Acid-base and lipophilic properties of peptide nucleic acid derivatives
Prmod Thkre , Frncesc Vsile , Mur Vllro , Sonj Visentin ,**, Giuli Cron ,Emnuel Licndro , Silvi Cuteruccio ,*
a Department of Chemistry, University of Milan, 20133, Milan, Italy
b Molecular Biotechnology & Health Sciences Department, University of Turin,10135, Turin, Italy
Keywords:Peptide nucleic acids Ionization properties Potentiometric pH titration Immobilized artificial membrane chromatography Lipophilicity
ABSTRACT The first combined experimental and theoretical study on the ionization and lipophilic properties of peptide nucleic acid (PNA) derivatives, including eleven PNA monomers and two PNA decamers, is described. The acidity constants (pKa) of individual acidic and basic centers of PNA monomers were measured by automated potentiometric pH titrations in water/methanol solution,and these values were found to be in agreement with those obtained by MoKa software. These results indicate that single nucleobases do not change their pKa values when included in PNA monomers and oligomers.In addition,immobilized artificial membrane chromatography was employed to evaluate the lipophilic properties of PNA monomers and oligomers, which showed the PNA derivatives had poor affinity towards membrane phospholipids, and confirmed their scarce cell penetrating ability. Overall, our study not only is of potential relevance to evaluate the pharmacokinetic properties of PNA, but also constitutes a reliable basis to properly modify PNA to obtain mimics with enhanced cell penetration properties.
1. Introduction
Ionization and lipophilicity have a pivotal role in governing not only drug-receptors interactions but also absorption, distribution,metabolism,excretion and toxicity of drugs.Ionization impacts pHdependent solubility, lipophilicity, permeability, stability and protein binding. For instance, aqueous solubility and membrane permeability both depend on the charge state of the molecules;therefore, the pKavalue of drugs governs oral bioavailability and absorption in the different compartments of the human body [1].The ionization properties of the nucleobases also play a crucial role in the self-assembly process of DNA and RNA, which are mainly governed by stacking and hydrogen-bonding interactions [2,3]. In particular,intermolecular hydrogen bonding between nucleobases in nucleic acids duplexes is essential in determining the tertiary structure and functions of natural DNA and RNA in cellular systems[3].The proton accepting and donating ability of the nucleobases in forming these hydrogen bonds and the corresponding base-pairing strength were found to be strictly associated with the ionization constants (pKa) of the complementary base-pairing partners [4].Thus, the evaluation of pKavalues of nucleobases in nucleosides and nucleotides is an important tool to disclose their hydrophobic and hydrophilic natures as well as their biological behavior [5].Different experimental techniques have been employed to measure the pKavalues of unmodified nucleobases and properly modified DNA and RNA monomeric building blocks, such as UV absorption spectroscopy [6,7], fluorescent methods [8],1H NMR shift experiments [9-13], potentiometric pH titration [14] or pH-dependent high-field NMR measurements [15,16]. Computational methods have also been used to estimate pKavalues of nucleobases in gasand solution-phases [17-25]. Both experimental and theoretical studies showed that the nature of sugar backbone and the position in the DNA and RNA strand significantly affect the pKavalues of nucleobases [26,27].
On the other hand, lipophilicity is a key physicochemical property with a major role as a predictor of eventual compound success as a drug. It is commonly measured by its distribution behavior in a biphasic system, mostly 1-octanol/water [28].Because of the limits of this traditional system to mimic the electrostatic interactions between drugs and membranes,immobilized artificial membrane (IAM) chromatography, which combines membrane simulation with rapid measurements, is nowadays considered as a valid alternative to log P/log D measurements[29-31].IAM stationary phases are based on phosphatidylcholine,which is the major component of biological membranes, and the determination of chromatographic retention coefficients of the analytes on such stationary phases is assumed as direct measures of their phospholipophilicity, i.e., the affinity that the analytes have for phospholipids [32]. Furthermore, IAM chromatography has been applied not only to small molecules but also to peptides of pharmaceutical interest[33]. Notably, the potential of IAM indices to predict passive transport through biological barriers can be of particular relevance in near future to estimate pharmacokinetic properties of compounds with potential pharmacological applications. Overall, to the authors’ best knowledge, no study on the ionization properties and lipophilicity of peptide nucleic acid monomers and oligomers has so far been reported[34].
Peptide nucleic acids(PNAs),introduced by Nielsen et al.[35]in 1991, are synthetic DNA/RNA mimics in which the neutralN-(2-aminoethyl)glycine (aeg) unit replaces the sugar phosphate backbone, and the pseudopeptide chain is covalently linked to nucleobases through a carboxymethylene spacer(Fig.1).
Since the distance between two nucleobases in PNA is comparable to the spacing between bases in DNA or RNA,single-stranded PNAs bind to complementary DNA, RNA and PNA sequences following the Watson-Crick base-pairing rules [36]. Unlike natural nucleic acids, the PNA backbone is neutral, thus providing strong hybridization properties with their complementary DNA/RNA targets in a high sequence specific manner [36]. Indeed, they are capable of forming PNA-DNA,PNA-RNA,and PNA-PNA duplexes or more complex structures(e.g.,triplexes,quadruplexes,or hairpins)that are generally of higher stability and are endowed with greater sequence discrimination than the corresponding DNA-DNA or DNA-RNA duplexes[37-39].Moreover,the nonstandard backbone makes PNAs resistant to enzymatic degradation by nucleases and proteases[40].Thus,PNAs are ideal candidates for therapeutic and biomedical applications, especially within gene therapy and diagnostics[41,42].However,it is found that PNA derivatives should fully overcome limitations in the efficiency of delivery to the nuclei of the desired cells to gain a major relevance in drug discovery.Indeed, these compounds often show poor pharmacokinetics which significantly limits their potential clinical use [43,44].

Fig.1.Structure of peptide nucleic acid (PNA) and DNA.
Since artificial systems like PNA may have different ionization and lipophilicity characteristics from those of DNA, to fully exploit their potential as drug candidates, there is a need for characterization of PNA derivatives in terms of ionization and lipophilicity.The aim of such characterization is to determine the pKaand lipophilicity values of monomers used to build PNA oligomers and to rationalize the poor internalization properties of these compounds in order to design more permeable PNA derivatives. For these reasons, in this paper we report a systematic study on the ionization and lipophilic properties of eleven PNA monomers1-11(Fig. 2) that could be divided into three main groups: (1) four commercially available PNA monomers1-4, in which the amino group of aminoethylglycine portion is protected with anN-tertbutyloxycarbonyl(N-Boc)group,and the exocyclic amino groups of adenine, cytosine and guanine are protected with anN-benzyloxycarbonyl (N-Z) protecting group; (2) four PNA monomers5-8containing the free terminal amino group on theaeg-backbone,obtained by Boc deprotection of1-4; (3) three fully deprotected PNA monomers9-11in which the free exocyclic amino groups of adenine, cytosine and guanine are also present. The study of PNA monomers with different level of amine groups protection should allow to attribute the correct pKavalue to each potential ionizable groups in the monomers.
Monomers5-11were synthesized for the first time and,in this paper, they were fully characterized by NMR experiments also in terms of the estimation of their equilibrium mixture of thetransandcis-rotamers. Next, the ionization properties of monomers1-11and the model PNA decamer12(Fig. 3) were investigated through the measurement of their pKavalues by means of automated potentiometric pH titration using the Sirius T3 apparatus.Finally,the lipophilic properties of monomers1-11and the model PNA decamers12and13(Fig. 3) were evaluated by IAM chromatographic technique.
2. Experimental
2.1. Materials
PNA monomers1-4were purchased from ASM Research Chemicals GmbH (Hannover, Germany), and used as received.Other chemicals and solvents were obtained from commercial sources and used without further purification. The homothymine decamer12was synthesized according to the literature [45]. The details of the synthetic procedures of the PNA monomers5-11and the PNA decamer13(Scheme S1) as well as their characterization data can be found in the Supplementary data.
2.2. NMR study
NMR experiments were performed at 303 K on a Bruker Avance 600 MHz spectrometer (Milan, Italy). The NMR experiments were carried out in 600 μL of deuterated DMSO at a concentration range of 30-50 mM.In some cases,in proton spectra,the water signal at 3.3 ppm was reduced using a presaturation pulse (58 db) during relaxation delay.13C experiments were performed using attached proton test sequence (Figs. S1-S7). For quantitative13C analysis,the sequence with inverse gated decoupling(which provide proton decoupling without nuclear Overhauser effect (NOE) was applied using 100 s as relaxation delay. All proton and carbon chemical shifts were assigned unambiguously using bi-dimensional experiments (COSY, NOESY, HSQC and HMBC) and the assignments are reported in Tables S1-S7.NOESYexperiments were performed with a mixing time of 400 and 700 ms; HMBC spectra were acquired applying 8 Hz as long range coupling constant1H-13C.
2.3. pKa measurements
To measure pKavalues,a potentiometric approach was applied.All measurements were performed using the Sirius T3 apparatus(Sirius Analytical Instruments Ltd.,Forest Row, UK) equipped with an Ag/AgCl double junction reference pH electrode (Forest Row,UK)and a turbidity sensing device(Forest Row,UK).All tests were performed in triplicate using standardized 0.5 M KOH and 0.5 M HCl as titration reagents.The titration experiments were conducted in 0.15 M KCl solution either in water or in co-solvent/water mixtures under nitrogen atmosphere at a temperature of (25 ± 1)°C.Aqueous pKavalues were extrapolated using the Yasuda-Shedlovsky method [46].

Fig.2.Chemical structures of PNA monomers 1-11 (Boc: tert-butyloxycarbonyl; Z: benzyloxycarbonyl).
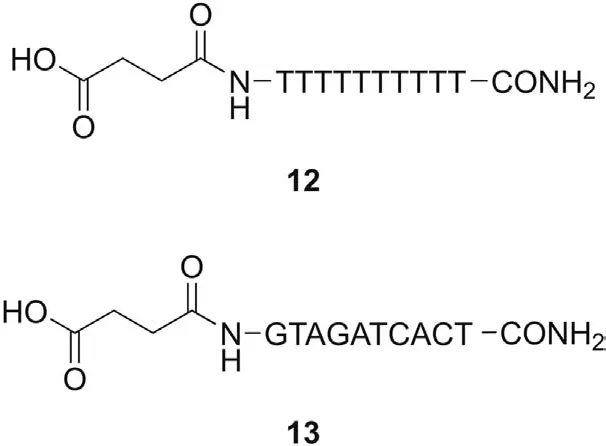
Fig.3.Structure of PNA decamers 12 and 13.
Shortly, in this pH-metric method, pKawas measured by titrating a solution of the sample with acid and base,and the results were obtained by a complex computational process.The pH of each point in the titration curve was calculated using equations that contain pKa,and the calculated points were fitted to the measured curve by manipulating the pKavalue.The pKathat provided the best fit was taken to be the measured pKa. This sophisticated method enables high precision in the determination of multiprotic compounds with pKain the 2-12 pH range[46].
2.4. Computational studies
The pKavalues were calculated with MoKA version 2.5.6 (Molecular Discovery Ltd.).
2.5. Chromatographic indexes
The applied method has already been described elsewhere[47].The analyses were performed at 30°C with 20 mM ammonium/acetate at pH 7.0 in mixture with acetonitrile at various percentages. The stationary phase was IAM.PC.DD.2 (Regis Technology,10 cm×4.6 mm,10 μm,packing 300 Å pore size).The flow rate was 1.0 mL/min, and the injection volume was 10 μL. Wavelength of detection was 260 nm.
Chromatographic retention data at a given amount of cosolvent,expressed as log kIAM(the logarithm of the retention factor),were calculated with the following expression:

wheretrandt0are the retention time of the drug and a nonretained compound (citric acid), respectively (data not shown).All log kIAMvalues are the average of at least three measurements.To avoid that the experimental measurements were affected by retention changes due to column aging,the retention times of five gold standard compounds (caffeine, carbamazepine, ketoprofen,theobromine and toluene)was checked daily.
An HPLC Varian ProStar instrument (Leini, Torino, Italy) equipped with a 410 autosampler, a PDA 335 LC detector and Galaxie Chromatography Data System version 1.9.302.952 was used to perform all the measurements.
3. Results and discussion
3.1. Synthesis and NMR studies of PNA monomers 5-11
Monomers5-8were synthesized in 82%-92%yield through the selective removal of theN-Boc group from the corresponding commercially available PNA monomers1-4, using a mixture of trifluoroacetic acid (TFA) and dichloromethane or tetrahydrofuran at room temperature(Scheme 1).
To obtain the fully deprotected monomers9-11, the simultaneous cleavage ofN-Boc andN-Z groups was performed by treating monomers1-4with a mixture of TFA and thioanisole at room temperature,according to the well-known“push-pull”mechanism[48], in which the combination of TFA and thioanisole efficiently ensures the removal ofN-Z group under mild conditions.The fully deprotected monomers9-11were obtained in 69%-79% yield(Scheme 2).
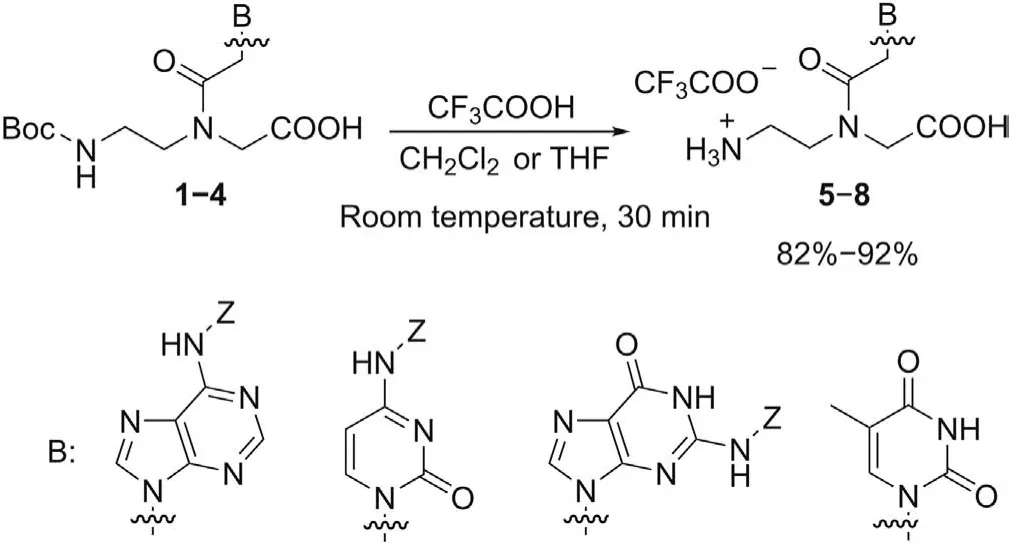
Scheme 1.Boc-deprotection from PNA monomers 1-4.
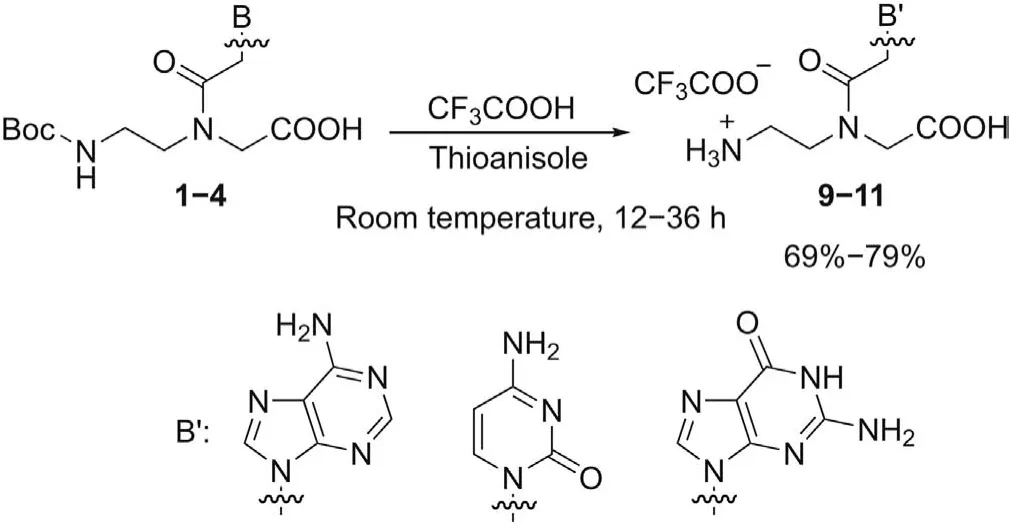
Scheme 2.Simultaneous Boc- and Z-deprotection from PNA monomers 1-4.
Monomers5-11were isolated as TFA salts after crystallization with MeOH and diethyl ether,and they were fully characterized by means of NMR and MS techniques.In particular,NMR experiments were used to characterize all PNA monomers, obtain additional information about the protonation of amine and carboxylic groups,and analyze their conformational features. Indeed, the monomers exist as mixture of two amide rotamers, namely, theE- andZrotameric forms [49], that slowly interconvert on the NMR timescale at 303 K.Thus,for each compound,we performed a complete1H and13C NMR analyses, and we identified two sets of signals corresponding to the two rotamers that, in solution, interchange between major and minor conformers. The assignments are reported in Tables S1-S7 for both major and minor forms(indicated with M and m, respectively). In order to distinguish between the two rotamers, we made use of NOESY spectra that yield through space correlations and they can be used to estimate the molecule conformations in solution [50,51]. Making use of bidimensional NOESY experiments, we were able to assign the NOE contacts typical of the two rotamers: the NOE contact between protons 10 and 12 (Fig. 4) was considered diagnostic for the presence ofErotamer, while the interaction between protons 10 and 14 identified theZ-rotamer.
From the integration of their1H spectra, we can evaluate the rotamer population distribution for each monomer as reported in the Supplementary data. Fig. 4 shows NOESY experiment for monomer8. We assigned the resonances relative to the more intense signals (70% populated) to theE-rotamer while theZrotamer was 30% populated. We observed thatE-rotamer was the major conformer for all compounds with the exception of monomer10that existed as 50:50 mixture of two amide isomers. NMR analysis of PNA demonstrated that the range ofE-andZ-rotamers’population was between 70:30 and 50:50,and it depended on the nature of the nucleic base.
13C NMR experiments were used in order to verify and quantify the presence of TFA. In a13C NMR spectrum, the area under the signal is not simply proportional to the number of carbons giving rise to the signal, because the NOE from proton decoupling is not equal for all the carbons. To exclude the contribution of NOE, we acquired monodimensional13C spectra using inverse gated decoupling sequence applying 100 s as relaxation delay in order to allow the relaxation of all13C before the acquisition of the next experiment scan. High-resolution spectra and accurate quantification using peak area integration permitted accurate measurements of relative ratios betweenE- andZ-rotamers and that of the ratio between PNA and TFA. We observed that for each analyzed PNA monomer(monomers8and9)the ratio of monomer-TFA was 1:1.
3.2. Ionization properties
Potentiometry, as implemented in the automatic Sirius T3 instrument, was applied to measure pKavalues of monomers1-11(Table 1). Most of the compounds under study are multiprotic molecules bearing both acidic and basic centers. In a multiprotic molecule it is crucial to identify the acidic/basic nature of different pKavalues. For instance, for monomer11we measured three pKavalues: 2.90, 8.67 and 9.79. In order to establish the acidic/basic nature of these pKavalues, two combined strategies can be used:predicted values and pKadetermination in the presence of a cosolvent. There are a number of commercial software and online websites that provide pKacalculations.The methods’performances strongly depend on the chemical nature of the investigated compounds and on the availability of experimental values for similar structures. Here, we used MoKa [52,53] after a preliminary validation through the prediction of the pKaand evaluation of the most stable tautomer of the standard free nucleobases (i.e., adenine,cytosine,guanine and thymine,see Fig.S9).Since predictions are in excellent agreement with the experimental values[25],we decided to use MoKa also for predicting pKavalues of PNA monomers (an example of MoKa predictions is provided in Fig.S10).Thus,as far as monomer11,MoKa predictions indicated two acidic and one basic centers(Table 1 and Fig. 5A).
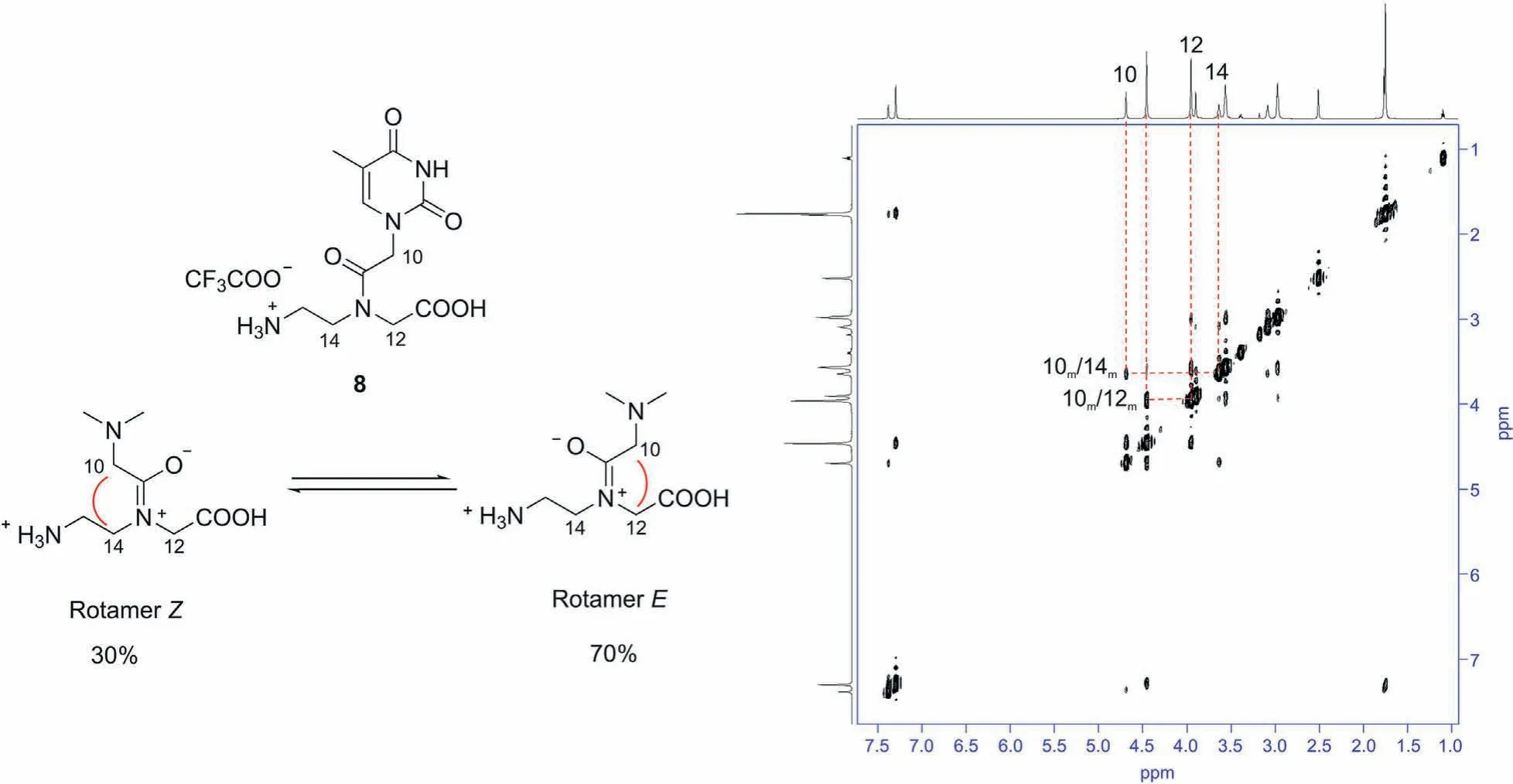
Fig.4.2D-NOESY experiment obtained for monomer 8 in DMSO-d6. Different nuclear Overhauser effect (NOE) cross peaks are observed, as evidenced in the spectrum: a NOE contact between protons 10-12 suggested that E-rotamer is the most populated, while the NOE contact between protons 10-14 indicated that Z-rotamer is the less populated.
Reasonably, the COOH moiety has a pKaof 2.90. Calculated values cannot be used to assign the second acidic pKa. The calculation is unreliable because the values of 8.67 and 9.79 are too close.The second strategy to evaluate the acidic/basic nature of pKaconsists in measuring pKain water and methanol as co-solvent.As the dielectric constant of the solvent mixture decreases by increasing the amount of the co-solvent, the pKaof an acid increases,whereas for a base the reverse is true.The variation of pKain the presence of the co-solvent for monomer11(Table 1 andFig. S11) clearly shows that the pKaof the second acidic center is 9.79(positive slope),whereas 8.67 is a basic center(negative slope).

Table 1 Predicted and experimental pKa for peptide nucleic acid (PNA) monomers 1-11. Standard deviation is reported.
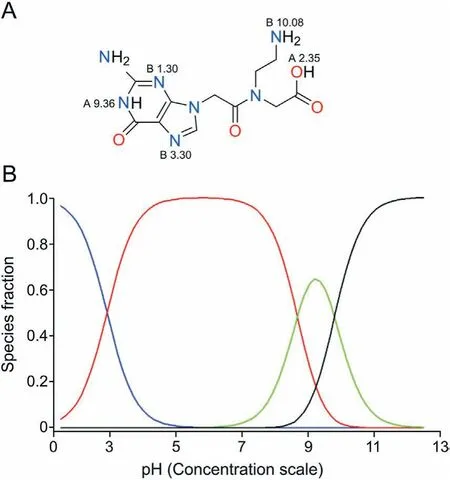
Fig.5.Monomer 11.(A)MoKa output;(B)the ionization profile(in red the zwitterionic species).
Fully protected monomers1-4and Boc-deprotected monomers5-8show a lower number of ionizable centers than monomers9-11and were used to confirm the acidic/basic nature of pKadiscussed above.For instance,we compared the ionization behavior of cytosine derivatives2,6,and10by experimental measurements in both water and water/methanol mixtures.In particular,for the fully protected monomer2we observed the presence of two acidic groups corresponding to pKavalues of 3.62 and 10.27. The first proton (pKa3.62) of monomer2was mainly released from the-COOH group on theaegbackbone,while the second constant(pKa10.27) was presumably due to proton loss from the -NH(Z) group of cytosine, as also suggested by Moka simulations. Monomer6displayed three experimental pKavalues: a basic center (pKa8.89)related to the free aliphatic amino group on the backbone,and two acidic centers with pKavalues of 2.84 and 10.39 related to the-COOH group on theaegbackbone and the -NH present in the Z group of cytosine, respectively. These data were in reasonable agreement with the values determined for monomer2and with MoKa predictions. A major change of the ionization profile was found for the fully deprotected monomer10when compared to monomer6.Indeed,as the consequence of the removal of Z group from the exocyclic-NH2of cytosine,the acidic center involving the-NH in the Z group was lost,and a weak basic moiety(pKa=4.15)appeared. This basic center can be associated with the nitrogen atom N(3) of the cytosine ring. For adenine derivatives1,5and9,the evolution of the ionization behavior when passing from the most protected monomer1to the fully deprotected monomer9was similar to that described for the cytosine derivatives (more details in the Supplementary data).
Slightly different ionization properties were found for the guanine derivatives3,7and11. As expected, the fully protected monomer3showed two acidic centers with pKaof 3.19 and 8.24,corresponding to the loss of proton from the-COOH group on theaegbackbone and the NH(1) of the guanine ring, respectively. The removal of the Boc protecting group from theaegbackbone from monomer3provided monomer7, in which a strong basic center(pKa9.44)appeared owing to the free aliphatic amine group.Fully deprotected monomer11showed the acidic center (pKa2.90), the basic center (pKa8.67), and a slightly acidic nitrogen (pKa9.79)related to the NH(1) of the guanine ring. Finally, the two thymine derivatives4and8differed by the protection of the aliphatic amino group on the backbone. As the consequence of the Bocdeprotection, monomer8showed a basic center (pKa8.96) due to the free amino on theaeg,besides the two acidic centers(pKa2.54 and 9.94 for -COOH and -NH(1), respectively) similar to those found in monomer4.
Overall,three main findings are obtained from ionization data of PNA monomers.Firstly,data shown in Table 1 support that partially deprotected monomers5-8and fully deprotected monomers9-11are present as zwitterions at physiological pH (the ionization profile of monomer11is shown in Fig. 5B, red line). The third ionization center has a weak acidic nature(pKa8-10)in monomers5-8and11and a weak basic center (pKa3.9-4.1) in monomers9and10;thus these sites have a poor relevance on the ionization profile.Secondly, the 2-ethyl amino group on theaegbackbone of monomers5-11is characterized by pKavalues ranging from 8.67 to 9.44.For the free-NH2of the alanine we found a basic pKavalue of 9.72 using the same potentiometric equipment. This means that the 2-ethyl amino group shows slightly lower basicity than that of-NH2in α-aminoacids. Finally, our data suggest that nucleobases do not significantly alter their pKavalues when included in PNA monomers, although the basicity of adenine N(1) center and cytosine N(3) center slightly decreases.
Once we obtained a complete characterization of the ionization properties of monomers1-11, we determined the pKavalues of decamer12that had been selected as model PNA oligomer for this study.In particular,decamer12,endowed with a terminal-COOH group useful for the potentiometric titration, was synthesized on solid phase according to the literature[45].Taking into account its structure,decamer12is expected to show eleven acidic pKavalues,being one around 4.00 for the -COOH group and the others ≥9.0 related to NH(1) of the thymine ring. We then submitted decamer12to potentiometric measurements and the obtained results confirmed our expectations(pKa1=5.08,pKa2=8.51,pKa3=9.56,pKa4=9.56,pKa5=10.22,pKa6=10.23 and pKa7-pKa11>10.3).The solubility of decamer13was too low for potentiometric measurements.
3.3. Lipophilicity
As discussed above, IAM chromatography offers a promising alternative to octanol/water partitioning as a tool to mimic specific interactions of compounds with membrane phospholipids. Lipophilicity data of the monomers1-11and the two model PNA decamers12and13expressed as log kWIAMare reported in Table 2.

Table 2 Lipophilicity data of PNA monomers 1-11 and PNA decamers 12 and 13 (for monomers 1-11 SD <0.08, for decamers 12 and 13 SD <0.20).
The fully protected PNA monomers1,2and3show very similar positive log(1.43-1.76), and they are the highest values obtained in the PNA monomers series.Thus,monomers1-3are the most lipophilic systems. As expected, this is due to the simultaneous presence of the hydrophobic butyloxycarbonyl protecting group on theaegbackbone and the benzyloxycarbonyl protecting group on the nucleobases. As the consequence of the Boc deprotection,monomers4,6and7have shorter IAM retention time,with logvalues ranging from -0.49 to 0.97, that is about half of those obtained for monomers1-3.Negative logvalues were obtained for the fully deprotected monomers8-11(from -0.68 to-0.75),which are the most hydrophilic ones.These data seem to be in agreement with the results obtained from a systematic study on the retention properties of nucleobases in IAM chromatographic systems [54]. Indeed, a set of structurally congeneric purines possess weaker IAM retention,and their logvalues are closeor smaller than 0, except for those with specific H-bond and/or electrostatic interactions. Since similar values were also obtained for PNA monomers, the presence of theaegbackbone does not significantly affect the lipophilic properties of the nucleobases.
Finally, we evaluated the lipophilicity of two model PNA decamers12and13, containing ten thymine monomers and all four nucleobases (GTAGATCACT), respectively, in order to verify the influence of the nucleobases’ nature on the absorption properties of PNAs. Oligomers12and13have very similar lipophilic properties since they display negative logvalues of -1.00 and -0.97,respectively.These are the shortest IAM retention time obtained in this study, which suggests that PNA decamers are slightly more hydrophilic than the single PNA monomers.Moreover,the nature of the nucleobases seems not to influence the lipophilicity of the oligomer. The poor affinity of PNA decamers towards IAM surface,which is a good model of the membrane lipid barrier, could rationalize the well-documented limited pharmacokinetic properties of PNA oligomers. Indeed, commercial drugs with good properties generally (at least when passive diffusion is known to be the dominant permeation mechanism)show positive logvalues(e.g.,caffeine 0.31,dexamethasone 1.71,and diltiazem 2.83)[47,55],while PNA decamers 12 and 13 display negative values. However,very high logvalues should also be avoided since they are potentially connected with undesired effects such as toxicity.
4. Conclusion
In this paper we reported the synthesis,the NMR analysis of the conformational properties, the ionization and the lipophilicity features of eleven PNA monomers1-11along with the study of the ionization and lipophilicity properties of two model PNA decamers12and13. From the conformational point of view, NMR study confirms that PNA monomers exist as a mixture ofE-andZ-rotamer in 70:30 or 50:50 ratio,depending on the nature of the nucleic base.Moreover,NMR experiments were also used to determine the ratio between the cationic monomer and the anion CF3COO-in monomers5-11, thus confirming their molecular formula.
The multiprotic nature of PNA derivatives imposed to set up a complex strategy to measure pKavalues and assign their acidic/basic nature. Therefore, we combined a sophisticated potentiometric method to measure the ionization constants with a convenient computational tool to guide the pKaassignment. Findings supported that acid-base properties of single nucleobases are maintained in the PNA decamer12. The lipophilicity properties of PNA monomers and oligomers were investigated by means of IAM chromatography. Results show that the investigated PNA derivatives show poor affinity towards phospholipid-based membranes.
Overall, we provide for the first time an insight into the physicochemical properties responsible for the modulation of the pharmacokinetic behavior of PNA derivatives. Our data in fact support the well-known inefficient cell penetration of PNA that restricts its clinical application. In this scenario, our study represents an important milestone since it delivers efficient propertybased drug design tools required to discover new drug-like PNA derivatives.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Pramod Thakare thanks the University of Milan for the Ph.D.fellowship. Giulia Caron, Maura Vallaro and Sonja Visentin acknowledge the financial support from the University of Turin(Ricerca Locale ex-60%, Bando2019).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.07.007.
 Journal of Pharmaceutical Analysis2021年5期
Journal of Pharmaceutical Analysis2021年5期
- Journal of Pharmaceutical Analysis的其它文章
- A sensitive electrochemical detection of metronidazole in synthetic serum and urine samples using low-cost screen-printed electrodes modified with reduced graphene oxide and C60
- Predicting the grades of Astragali radix using mass spectrometrybased metabolomics and machine learning
- Capsid destabilization and epitope alterations of human papillomavirus 18 in the presence of thimerosal
- Transformation of berberine to its demethylated metabolites by the CYP51 enzyme in the gut microbiota
- Simulation of the oxidative metabolization pattern of netupitant, an NK1 receptor antagonist, by electrochemistry coupled to mass spectrometry
- One extraction tool for in vitro-in vivo extrapolation? SPME-based metabolomics of in vitro 2D,3D,and in vivo mouse melanoma models
