL−1(Fig. 3),which indicates that the polymers can self-assemble into micelles above CMC. The obtained CMC is lower than the preparation concentration, indicating that it is the micelles rather than the individual polymer that kill the bacteria.

Fig. 3 CMC of the block copolymer.
3.4 In vitro cytotoxicity activity
The cytotoxicity of the micelles without and with tobramycin was assessed according to a previous method44. The concentration of micelles without and with tobramycin varies from37.5 to 600 μg·mL−1, respectively. In this range, the polypeptide-based micelles did not show obvious cytotoxicity(Fig. 4).
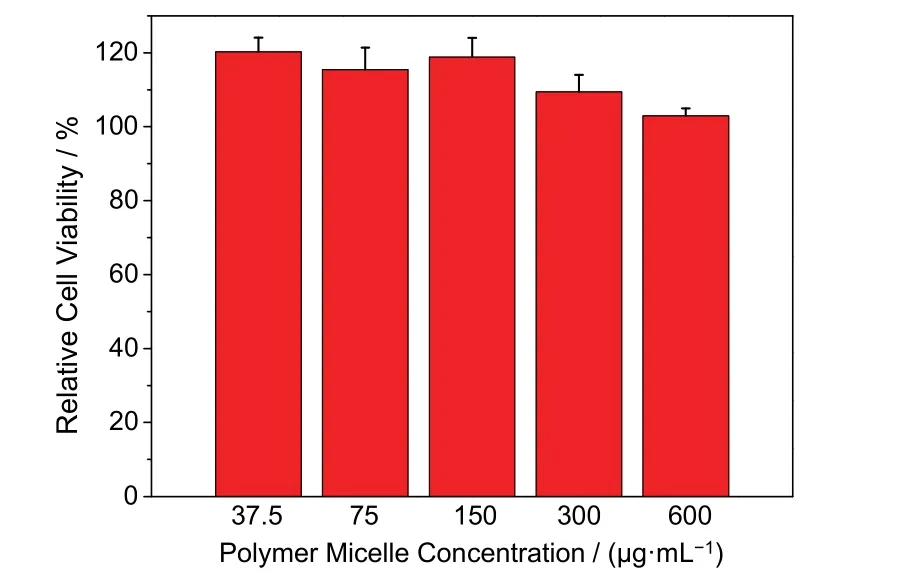
Fig. 4 Cytotoxicity evaluation of the polymer micelles against L02 cells of different concentrations. Relative cell viability was determined through CCK-8 assay (n = 5).
The micelles with tobramycin also did not show obvious cytotoxicity below 300 μg·mL−1(Fig. 5). This is because the negative charge of PGA neutralizes the positive charge of lysine.Moreover, the micelle morphology of nanoparticles can reduce cytotoxicity and improve the blood compatibility with eukaryotic cells to some extent20.
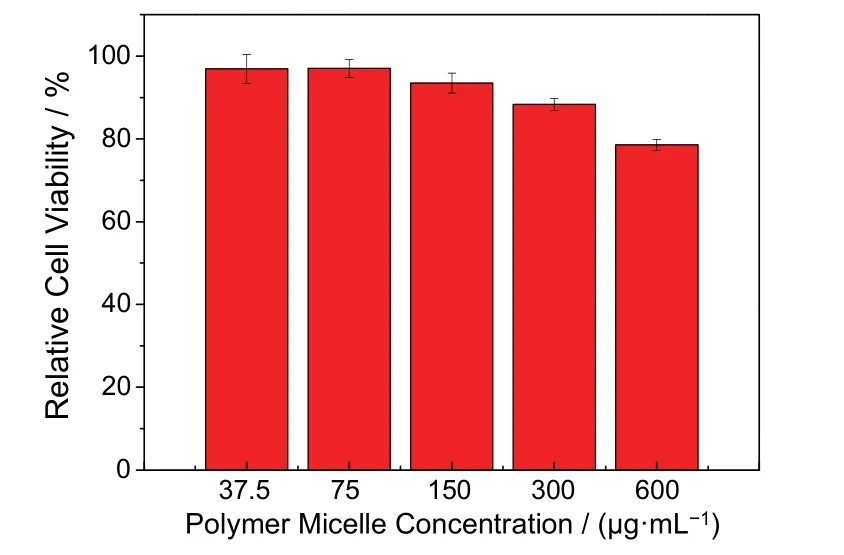
Fig. 5 Cytotoxicity evaluation of the tobramycin-loaded polymer micelles against L02 cells of different concentrations. Relative cell viability was determined through CCK-8 assay (n = 5).
3.5 Drug release behavior of tobramycin-loaded micelles
Tobramycin was verified to be successfully loaded in the micelles by measuring the solution after dialysis with a fluorescence spectrometer. The polymer contains negatively charged carboxyl groups, and tobramycin contains positively charged amino groups. Due to electrostatic adsorption,tobramycin can be adsorbed on the carboxyl groups of the polymer and th us entrapped on the coronas of the micelles.The DLE and DLC are 24.3% and 5.2%, respectively. Over time,antibiotics will be captured when reaching the outer core of micelle to slow down the releasing process. After 8 hours, the release rate reached about 85% (Fig. 6), demonstrating a sustained release characteristic of the polymer micelles.

Fig. 6 Cumulative release profile of tobramycin-loaded micelles.
3.6 In vitro antibacterial activities of micelles
The MIC of PCL34-b-PGA30-b-P(Lys16-stat-Phe12)micelles with antibiotics was determined by the broth microdilution method. After 24 h, we observed visually the turbidity of the bacterial solution in the wells. The minimum concentration of micelles in the wells which completely inhibited the growth of bacteria was determined as the MIC value of the antibacterial micelles.
We first tested the MIC of the micelles againstE. coliandS.aureus. The MIC value of this micelle is greater than 2000 μg∙mL−1, indicating negligible antibacterial effect. Due to the low degree of polymerization of antibacterial segment (segment of random copolymerization of Lys and Phe)and the high degree of polymerization of PGA segment, the positive and negative properties of the two segments are mutually offset, and the overall copolymer is negatively charged (−26.7 mV)in a neutral environment. Therefore, the micelles can not adhere to the surface of the bacteria.
In order to verify whether this micelle could improve the antibacterial efficiency of antibiotics, we tested the MICs of this antibiotic-loaded micelles and free tobramycin. Tobramycin is an aminoglycoside antibiotic with broad-spectrum antibacterial activity. It is clinically applied for the treatment of neonatal sepsis, sepsis, central nervous system infection, genitourinary infection, lung infection and other diseases caused by Gramnegative bacteria. The copolymer was dissolved in a DMF and co-assembled with tobramycin to load it. The results of the MIC value of the antibiotic-loaded micelles and free tobramycin by the broth microdilution method are shown in Table 1.

Table 1 Minimum inhibitory concentrations of tobramycin, micelles without and with tobramycin against E. coli and S. aureus.
The MICs of the tobramycin-loaded micelles againstE. coliandS. aureusare 7.8 and 18.2 μg∙mL−1, respectively. The MICs of the free tobramycin againstE. coliandS. aureusare 3.9 and 1.0 μg∙mL−1, respectively. Since DLC is 5.2%, the MICs of tobramycin loaded in the micelles againstE. coliandS. aureusare 0.4 and 0.9 μg∙mL−1. It is indicated that the polymer micelles can obviously enhance the antibacterial effect of the antibiotics due to the sustained release property. Under basic conditions,PGA blocks take a coil conformation. In the acidic microenvironment produced by bacteria, its conformation changed and the PGA segments shrink46–50. Meanwhile, the carboxylic side chains protonate in the acidic environment and the hydrophobicity of the micelles increases48. Due to the PGA block connecting to the micelle core composed of PCL, the hydrophobicity of the micellar core is increasing, leading to the change of composition48. Antibiotics tend to be trapped when they reach the outer core which results in a slow release of the antibiotics. Sustained release of antibiotics can reduce the frequency of antibiotic administration, improve the utilization of antibiotics, and improve patient compliance and therapeutic efficacy51. Therefore, the same therapeutic effect can be achieved with fewer antibiotics. On the other hand, poly(lysinestat-phenylalanine)[P(Lys-stat-Phe)] provides micelles with broad spectrum intrinsic antibacterial activity. This segment can insert and penetrate the bacterial cell membrane, causing the contents of the cells to leak, and finally leading to bacterial death44. Therefore, this antibiotic-loaded micelle can significantly reduce the dosage of antibiotics.
4 Conclusions
In conclusion, we synthesized an amphiphilic triblock copolymer PCL34-b-PGA30-b-P(Lys16-stat-Phe12)by ROP ofεcaprolactone and NCAs to improve the antibacterial efficiency of antibiotics. This block copolymer can be self-assembled into micelles, as confirmed by TEM and DLS. These antibioticloaded micelles did not show obvious cytotoxicity below 300 μg·mL−1against human liver L02 cells. After loading antibiotics,the micelles demonstrated very low MICs against both GramnegativeE. coli(7.8 μg∙mL−1)and Gram-positiveS. aureus(18.2 μg∙mL−1).The DLE and DLC of this micelle are 24.3% and 5.2%, which means only 0.4 and 0.9 μg∙mL−1tobramycin was contained in the micelles againstE. coliandS. aureus. A sustained release characteristic of loaded antibiotics was observed from the micelles. The conformation of the PGA changes in the acidic environment caused by bacteria, and the segments shrink toward the micelle core. Antibiotics tend to be captured when they reach the outer core, causing sustained release of antibiotics. In addition, the P(Lys-stat-Phe)] can provide broad spectrum intrinsic antibacterial activity. Overall,our study indicated that this micelle can enhance the antibacterial efficiency of the antibiotic, providing new strategies for reducing the dosage of antibiotics clinically and avoiding the side effect of antibiotics.
Supporting Information:1H NMR spectrum, SEC curve and calibration curves (Fig. S1–Fig. S11)areavailable free of chargeviathe internet at http://www.whxb.pku.edu.cn.

