Microbiota-gut-brain axis and major depressive disorder:implications for fecal microbiota transplantation therapy
Yu Li,Xiao-Jun Cai*,Qin Wang,Yuan-Yuan Wu ,Yan-Peng Xie,Xue Wang
1Department of Endocrinology,Heilongjiang Provincial Academy of Chinese Medical Science,Harbin,Heilongjiang,China.
Abstract Major depression disorder(MDD),which can affect individuals of any age,is one of the most common diseases,affecting an estimated 350 million people worldwide and placing a significant burden on individuals and society.MDD is heterogeneous.The conventional antidepressants are only partially effective and only 44% of patients are in remission during treatment.Therefore,improving the efficacy of MDD therapy has become a key research focus.An increasing number of studies have shown that the microbiota-gut-brain axis is closely related to the physiological and pathological processes of depression,suggesting that the gut microbiota may have protective or pathogenic effects on the development of MDD.Gut microbiota-oriented treatment is one of the most promising approaches.Fecal microbiota transplantation(FMT)has great potential to improve MDD more directly and effectively,although few research results in this area has been conducted.To assess the gut microbiota's connection with MDD,the efficiency of the nodes and the prospects of FMT therapy for MDD have been reviewed in this paper.
Keywords:microbiota-gut-brain axis;depression;fecal microbiota transplantation;gut microbiota;neurotransmitters
Introduction
Major depression disorder(MDD)is a common neuropsychiatric disease with a global prevalence of up to 4.4%[1].As the fourth primary reason for disability[2],MDD accounts for 10.3% of the global burden of disease[3-4].MDD is not just a mental disorder but also a physiological disease.Certain genes and psychological features might contribute to some patients’ depression,but the latest research has indicated that gut microbiota probably plays a crucial part in the pathophysiology of depression.Patients with MDD exhibit an imbalance of the gut microbiota that manifests as significant changes in fecal microbial alpha and beta diversity[5].Alpha diversity was used to analyze the complexity and species diversity of the fecal microbiota.Beta diversity analysis was utilized to evaluate the differences in species complexity[6].Nevertheless,it is still unclear how the gut microbiota participates in the pathogenesis of MDD.The two-way communication between the gut microbiota and the brain-termed the “microbiota-gut-brain axis”(MGBA)-is involved in brain function,immune inflammation,neural development,and aging[7].MGBA is an information exchange network that connects the gut and brain,which includes the vagus nerves,the hypothalamic-pituitaryadrenal(HPA)axis,intestinal immune system,synthesis of relevant neurotransmitters,intestinal mucosal barrier,and blood-brain barrier(BBB)pathways[8].It may change brain emotion and cognitive behavior through the gut microbiota,and influence the structure of the gut microbiota[9].A great number of studies suggest that dysfunction of the MGBA may be an immediate cause and a key risk factor for MDD[10-12].There is still a lack of complete relevant data analysis and scientific research and emerging technology,fecal microbiota transplantation(FMT)[13],makes it possible to systematically study the MGBA.The purpose of this paper is to review the recent research progress on the relationship between the MGBA and MDD and to discuss the prospects of FMT in the treatment of MDD.
Gut Microbiota and MGBA
People start to establish lifelong contact with a large number of microbes that exist in the skin,oral cavity,vaginal mucosa and gastrointestinal tract after birth[14].The density and diversity of gut microbiota are the highest,and the gut microbiota can respond to and even affect other organs overall[15].Microbiota in the gut accounts for 90-95% of the total number of cells,including bacteria,archaea,fungi,and viruses.They are widely distributed in the colon,mainly represented byFirmicutesandBacteroidetes,which account for approximately 70 - 75% of the microbiota[16].In addition to their role in the development and maintenance of digestive,metabolic and immune functions of the host,these symbiotic microbes also play an important role in regulating brain emotion and cognitive behavior[17].In recent years,the potential mechanisms of the MGBA’s participation in MDD have received extensive attention,including central nervous system(CNS),enteric nervous system(ENS),HPA axis,intestinal immune system,neurotransmitters,intestinal mucosal barrier,and BBB pathways.Microbiota are effective producers of various monoamine neurotransmitters,such as 5-hydroxytryptamine(5-HT),dopamine(DA),and γ-aminobutyric acid(GABA)[18-20].These microbes are supposed to be of great importance as they may affect the intestinal homeostasis and the plasticity of neural circuits,which involves affective disorders such as depression[21].The HPA axis is a significant component of the neuroendocrine system.And overactivation of the HPA axis can lead to the up-regulated secretion of cortisol,adrenocorticotropic hormone(ACTH),and corticotropic hormone-releasing hormone(CRH),which proved to be a central factor in triggering depression[22].Insufficient brain-derived neurotrophic factor(BDNF)is also a high riskfactor for impaired neuroplasticity and depression[23].A growing body of research has stated that the pathogenesis of many immune-mediated diseases is closely related to the gut microbiota[24].Inflammatory cytokines such as IL-6,IL-1β,and TNF-α may increase neurotoxic effects[25],leading to depression-like behavior by disrupting neurotransmitter synthesis and signaling.The increased permeability of the BBB and intestinal mucosal barrier associated with depression makes it easier for microbial metabolites and toxins to penetrate blood circulation and CNS[26],leading to an excessive immune-inflammatory response.In addition,the closer ties between the gut microbiota and depression may be related to the liver,where metabolites of the gut microbiota(such as lipopolysaccharides(LPSs)and alkaline phosphatase(AP))promote liver injury through a cascade of blood circulation and cytokines[27].However,these metabolites induce inflammation in the brain and further affect mood and cognition[28].The gut microbiota can convert oligosaccharides in dietary carbohydrates into short-chain fatty acids(SCFAs),which participate in the synthesis of neurotransmitters by activating G-protein-coupled receptors and have neuroprotective effects[29 - 30].Micronutrient deficiencies can also lead to depression,while a variety of water-soluble vitamins can also be produced by the gut microbiota and absorbed in the colon.Bifidobacterium,for example,synthesizes riboflavin,niacin,and folic acid[31],and an epidemiological study has shown that folic acid levels are negatively correlated with the severity of depressive symptoms[32].
Gut Microbial Dysbiosis and MDD
Introduction to Dysbiosis
MDD is not only a mental disorder or brain disease but also a systemic disease.Patients with MDD usually have intestinal dysfunction,such as loss of appetite,metabolic disorders,functional gastrointestinal diseases,and intestinal microbiome abnormalities[33].More recently,Felice and O’Mahony explained the origin of the high overlap between stress-related psychiatric disorders and gastrointestinal symptoms[34].A growing number of studies have revealed that MDD is linked with gut microbial dysbiosis in humans and animals.Moreover,the regulation of the MGBA provides new avenues for the treatment of MDD[35].
Animal Studies
Multiple animal studies have shown significant differences in the gut microbiota between MDD model animals and healthy controls(Table 1).Depression models include the bilateral olfactory bulbar resection model,maternal separation model,social disruption model,chronic unpredictable stress model and chronic restraint stress model[36-40].Daugé et al.found that the β diversity of the gut microbiota significantly changed,which involved naturally stress-sensitive Fischer rats and maternal separation-induced rats.The number ofBacteroidetes,FirmicutesandProteobacteriachanged dramatically[41].In addition,studies have shown that the abundance ofBifidobacteriumin mouse feces induced by mild social defeat stress(CSDS)is reduced,and oralBifidobacteriumpreparation can restore the stress resistance of CSDS mice to normal[42].Matsuda Y et al.observed an increase in the relative abundance ofBetaproteobacteriaandFlavobacteriumin rat feces on the 11th day of 14-day social defeat stress(SDS)-induced MDD model rat,as well as a decrease in the relative abundance ofClostridia.Oral administration of L-ergothioneine to MDD model rats improved rapid eye movement(REM)sleep-related abnormalities[43].
Schmidtner AK et al.used an elevated cross maze to induce high anxiety-related behavior in rats and found that butyrate may reduce microglial density in the prefrontal cortex(PFC)of HAB rats by mediating microglial apoptosis.The relative abundance ofLachnospiraceaeandClostridialesFamily XIII was positively correlated with butyrate levels[44].Song J et al.induced a depression-like phenotype by subcutaneous injection of adrenocorticotrophic hormone(ATCH)into male Wistar rats,and analysis of microbial community changes found that the relative abundances ofRuminococcusandKlebsiellaincreased and the relative abundances ofAkkermansiaandLactobacillusdecreased in the intestinal tract of MDD model rats.This may be closely related to the metabolite inositol and hippurate[45].Ma et al.used GC-MS urine metabolomics and 16S rRNA gene sequencing to observe the effects of chronic contradictory sleep deprivation(PSD)on host metabolism and the gut microbiota,and it was found that the relative abundances ofAkkermansia,Oscillospira,Ruminococcus,Parabacteroides,AggregatibacterandPhascolarctobacteriumchanged significantly in the intestinal tract of 7D-PSD rats.This change is closely related to host energy metabolisms,such as arginine and proline metabolism,serine and THR metabolism,and pyruvate metabolism[46].Taken together,these results from different animal studies explain the role of gut microbiota in the pathogenesis of MDD.In order to determine which bacteria can treat depression and which can cause it,more research is needed to be implemented.At the same time,further research needs to place more emphasis on metabolomics and its role in pathophysiology.
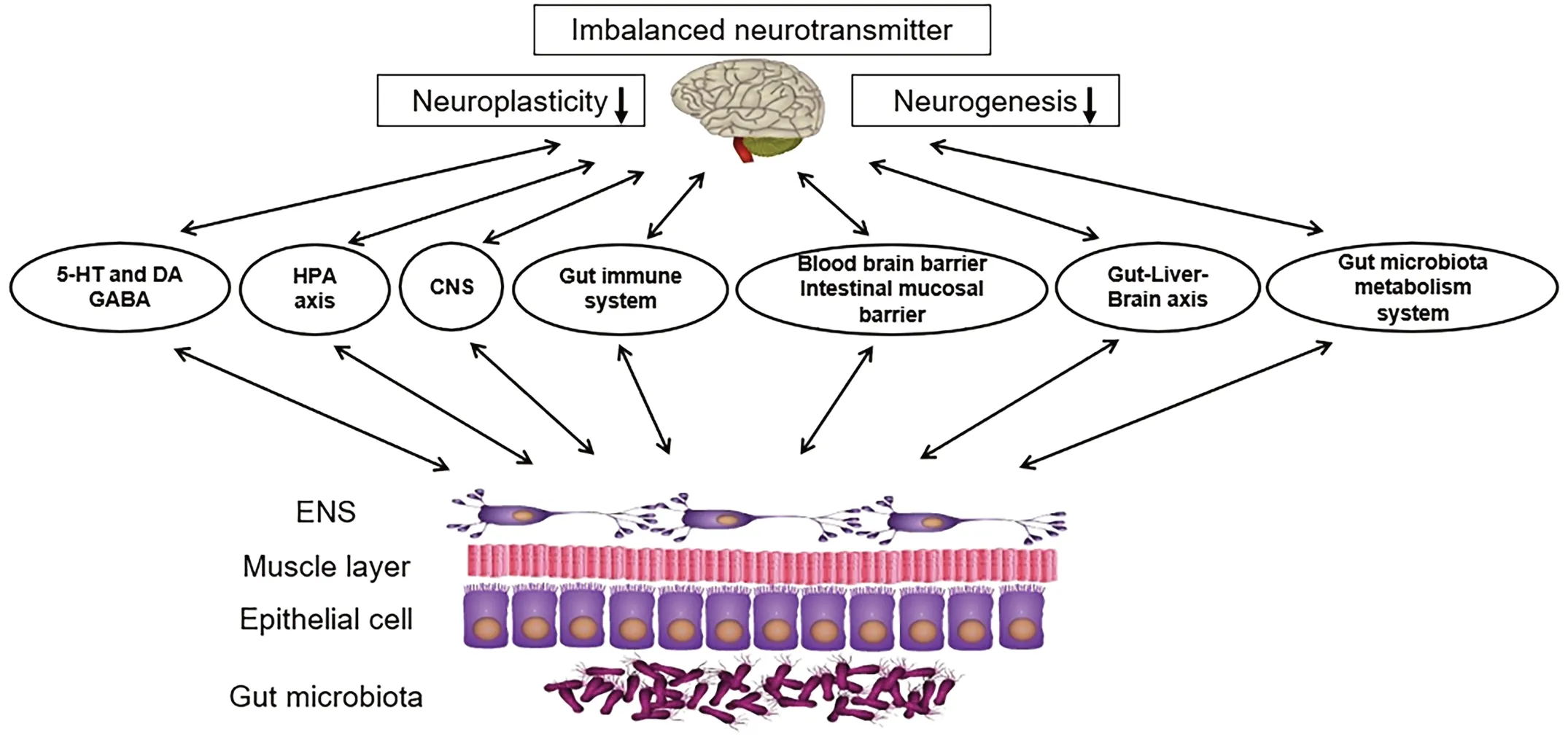
Figure 1.Feasible Mechanisms Associated with the Relationship Between Gut Microbial Dysbiosis and MDD.The MGBA is a major pathophysiological and potential therapeutic target for MDD.CNS:central nervous system;ENS,enteric nervous system.
Human Studies
A growing body of evidence suggests that gut microbiota is of vital importance to the physiological and pathological processes of MDD.Gut microbiota disorders,in addition,are also present in MDD patients(Table 2).Independent studies have shown decreased tryptophan metabolic bacteria levels in MDD patients,such asClostridiumspp.,Bifidobacteriumspp.,Escherichiaspp.,Ruminococcusspp.,andLactobacillispp.[48].Cheung SG’s study found that compared to those in a blank control,the relative abundancesofAnaerostipes,Blautia,Clostridium,Klebsiella,Lachnospiraceae,Parabacteroides,Parasutterella,PhascolarctobacteriumandStreptococcusincreased,and the relative abundance ofBifidobacterium,Dialister,Escherichia,Shigella,FaecalibacteriumandRuminococcusdecreased in MDD patient intestines[25].Jiang H analyzed fecal samples from 46 patients with depression and 30 healthy controls with a highthroughput pyrosequencing method and found that the α diversity of the fecal microbiota increased in the MDD group.Bacteroidetes,Proteobacteria,andActinobacteriastrongly increased in level,whereas that ofFirmicuteswas significantly reduced,and a negative correlation was observed betweenFirmicutesand the severity of depressive symptoms[49].The results of a meta-analysis showed that compared with healthy controls,the relative abundance ofPrevotellaceae,CryptococcusandFaecalibacteriumwas reduced in MDD patient intestines,and symptoms of depression improved after probiotic intervention[50].

Table 1 Variations on Gut Microbial Composition Associated with MDD in Animals
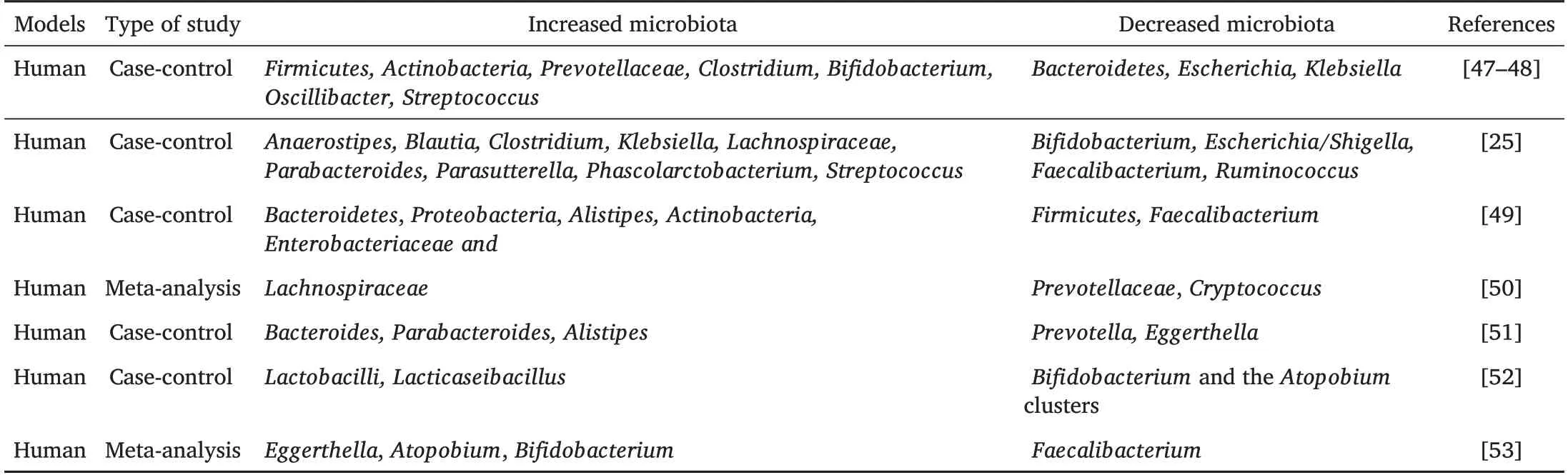
Table 2 Variations on Gut Microbial Composition Associated with MDD in Humans
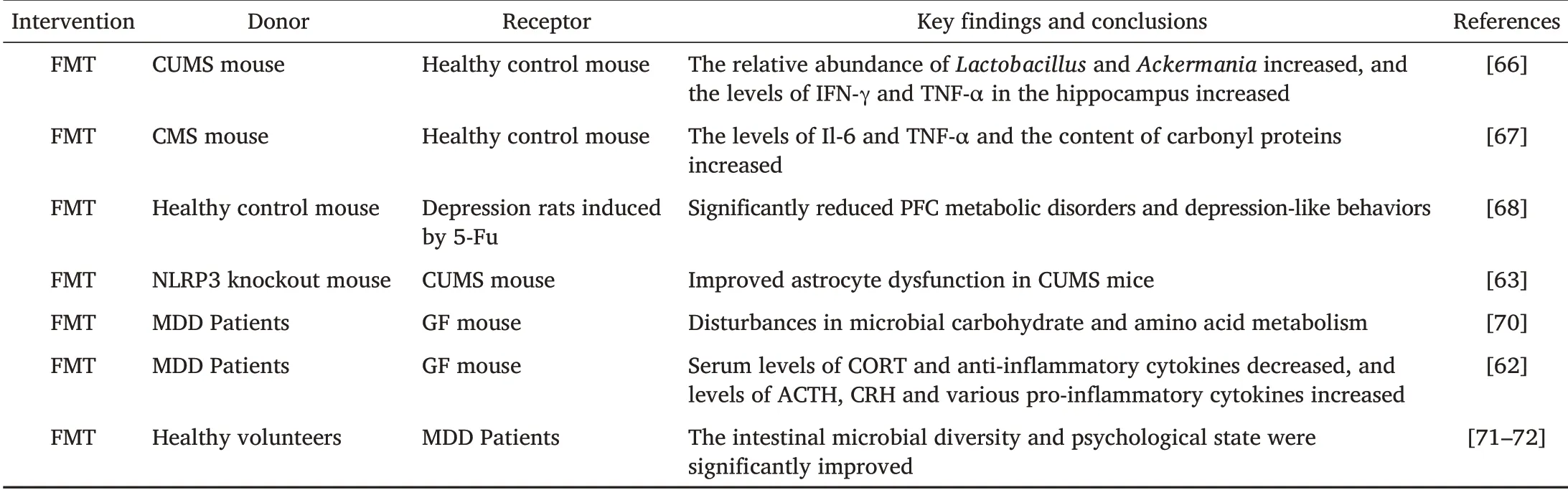
Table 3 Studies Associated with MDD and FMT Therapeutic Strategies
Consistent with these results,Zhang Q et al.analyzed the gut microbial composition,sleep quality in MDD patients,and found significant differences in 48 microbial targets between MDD patients and healthy controls.At the genus level,Doreawas simultaneously related to depression and sleep quality,whileCoprococcusandIntestinibacterwere associated with sleep quality but were independent of the severity of depression[51].In addition,studies have found that the relative abundance ofBifidobacteriaandLactobacillusin the intestinal tract of MDD patients is significantly decreased,and intervention withLacticaseibacillus paracaseistrain Shirota can significantly reduce depressive symptoms.Symptom improvement was more significant when the number ofBifidobacteriumand theAtopobiumclusters of theActinobacteriaphylum remained high[52].Knudsen JK’s paper made a comparison from four aspects,including demographics,clinical characteristics,application methods and the observed gut microbial structure,then found that the α diversity of gut microbiota and the relative abundance ofFaecalibacteriumin MDD patients were significantly decreased,compared to the increase ofEggerthella,Atopobium,andBifidobacterium.[53].
These observations discussed above provide additional insight into the dysregulation of the gut microbiota in MDD patients,which could be used to guide the diagnosis and treatment of the disease by intervening with the gut microbiota.However,existing studies on depression and the human gut microbiome have yet to reach a consensus on which bacteria are most associated with depression,possibly due to differences in study design.In order to identify which bacteria or metabolites can be used as biomarkers to precisely distinguish people with or without depression,further research needed to be implemented.
Prospects of FMT Therapy for MDD
Introduction to FMT
FMT,as a treatment for a variety of diseases,involves transferring fecal microbiota from healthy donors to the gut of recipients[54].FMT was first used in China in the 14th century to treat severe food poisoning and related symptoms such as diarrhea[55].In 1958,Eiseman et al.used fecal enemas to treat colitis caused byClostridium difficileinfection,which was considered to be the initiation of FMT’s entry into modern medicine[56].The adoption of antibiotics frequently damages healthy bacteria and gut microbial stability in the gastrointestinal tract,and may even lead to gut microbiota dysfunction.FMT restores a healthy microbiome by refilling the gut with healthy bacteria.The effect of this method is similar to that of probiotics,which both help maintain bacterial balance and function[57].However,unlike the transient colonization of probiotics,FMT can provide long-term colonization of donor strains[58].In addition to gastrointestinal diseases,FMT,which has already received widespread attention,is believed to have the potential to combat psychiatric disorders like depression,generalized anxiety disorder(GAD),and Parkinson’s disease[59-60].The candidate mechanisms of FMT underlying MDD are manipulation of gut microbial composition,intestinal mucosal barrier fortification,pathogen suppression,and immunomodulation.FMT therapy mitigated gut microbial dysbiosis mainly by reducing the production of fecal SCFAs,mitigating physical dysfunction,and boosting the levels of 5-HT,DA,noradrenaline,serum CORT and anti-inflammatory cytokine in MDD mice[61 - 62].Furthermore,FMT decreased the activation of astrocytes and microglia in substantia nigra,and down-regulated the components in the Toll-like receptor 4(TLR4)/ interferon-c(IFN-c)/TNF-a signal transduction pathway and hippocampal endocannabinoid(eCB)signaling system[63-65].The above findings revealed the effect of FMT on protecting MDD mice through inhibiting neuroinflammation while decreasing the TLR4/IFN-c/TNF-a and the eCB signal transduction.
Animal Studies
Li N et al.transferred fecal microbiota from mice induced by chronic and unpredictable mild stress(CUMS donor)to healthy control mouse,then found that both CUMS donor and CUMS recipient mouse exhibited high levels of anxiety and depression-like behavior,increased relative abundances ofLactobacillusandAckermania,and increased levels of interferon-γ(IFN-γ)and tumor necrosis factor-α(TNF-α)in the hippocampus[66].Marcondes et al.found that animals submitted to chronic mild stress(CMS)protocol or that received FMT from stressed animals showed behavioral changes and changes in neuroactive substances.The levels of IL-6 and TNF-α and the content of carbonyl proteins were also increased.FMT from healthy donors can reverse these changes[67].To illustrate the relationship between the gut microbiota and its effect on depression-like behavior induced by 5-fluorouracil(5-FU),Zhang F et al.conducted an FMT experiment.The results showed that 5-FU could induce metabolic disorders in the PFC of rats,meanwhile to a large extent it also could change the diversity and abundance of gut microbiota.Transplantation of fecal microbiota from healthy controls into rats induced by 5-FU significantly reduced PFC metabolic disorders and depression-like behavior[68].Zhang Y et al.proposed for the first time that the NLRP3 inflammasome was involved in lipinduced depression-like behavior in mice,and the gut microbiota from NLRP3 knockout mice was transplanted into germ-free(GF)mice to prevent depression-like symptoms in mice[69].Subsequently,FMT from NLRP3 knockout mouse significantly improved depression-like behavior in recipient mouse induced by chronic unpredictable mild stress(CUMS).FMT can improve astrocyte dysfunction induced by CUMS in mice by inhibiting the expression of circinate HIPK2(circHIPK2)[63].
Animal Studies with Human Donors
FMT from MDD patients to GF mice has been shown to induce depressionlike behavior in receptor mice.By identifying 367 proteins in the olfactory bulb,Huang C et al.found that the downregulated CACNA1E in the FMT model may be the promoter of microbe-induced depression[73].Zheng et al.believed that the gut microbiota is an increasingly recognized environmental factor.The intestinal microbiota structure of MDD patients was significantly different from that of healthy controls,which was characterized by significant changes in the relative abundances of
Firmicutes,ActinomycesandBacteroidetes.Depression-like behavior can be induced by transferring the “depression microbiota” from MDD patients to GF mice,mainly manifested as disturbances in microbial carbohydrate and amino acid metabolism[70].Consistent with this study,Liu S et al.transplanted fecal microbiota from MDD patients and healthy individuals into GF rats by FMT technology and found that rats receiving fecal microbiota from depressed patients showed depression-like behavior,accompanied by decreased levels of serum CORT and anti-inflammatory cytokines and increased levels of ACTH,CRH,and various proinflammatory cytokines.These results suggest that the gut microbiota may induce depression-like behavior through the neuroendocrine-immune-mitochondrial pathway,which is related to the inflammatory response and mitochondrial damage[62].
Human Studies
On the contrary to animal studies with human donors,fecal microbiota from fit volunteers is generally transplanted to people with diseases such as depression in clinical studies.Microbial ecosystem therapy-2(MET-2),alternative therapy to FMT,consists of microorganisms procured from fecal samples of healthy donors,which were purified and cultured in the laboratory before freeze-drying and oral uptake by patients(Meyyappan et al.,2020).As an alternative therapy for FMT,MET-2 has been widely used in clinical practice[74].In the cause of assessing the security and efficacy of FMT,our researchers used the Hamilton Depression Scale(HAM-D)to evaluate the changes in the gut microbiota and psychological status of patients with irritable bowel syndrome(IBS)4 weeks after FMT.Our research found that the gut microbiota and psychological status of entire patients were distinctly improved after FMT,and it is proposed that FMT has relatively effective safety[75].Most studies have found significant short-term improvement in depressive symptoms after treatment with FMT,while the long-term effects were inconsistent[72].Xie WR et al.found that after the last round of FMT,depressive symptoms continued to decrease for up to 17 months[76].In an open-label randomized trial,patients with MDD received daily FMT for 7 - 8 weeks by mixing standardized human gut microbiota with a drink or via enema.Gastrointestinal symptoms and depression-like behaviors significantly improved,which persisted until 2 years after treatment[77-78].Although current evidence agrees that FMT is a generally safe therapeutic method with few adverse effects,the long-term efficacy of FMT has not been completely elucidated.Therefore,establishing periodicity and length of regular follow-up after FMT to monitor the clinical outcomes and longterm adverse events are other essential issues.
Conclusion
The progress of research on the treatment of MDD has been challenging because depression symptoms and prognosis vary widely among individuals and are influenced by genetic,environmental,and other factors.FMT is considered to be a promising potential treatment because of its considerable effectiveness and tolerability,whereas in some cases the use of FMT is frequently restricted in clinical settings.At present,we still do not know what a “healthy microbiota” is,and it is quite difficult to make sure of specific effectiveness and safeness,which is due to the deficiency of large,double-blind,randomized controlled trials.Treatment costs for FMT are comparable to those of antidepressants,but they are still relatively expensive,and no standardized FMT protocol has been established.While research on this area is atelic to a great degree,the potential to use FMT to target the MGBA to reduce depressive symptoms is promising.
- Life Research的其它文章
- The impact of the COVID-19 outbreak on Australia’s public health and medical industry in 2020
- Coronary heart disease-related fatigue:risk factors,assessment and treatments
- The mechanism of DDR genes in tumor progression
- The therapeutic potential of stem cell therapy for myocardial infraction
- ZMYND10 downregulates cyclins B1 and D1 to arrest cell cycle by trimethylating lysine 9 on histone 3
- Preoperative traction is a risk factor for osteonecrosis of femoral head in patients with stable femoral neck fractures