Mechanism underlying treatment of ischemic stroke using acupuncture: transmission and regulation
Bing-Qian Cao, Feng Tan, , Jie Zhan, Peng-Hui Lai
Abstract The inflammatory response after cerebral ischemia/reperfusion is an important cause of neurological damage and repair. After cerebral ischemia/reperfusion, microglia are activated, and a large number of circulating inflammatory cells infiltrate the affected area.This leads to the secretion of inflammatory mediators and an inflammatory cascade that eventually causes secondary brain damage, including neuron necrosis, blood-brain barrier destruction, cerebral edema, and an oxidative stress response. Activation of inflammatory signaling pathways plays a key role in the pathological process of ischemic stroke.Increasing evidence suggests that acupuncture can reduce the inflammatory response after cerebral ischemia/reperfusion and promote repair of the injured nervous system.Acupuncture can not only inhibit the activation and infiltration of inflammatory cells, but can also regulate the expression of inflammation-related cytokines, balance the effects of pro-inflammatory and anti-inflammatory factors, and interfere with inflammatory signaling pathways. Therefore, it is important to study the transmission and regulatory mechanism of inflammatory signaling pathways after acupuncture treatment for cerebral ischemia/reperfusion injury to provide a theoretical basis for clinical treatment of this type of injury using acupuncture. Our review summarizes the overall conditions of inflammatory cells,mediators, and pathways after cerebral ischemia/reperfusion, and discusses the possible synergistic intervention of acupuncture in the inflammatory signaling pathway network to provide a foundation to explore the multiple molecular mechanisms by which acupuncture promotes nerve function restoration.
Key Words: acupuncture; central nervous system; factor; inflammation; ischemic stroke;pathways; protein; stroke
Introduction
Stroke is the second leading cause of death worldwide, and it is a primary cause of death and disability, thus endangering human health (Feigin and Brainin, 2019). The ischemic penumbra can be spared by restoring blood perfusion. At present, the most effective treatments are thrombolysis and interventional therapy, but both of these treatments have a limited time window. Therefore, an alternative treatment strategy for cerebral ischemia/reperfusion(CI/R) injury is urgently needed to promote recovery of neurological function through multiple targets using multidirectional comprehensive treatments and alleviate the burden of stroke on society and the family. The pathological mechanism of CI/R is complex. Energy metabolism disorders,excitatory amino acid toxicity, inflammatory reactions,penumbra depolarization, and apoptosis are involved in the pathophysiological process (George and Steinberg, 2015). The cytokine-mediated inflammatory response plays a crucial role in the pathological injury of CI/R (Kawabori and Yenari, 2015).Many animal studies have found that damage-associated molecular patterns (DAMPs) recognize the widely expressed Toll-like receptors (TLRs), endogenous cannabinoid system receptors, and Notch receptors on microglia, perivascular macrophages, and vascular endothelial cells and activate multiple inflammatory signaling pathways. On the one hand,DAMPs activate the innate immune system, which prevents immune cells from producing anti-inflammatory factors, clears dead cells and extracellular matrix fragments in infarcted areas,and alleviates inflammatory responses. On the other hand,the resulting dynamic ion imbalance and brain dysfunction causes an excessive increase in inflammatory cell chemotaxis,adhesion, and infiltration of damaged brain tissue, resulting in the production of pro-inflammatory factors. Subsequently,these inflammatory factors increase inflammatory cell infiltration, resulting in further release of inflammatory mediators and reactive oxygen species, triggering an inflammatory cascade reaction. This leads to neurovascular injury, blood-brain barrier (BBB) damage, and aggravation of neuronal injury. Furthermore, peripheral immune cells, such as neutrophils, lymphocytes, and macrophages, can penetrate brain tissue through the damaged BBB to exacerbate ischemic brain damage (Jin et al., 2010; Kawabori and Yenari, 2015).These two processes promote and antagonize each other,and their coordinated relationship is complex. The outcome of nerve repair or necrosis after cerebral infarction depends on the balance between the two processes. Therefore,early control of glial cell activation, reduction of leukocyte infiltration and inflammatory mediator release, and inhibition of the inflammatory response are essential for reducing stroke-induced damage and promoting restoration of neurological function.
Chinese acupuncture has been used to treat stroke for thousands years and has achieved remarkable benefits. It mainly stimulates acupoints, clears the meridians, reconciles the Qi, blood, Yin and Yang, adjusts the function of the viscera,stimulates the body’s ability to resist disease, and restores the non-pathological, physiological state. Acupuncture is a benign process that has biphasic effects on the overall immune regulation. An increasing number of basic research studies have shown that acupuncture regulates expression of inflammatory mediators, factors, and adhesion molecules by mediating inflammatory signaling pathway molecules, such as TLRs, endocannabinoid receptors, and Notch receptors,inhibiting inflammatory responses, reducing the infarction volume, promoting restoration of neural function, and alleviating CI/R injury (Wang et al., 2009; Lan et al., 2013; Geng et al., 2015; Han et al., 2015). Clinical studies suggest that acupuncture can reduce expression of various inflammatory cytokines and promote rehabilitation of nerve function by controlling the immune response of cerebrovascular inflammation, including molecules such as high-sensitivity C reactive protein and interleukin (IL)-1β (Wang et al., 2016).However, current studies are focused on single proteins,cells, and channels, while acupuncture is focused on multiple targets and multi-channel interventions in its overall effect on inflammatory cells, mediators, and pathways after cerebral infarction. It is not clear how these inflammatory cells,mediators, and pathways synergistically or differentially affect the dynamic balance between anti-inflammatory and proinflammatory processes and aggravate CI/R injury. Therefore,it is not known whether signal transmission pathways containing these factors can form a signaling pathway network and how this network synergistically or differentially affects the dynamic balance between anti-inflammatory and proinflammatory processes in the aggravation of CI/R injury. It is unknown whether acupuncture can restore homeostasis under various pathological conditions by regulating similar signaling pathway networks to activate different response cascades in specific tissues to manage pathological insults. Therefore, this article examines inflammatory cells, summarizes the overall conditions of inflammatory cells, mediators, and pathways after CI/R, and discusses the possible synergistic intervention of acupuncture in the inflammatory signaling pathway network to enrich the theory of acupuncture and provide a foundation to explore the multiple molecular mechanisms by which acupuncture promotes nerve function restoration.In this manner, acupuncture can be better transformed into clinical practice and used to guide the rehabilitation and treatment of patients. We performed a literature search of the PubMed database from 1900 to the present using the following search strategy: (CI/R OR ACI OR stroke[MeSH Terms]) AND (acupuncture OR electroacupuncture[MeSH Terms] AND inflammation[MeSH Terms]).
Biphasic Regulation of Inflammatory Cells via Acupuncture
Microglia/macrophages
Microglia are important immune cells of the central nervous system (CNS). They can be activated rapidly within a few minutes of CI/R, reaching a peak at 6–12 hours after injury and decreasing after several days. Although microglia and macrophage populations have a certain genetic heterogeneity,both originate from the original medullary progenitor cells of the yolk sac (Greter and Merad, 2013), Ginhoux et al. (2010)showed using fate mapping analysis that microglia may be differentiated from a specific population of macrophages;therefore, they have certain similarities with resident macrophages regarding their molecular morphology and biological functions. After cerebral ischemia and hypoxia,damaged brain cells release DAMPs, which are recognized by TLR4 and other pattern recognition receptors on the surface of microglia. On the one hand, microglia can provide neuroprotective factors after nervous system injury, remove cell debris, and regulate the neural repair processes. On the other hand, microglia produce high levels of proinflammatory and cytotoxic mediators that hinder CNS repair and contribute to neuronal disability and cell death. These dual characteristics may be related to their phenotype and the functional response after injury (Kim et al., 2014; Ma et al., 2017; Qin et al., 2017). Microglia are divided into a proinflammatory M1 phenotype and an immunosuppressive M2 phenotype. The former is designated as activated microglia and can be stimulated by interferon-γ and lipopolysaccharide to produce a variety of pro-inflammatory mediators (such as IL-1β, IL-6, and tumor necrosis factor (TNF)-α), reactive oxygen species, nitric oxide, and matrix metalloproteinase-9 (Varnum and Ikezu, 2012; Hu et al., 2015; Garaschuk and Verkhratsky,2019; Zheng et al., 2020). These factors decrease neuronal survival and aggravate neuronal damage (Hu et al., 2012).The M2 phenotype, also known as the anti-inflammatory phenotype, is mainly expressed early in ischemia. These cells can produce a variety of anti-inflammatory cytokines, such as IL-10, transforming growth factor-β, IL-4, IL-13, and insulin-like growth factor-1, and are thought to have protective functions that inhibit inflammation and promote tissue repair (Cherry et al., 2014). The microglia phenotypes expressed at different cerebral ischemic stages are time-dependent and can mutually transform (Xia et al., 2015; Xiong et al., 2016). Triggering receptor expressed on myeloid cells 2 (TREM2) is an important cell surface innate immune receptor that is expressed in microglia in the CNS. It binds to the transmembrane signal adaptor DNAX activator protein 12 and participates in a variety of pathophysiological responses, such as immune surveillance, cell interactions, phagocytosis of tissue debris,and inflammatory responses (Kiialainen et al., 2005). TREM2 is a negative regulator of TLR4/nuclear factor kappa-B(NF-κB) signaling that inhibits lipopolysaccharide-induced neuroinflammatory responses by negatively regulating TLR4-mediated activation of the NF-κB pathway and promotes the transformation of microglia phenotypes (Gawish et al., 2015;Zhang et al., 2019). Moreover, some studies have found that the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)pathway is mediated by TREM2. TREM2 may contribute to the M1 to M2 transformation through the above two major microglial pathways and then induce phosphorylation of DNAX activator protein 12 and extracellular signal-regulated kinase(ERK), which promotes cytoskeletal reorganization, enhances phagocytosis and reduces TNF-α levels, nitric oxide synthase gene transcription, and inflammatory responses (Takahashi et al., 2005; He et al., 2019).
Microglial activation is regulated by complex signaling networks formed by many factors at the cellular, molecular,and gene levels. Acupuncture can regulate the direction of activation and phenotypic transformation of microglia,which are related by the corresponding signaling pathways.Wang et al. (2015a) found that acupuncture up-regulates the nuclear translocation of nuclear factor erythroid2-related factor 2 (Nrf2) in neurons as well as the expression of Nrf2 and the downstream target genes NADPH quinone oxidoreductase-1 and heme oxygenase-1 in hippocampal neurons, reduces Nrf2-dependent activation of microglia,improves cognitive impairment caused by bilateral carotid ischemia, and exerts neuroprotective effects. Studies have found that limb ischemia/reperfusion injury can mediate microglial activation through circulatory inflammation, thus aggravating brain damage (Bianco-Batlles et al., 2008).However, electroacupuncture (EA) preconditioning can interfere with the important target of this process, activation of microglia, to further improve pathological damage in the brain caused by limb ischemia/reperfusion (Chen et al.,2012b). Acupuncture inhibits microglial activation through the p38 mitogen activated protein kinase (MAPK) and ERK pathways and down-regulates IL-1β, IL-6, TNF-α, matrix metalloproteinase-9, and prostaglandin E2 expression, thereby reducing the inflammatory response (Choi et al., 2012). EA improves motor behavior and memory by inhibiting the inflammatory response mediated by microglia/macrophage P2 purinoceptors (P2Y purinoceptor 7 and 1) and reduces cerebral astroglial and microglial/macrophage hyperplasia in the hippocampal CA1 region and sensorimotor cortex around the infarction (Huang et al., 2017). In addition, studies have confirmed that EA treatment of focal CI/R injury in rats can significantly up-regulate TREM2, promote the microglial M1 to M2 transformation, and increase the phagocytic activity of microglia to reduce inflammatory damage (Xia et al.,2015; Xu et al., 2018). Cannabinoid type II (CB2) receptor, a G-protein-coupled receptor in the endocannabinoid system(ECS), mediates the microglial M1 to M2 transition via the CB2 receptor-protein kinase C (PKC) pathway, which may be associated with a reduced inflammatory response (Ma et al.,2015).
In addition to the dual characteristics of phagocytotic clearance and activation of pro-inflammatory factors in microglia described above, acupuncture also has a biphasic regulatory effect on microglia, which not only increases microglial phagocytosis in the ischemic penumbra of brain tissue, but also down-regulates the inflammatory damage caused by excessive activation of microglia. Table 1 summarizes the evidence of acupuncture-induced downregulation of microglial activation in inflammatory cascade reactions.
Astrocytes
Astrocytes are more than five times as numerous as neurons in the CNS. They have many intermediate and long protrusions that serve to separate, support, and connect neurons.Astrocytes, as important in situ cells of the neuroinflammatory response after stroke, are responsive to various inflammatory factors in the injured area within 1–2 weeks after stroke,showing cellular hypertrophy, proliferation, and intermediate increased expression of filament proteins, including glial fibrillary acidic protein (GFAP), vimentin, and nestin. After 2 weeks, astrocytes form scars around the ischemic necrosis area (Cekanaviciute and Buckwalter, 2016). In the early stage of ischemia, scars form a tight barrier to prevent the spread and aggravation of inflammation, while chondroitin sulfate proteoglycans in the mature scar can inhibit axon growth and affect nerve regeneration during the recovery period.However, researchers have also found that astrocytes are beneficial for axonal regeneration in the CNS using three genetically targeted loss-of-function manipulations (Anderson et al., 2016).
GFAP is a skeletal protein unique to astrocytes that helps maintain the integrity of the BBB and myelin. EA can significantly activate astrocytes, up-regulate the expression of GFAP, improve long-term motor function defects, and promote nerve function restoration (Zhao et al., 2018). The mechanism involves up-regulation of astrocyte monocarboxylate transporter 1 expression and enhancing lactic acid energy metabolism of neurons in the ischemic zone, transforming it from an intracellular to an extracellular process (Lu et al.,2015). Tao et al. (2016) found that EA exerts neuroprotective effects by promoting expression of GFAP/vimentin/nestinpositive reactive astrocytes and secretion of brain-derived neurotrophic factor (BDNF) by reactive astrocytes. In addition,studies have confirmed that EA preconditioning can interfere with the expression of monocyte chemotactic protein-induced protein (MCPIP) molecules in astrocytes in ischemic brain tissue and reduce leukocyte infiltration and the secretion of inflammatory mediators such as TNF-α, IL-1β, and IL-6(Jin et al., 2013; Xu et al., 2020). Huang et al. (2017) found that EA regulates P2Y purinoceptor 1, an ATP-activated P2 purinoceptor family G-protein-coupled receptor, to inhibit astrocyte activation and reduces the release of inflammatory mediators. EA induces downstream molecules and type 2 excitatory amino acid transporters by activating CB2 receptors in astrocytes and exerts neurological effects by activating excitatory amino acid neurotransmitters such as glutamate(Zhu et al., 2013; Zhang et al., 2018b). These studies confirmed that acupuncture exerts neuroprotective effects through the biphasic action of astrocytes, reduces secondary inflammatory damage, and promotes nerve function restoration. Table 2 summarizes the evidence for the role of acupuncture in down-regulation of astrocyte infiltration in the inflammatory cascade.
Leukocytes
Circulating leukocytes are rapidly activated in large numbers after receiving cerebral ischemic stimulation signals. These cells then infiltrate the damaged brain tissue through the BBB and adhere to endothelial cells expressing adhesion molecules, thus impairing local microvascular blood flow and causing further release of pro-inflammatory factors,which amplifies the inflammatory response and aggravates brain damage (Jin et al., 2010). EA pretreatment significantly down-regulated leukocyte co-antibody CD45 expression in brain tissue of middle cerebral artery occlusion mice, but no significant change was observed in MCPIP1 knockout mice,suggesting that EA can reduce infiltration of white blood cellsafter cerebral ischemia in an MCPIP1-dependent manner (Jin et al., 2013). Under normal circumstances, intercellular cell adhesion molecule-1 (ICAM-1) is expressed in low levels in vascular endothelial cells, but its expression is significantly up-regulated by inflammatory signal stimulation after CI/R. P-selectin is an early mediator of polymorphonuclear migration and is beneficial for ICAM-1-mediated polymorphonuclear capture. One study found that the leukocyte-mediated pathological damage and infarct volume of cerebral infarction are closely related to ICAM-1 and P-selectin expression (del Zoppo, 2010). EA can significantly interfere with this process by inhibiting adhesion of ICAM-1 and P-selectin mRNA and protein in cerebral ischemic areas,thus inhibiting the adhesion molecule-mediated adhesion and infiltration of vascular endothelial cells and neutrophils, which can prevent or ameliorate the inflammatory reaction (Mao et al., 2007).
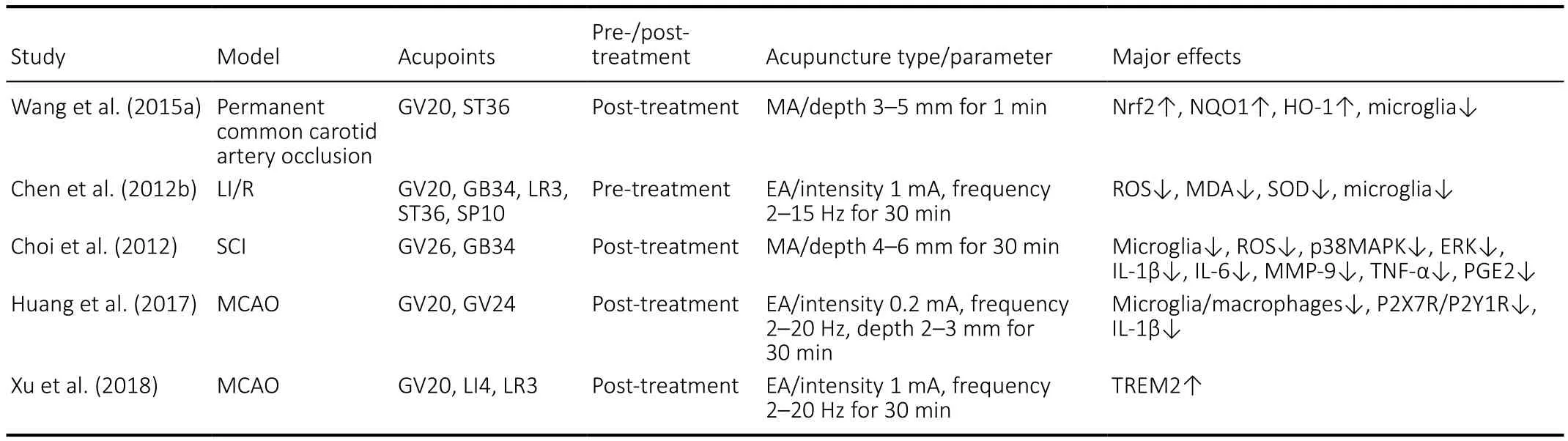
Table 1 |Acupuncture downregulates microglial activation in the inflammatory cascade
Both neutrophils and lymphocytes are important types of leukocytes that are recruited by brain immune cells early in CI/R. However, a recent report suggested that T lymphocytes play a crucial role in the inflammatory response in the nervous system and are closely related to targeted therapy after acupuncture intervention (Subra and Trautmann, 2019).T lymphocytes are classified as CD4+helper T cells (Th) and CD8+cytotoxic T cells according to the expression of antigens on their cell membrane. A study using fluorescence-activated cell sorting found that lymphocytes infiltrated ischemic brain tissue before neutrophils in the case of CI/R, and CD4+/CD8+T lymphocytes were expressed on the first day after stroke (Gelderblom et al., 2009). CD4+T cells are further divided into Th1/Th2 and Th17/regulatory T (Treg) cells according to their secreted cytokines (Gu et al., 2015). Under normal conditions, Th1/Th2 and Th17/Treg cells maintain a dynamic balance in the body. Th1 cells mainly secrete IL-2, interferon-γ, TNF-α, and other inflammatory mediators,mediate cellular immune responses related to cytotoxicity and local inflammation, and facilitate antibody production;Th2 cells mainly secrete IL-4, IL-5, and IL-10 and participate in other humoral immune responses that stimulate B cells. Th17 cells have pro-inflammatory effects and induce the expression of pro-inflammatory cytokines, chemokines, and matrix metalloproteinases; Treg cells inhibit CD4+T cells, CD8+T cell proliferation, cytokine secretion, dendritic cells, and monocyte function and have an immunosuppressive function.
EA atZusanli(ST36) can down-regulate Th1 and Th17 cell expression through the adreno-corticotropic hormone pathway, up-regulate Th2 and Treg cell expression, and achieve relative balance between Th1/Th2 and Th17/Treg cells, thereby inhibiting the proliferation of specific T cells and regulating the inflammatory response (Liu et al., 2010). It has also been suggested that inhibition of proliferation of specific T cells may be related to the effect of EA on the increase in endogenous opioid peptides and β-endorphin concentrations(Liu et al., 2013). Pretreatment with bee venom acupuncture regulated CD4+/interferon-γ and CD4+/IL-17+T cells, which are highly expressed in the spinal cord and lymph nodes of rats,decreasing their levels to less than those of the control group and inducing the transformation of primitive Th cells into Treg cells. This process alleviated certain inflammatory responses and enhanced immunity (Lee et al., 2016). Table 3 summarizes the evidence of acupuncture-induced down-regulation of leukocyte infiltration in the inflammatory cascade.
Signaling Pathway
The cascade of inflammatory reactions after the acute cerebral infarction (ACI) is accompanied by activation of many inflammatory cells and the release of inflammatory mediators. The signaling pathway is a critically important segment for these inflammatory cells; therefore, investigation of the effects of acupuncture regimens on the inflammatory signaling pathway after ACI is of great significance.
TLR4/NF-κB
TLRs are pattern recognition receptors that recognize different pathogen-associated molecular patterns and are indispensable for innate immune and inflammatory responses. The signaling pathways of TLRs are closely related to the precise regulation of cellular Toll-IL-1 receptor (TIR) domain adapters such as myeloid differentiation factor 88, TIR domain-containing adaptor protein, TIR domain-containing adaptor inducing interferon-β, and the related TIR domain-containing adaptorinducing interferon-β adaptor molecule. Mammals express atleast 11 subtypes of TLRs, of which TLR4 is closely related to inflammatory gene expression (Chen and Yu, 2016). After TLR4 activation, myeloid differentiation primary response 88 and TIR domain-containing adaptor protein are recruited through TIR-TIR interactions, and myeloid differentiation primary response 88 interacts with IL-1 receptor-associated kinase 4 through its death domain. In turn, IL-1 receptor-associated kinase-4 activates other members of the IL-1 receptorassociated kinase family, including IL-1 receptor-associated kinase 1, and TNF receptor-associated factor 6. Then, TNF receptor-associated factor 6, together with E2 ubiquitin protein ligase, activates a complex containing transforming growth factor-β-activated kinase 1 and transforming growth factor-β-activated kinase 11 proteins 1–3. This complex can activate the MAPK and NF-κB pathways (Hanamsagar et al., 2012). In addition, activated IL-1 receptor-associated kinase 1 can degrade I-κB (an inhibitor of NF-κB) and activate transcription of inflammatory cytokines such as TNF and IL-6(Yamamoto et al., 2003). Many pro-inflammatory cytokines and chemokines are activated in glial cells after a stroke, and TLR4 expression in neurons is significantly increased (Yang et al., 2008).

Table 2 |Acupuncture downregulates astrocyte infiltration in the inflammatory cascade

Table 3|Acupuncture downregulates leukocyte infiltration in the inflammatory cascade
EA in limb acupoints can significantly reduce the expression of key signaling molecules including TLR4, NF-κB p65, and phospho (p)-IκB in the TLR 4/NF-κB signaling pathway after ACI and inhibit secretion of inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Therefore, it is speculated that EA can mediate the TLR4/NF-κB signaling pathway to exert anti-inflammatory effects after cerebral ischemia (Lan et al.,2013). EA promoted TLR4/NF-κB pathway mRNA expression at 3 days and down-regulated its expression at 7 and 14 days after cerebral ischemia, suggesting that EA can mediate bidirectional regulation of the TLR4/NF-κB pathway to promote nerve damage repair. This bidirectional control may be associated with proliferation and differentiation of glial cells and scar formation (Lan et al., 2013; Jiang et al., 2016;Lin et al., 2018). EA can significantly improve neurological damage, reduce neuronal mortality, and attenuate inflammatory responses through inhibition of microglial TLR4/NF-κB pathway activity and attenuation of IL-1β, IL-6, TLR4,high mobility group box 1, TIR domain-containing adaptor inducing interferon-β 6, inhibitor kappa B kinase β, and NF-κB p65 levels (Han et al., 2015). EA at theBaihui(GV20) and Shenting (GV24) points inhibits activation of the NF-κB signal and brain cell apoptosis, reduces the expression of Bax and Fas, two downstream apoptotic genes, and improves learning and memory abilities and post-stroke recognition of objects(Feng et al., 2013). These studies suggest that acupuncture during the early stage of ischemia can up-regulate TLR4/NF-κB pathway expression, promote glial cell proliferation and differentiation and formation of early scars, and play a role in nutritional protection and limiting the inflammatory response.In the late stage, the TLR4/NF-κB pathway and TNF-α, IL-1β, IL-6, and other inflammatory cytokines were inhibited,which significantly improved nerve injury and reduced the inflammatory response.
ERK/JNK/p38
The MAPK family is an important group of signal-regulating enzymes linking cell membrane surface receptors and regulatory genes. The cellular responses to neuroprotection may involve CB1 receptors and related signaling elements,including ERK 1/2, c-jun N-terminal kinase (JNK), and p38, which are activated by phosphorylation and activate transcription factors to regulate cell survival, differentiation,and apoptosis (van der Stelt and Di Marzo, 2005). Hu and Wieloch (1994) first proposed activation of the MAPK signal transduction pathway in cerebral ischemia and hypoxic injury.Since then, many studies have been performed, finding that ischemia and hypoxia can activate the MAPK cascade in nerve cells through known pathways such as PKC, adenylyl cyclase,and tyrosine receptors, which in turn activate pathways such as ERK, JNK, and p38. Activation of the ERK1/2 pathway is mediated by Gi/o-dependent CB1 and is involved in synaptic plasticity and integrity. This pathway can enhance antioxidant activity and neurotrophin expression to induce ischemia tolerance in brain tissue (Du et al., 2010; Zhang et al., 2016a;Wang et al., 2018). The MAPK/ERK pathway is involved in the inflammatory response, apoptosis, angiogenesis, destruction of the BBB, and development of neuronal stem cells after CI/R, but its role is controversial. Activation of the MAPK/ERK pathway promotes proliferation and differentiation of endogenous neural stem cells (eNSCs) in the rat hippocampus and reduces neuronal apoptosis after cerebral infarction(Shioda et al., 2009; Liu et al., 2018b; Wang et al., 2019a).Additional studies have found that activation of the MAPK/ERK pathway aggravates brain tissue injury after CI/R (Alessandrini et al., 1999), and the mechanism may be related to activation of JNK/p38, leading to an increase in sodium-glucose transporter type 1 (Yamazaki et al., 2018).
The protective effect of EA may be related to activation of the ERK1/2 pathway by CB1 receptors, promotion of an endogenous protein kinase Cε (εPKC)-mediated anti-apoptotic effect, and enhancement of glycogen synthetase kinase 3β (Du et al., 2010; Wang et al., 2011; Wei et al., 2014). Studies have shown that EA can induce the ERK/JNK/p38 pathway to inhibit the up-regulation of caspase-3 and Bim, reverse inhibition of ERK and Bcl-2, and exert anti-apoptotic and neuroprotective effects (Wu et al., 2015a; Xing et al., 2018). Liu et al. (2018a)demonstrated through terminal deoxynucleotidyl transferase dUTP nick end labeling and transmission electron microscopy that EA at the Shenting and Baihui acupoints can inhibit activation of JNK and p38 in the ischemic peripheral zone,enhance the activation of ERK1/2, and up-regulate the expression of Bcl-2/Bax signaling pathway proteins to facilitate clearance of apoptosis. Wu et al. (2018) confirmed that EA can simultaneously up-regulate the ERK protective pathway,down-regulate the p38MAPK pro-apoptotic pathway, and promote crosstalk between these two signaling pathways to exert neuroprotective effects. Yang et al. (2013) found that EA can promote the proliferation of eNSCs in the hippocampus by activating the ERK pathway and promoting neurogenesis in damaged brain tissue. The p38MAPK signaling pathway not only promotes reactive astrocyte lesions but also aggravates progressive infarction of CI/R (Cheng et al., 2014;Roy Choudhury et al., 2014). Furthermore, EA-mediated activation of the ERK/p38 pathway activates cyclic adenosine monophosphate response element-binding protein, a selective nuclear transcription factor that regulates gene expression and nuclear translocation and is involved in cell survival, neurogenesis, and neural plasticity, and it inhibits reactive astrocytes and decreases the release of TNF-α, IL-1β, and other downstream inflammatory factors (Cheng et al.,2015).
PI3K/Akt
The PI3K/Akt signaling pathway regulates cellular activities in a variety of environments, including cell survival, proliferation,metabolism, and cancer progression (Fruman et al., 1998).CB1 receptors in the central ECS mediate activation of the PI3K/Akt pathway and increase phosphorylation of glycogen synthetase kinase 3β. Therefore, activation of the PI3K/Akt signaling pathway may be associated with neuroprotective properties (Ozaita et al., 2007). Similar to CB1 receptors, CB2 receptors also display neuroprotective functions by inhibiting PI3K/Akt pathway activation of the MAPK cascade reaction(Fernández-Ruiz et al., 2008). Increased microglia/macrophage expression and enhanced release of the inflammatory factors IL-6, IL-1β, TNF-α, CD14, CD44 and inducible nitric oxide synthase were found after cerebral ischemia (Chen et al.,2017; Wei et al., 2017). After CI/R, the expression of target genes such as Bad, p-Bad, Bcl-2, and Bax and the number of caspase-3 positive cells in the PI3K/Akt signaling pathway were significantly increased. After EA treatment, PI3K and p-Akt expression was significantly increased, and Bad, p-Bad, Bcl-2, Bax, and caspase-3 expression was significantly decreased.A PI3K inhibitor could reverse the above-mentioned expression, suggesting that the EA could interfere with the expression of downstream apoptosis genes through the PI3K/Akt signaling pathway (Xue et al., 2014). Chen et al. (2012a)suggested that the neurotrophic factors BDNF and glial cell derived neurotrophic factor modulate these apoptotic genes downstream of the PI3K/Akt pathway that are affected by EA. Zhang et al. (2018a) combined traditional Chinese acupuncture therapy with modern medical theory, using scalp acupuncture to stimulate the corresponding motor cortex projection area, and found that this treatment can inhibit the PI3K/Akt signaling pathway, reduce hippocampal neuronal apoptosis, and promote the recovery of function after nerve injury. The action of acupuncture on the PI3K/Akt signaling pathway may be mediated by CB receptors in the ECS, which down-regulate the expression of inflammatory cells.
BDNF/TrkB
BDNF is a biologically active signaling molecule secreted by microglia that is involved in synaptic connections between nerve cells and synaptic plasticity and acts as a neurotrophic factor for neuronal survival and growth (Binder and Scharfman, 2004; Kramár et al., 2012). After stroke, activated microglia secrete an increased amount of BDNF, which exerts its action by binding to its high-affinity receptor, TrkB. Some studies have also suggested that BDNF released after focal ischemia further activates microglia, promotes expression of the anti-inflammatory factors IL-10 and NF-κB, decreases TNF-α expression, and reduces cell necrosis and apoptosis(Jiang et al., 2010, 2011). However, BDNF-activated microglia promote inflammatory reactions during ACI and aggravate the pathological damage of brain tissue. BDNF plays a dual role in CI/R. Down-regulation of BDNF/TrkB signaling pathway protein expression is involved in neuronal apoptosis in the striatum. In a model of global cerebral ischemia,an inflammatory response was triggered by activation of microglia and astrocytes and up-regulation of the BDNF/TrkB pathway in the hypothalamus and amygdala, accompanied by release of the pro-inflammatory cytokine TNF-α. However,the trend of BDNF and TrkB mRNA and protein expression in the hippocampus was opposite to that of the hypothalamus and amygdala, exhibiting down-regulation after brain injury.This difference in expression may occur because of specific benefits at various sites (de la Tremblaye et al., 2017). Jin et al. (2018) found that brain inflammation is involved in the regulation of pentylenetetrazole-induced seizures in zebrafish. Gene expression profiling revealed that this inflammatory response is closely related to the BDNF/TrkB signaling pathway. Activation of the BDNF/TrkB pathway can further promote the infiltration of neutrophils and microglia/macrophages and the secretion of IL-1 and NF-κB (Jiang et al.,2010; Jin et al., 2018). Pro-inflammatory cytokines in the CNS induce depression-like symptoms by activating the BDNF-TrkB pathway in the prefrontal cortex, hippocampus, and nucleus accumbens (Zhang et al., 2016b).
Spinal microglia-mediated BDNF-TrkB signaling pathway interaction is critical in hyperalgesia following spinal cord injury. EA induces the BDNF/TrkB signaling pathway and reduces mechanical allodynia and thermal hyperalgesia by mediating microglial activity and BDNF expression (Tu et al., 2018). EA was shown to increase BDNF secretion in serum, activate the downstream P13K/Akt pathway, and exert neuroprotective effects against apoptosis (Chen et al.,2012a; Lin et al., 2014). In addition, Li et al. (2017) confirmed that EA could promote the continuous expression of BDNF after traumatic brain injury, overcoming the limitations of the endogenous quantity and short half-life of BDNF, and promote neurological function related to exercise, sensory function, cognition and synaptic plasticity. Application of the TrkB-specific inhibitor K252a inhibits the restoration of these neurological functions, suggesting that EA-induced upregulation of BDNF may be associated with activation of the BDNF/TrkB/p-Akt/p-ERK1/2 pathway.
JAK2/STAT3
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is an important pathway that regulates inflammation and oxidative stress in cells. DAMP-induced binding of cytokines to their receptors leads to dimerization of receptor molecules, causing receptorcoupled JAKs to be in close proximity and activated by interactive tyrosine phosphorylation. The activated JAKs catalyze tyrosine phosphorylation of the receptor itself, recruit STATs via the SH2 domain, and then increase transcription and expression of genes by forming a homodimers and binding to the corresponding target gene promoter (Chen et al., 2019). The JAK2/STAT3 pathway, which is the most closely related pathway to CNS disease, is important in the regulation of microglial activation. After CI/R, JAK2, p-JAK2,and p-STAT3 expression is increased in the brain, especially in activated microglia and astrocytes, which can aggravate brain edema and damage the BBB (Hou et al., 2018; Gong et al., 2019; Porro et al., 2019). Inactivation of the STAT pathway promotes M2 polarization of microglia/macrophages (Bi et al., 2020), indicating that abnormal activation of glial cells triggers neuroinflammatory responses that are closely related to overexpression of the JAK2/STAT3 signaling pathway. Cell experiments confirmed that down-regulation of JAK2/STAT3 pathway phosphorylation could reduce lipopolysaccharideinduced microglial secretion of TNF-α and IL-1β (Shrivastava et al., 2013). IL-1β activates JAK2/STAT3, promotes the expression of p-STAT3, GFAP, and vimentin and the release of inflammatory factors and adhesion molecules by binding to type IL-1β receptors on the cell surface, causing T cell immune responses (Liddelow et al., 2017). Overactivation of the JAK2/STAT3 pathway not only acts as an extramembrane signal of ischemia and hypoxia but also up-regulates the action of inflammatory factors and activates glial cells, forming a vicious circle that aggravates brain nerve tissue damage. Hence,identifying targets to down-regulate JAK2/STAT3 signaling may be a novel strategy to inhibit neuroinflammation.
Experimental results have shown that, under IL-6 induction,CD4+T lymphocytes differentiate into Th17 cells through JAK2/STAT3 regulation (You et al., 2017). Then, JAK2/STAT3 binds to the IL-17 genes to enhance its transcription and cause further secretion inflammatory factors. Acupuncture can notably reduce inflammatory cell infiltration, down-regulate CD4+T cell expression, improve neural function scores (Liu et al., 2010;Kim et al., 2012), reduce Th17 cell expression (Lee et al., 2016)and the pathological changes in brain tissue, and effectively inhibit secretion of the pro-inflammatory cytokine IL-17 in mice (Liu et al., 2013). The α7 nicotinic acetylcholine receptor is widely distributed in the nervous system. It regulates phosphorylation of JAK2/STAT3 pathway proteins after activation and participates in the inflammatory response (Yang et al., 2015). EA activates α7 nicotinic acetylcholine receptors and promotes balance between the pro-inflammatory cytokines IL-1β and IL-6 and the anti-inflammatory cytokine IL-10 by inhibiting JAK2/STAT3 signaling (Wang et al., 2019b).
Suppressor of cytokine signaling 3 (SOCS-3) belongs to the cytokine signaling inhibitory protein SOCS family, which is involved in negative feedback regulation of the JAK2/STAT3 signaling pathway. After EA, activation of central glial cells is inhibited, and SOCS-3 expression is up-regulated, thus providing negative feedback regulation of the JAK2/STAT3 signaling pathway and playing an anti-inflammatory role(Wang et al., 2015b). Acupuncture can regulate the release of inflammatory factors from microglia induced by amyloid β through down-regulation of the expression of JAK2 and STAT3 in the JAK2/STAT3 signal pathway in the cerebral cortex, thus reducing the chronic inflammatory damage (Chiba et al.,2009; Liu et al., 2019). In contrast to a previous study, one study found that IL-6 activation of the JAK2/STAT3 pathway increases superoxide dismutase 2 target gene expression and is involved in antioxidant and anti-inflammatory responses(Jung et al., 2011). However, knockout or blockade of IL-6 signals can aggravate cerebral ischemic injury (Yamashita et al., 2005). The IL-6 level in the hippocampus of rats with chronic cerebral hypoperfusion was increased with EA treatment, and the mRNA and protein expression of JAK2/STAT3 was significantly increased, indicating that EA can inhibit inflammatory responses by regulating the IL-6/JAK2/STAT3 signaling pathway, thus reducing hippocampal nerve cell injury and cognitive impairment (Jiang et al., 2018). Discrepancies in these results may depend on the different pathological states of the disease and whether the JAK2/STAT3 pathway is over activated and expressed during different periods or pathological states. Therefore, acupuncture can affect various targets in the JAK2/STAT3 pathway to inhibit the inflammatory response and reduce brain tissue damage.
Peroxisome proliferator-activated receptor (PPAR) is a liganddependent transcription factor of the nuclear hormone receptor family and includes three subtypes: PPARα, PPARβ/δ,and PPARγ (Michalik et al., 2004). Recent studies have shown that PPARγ is closely related to the JAK2/STAT3 signaling pathway after CI/R. Agonists can reduce p-JAK2 and p-STAT3 protein expression in activated astrocytes and microglia in the ischemia-reperfusion area of CI/R rats, decrease the neural function score of rats with ischemia-reperfusion injury,decrease apoptotic neurons in the ischemic penumbra of rats,and effectively inhibit the accumulation of activated microglia/macrophages around the infarct cortex, ultimately promoting nerve regeneration and the formation of neuronal networks(Park et al., 2003; Culman et al., 2012). The above studies suggest that the protective effect of PPARγ agonists against CI/R injury may be related to the JAK2/STAT3 signal transduction pathway in glial cells. In obese rat models, EA inhibited JAK2/STAT3 signal activation induced by PPARγ agonists,downregulated SOCS-3 and PPARγ expression, and increased leptin receptor and STAT3 levels, thereby reducing food intake and weight gain in rats. It is speculated that EA has a benign regulatory effect on SOCS-3 and PPAR-γ overexpression in obese rat models (Gao et al., 2013; Jing et al., 2016). The PPARγ-mediated receptor effect of acupuncture on the JAK2/STAT3 pathway after CI/R remains unknown. Further study is needed to determine how downstream proteins and gene expression in the JAK2/STAT3 pathway participate in pathological brain injury.
Notch
In mammals, the Notch receptor is a highly conserved membrane surface receptor that regulates neuronal cell development and includes intracellular, transmembrane,and extracellular segments. Notch receptors can be divided into four types, Notch 1–4. The Notch ligand of adjacent cells interacts with the receptor to activate the γ-secretase complex and cleave the Notch protein, and the intracellular domain of Notch (NICD) transports cellular signals to the nucleus. The transcription factor C-promoter binding factor-1/suppressor of hairless/LAG-1 family binds to form the NICD/RBP-JK/MAML transcriptional activation complex, regulating target gene expression, including adjacent neurons and glial cells (Bray, 2006; Alberi et al., 2013). The Notch 3 gene,which is closely related to stroke, was the first Notch gene discovered. Notch 3 mutations are involved in the occurrence of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy syndrome, and various Notch 3 mutations may be related to the severity of these diseases (Joutel et al., 1997; Arboleda-Velasquez et al., 2011;Papakonstantinou et al., 2019). Gradually, other members of the Notch family have also been found to be closely related to focal cerebral ischemia. Among them, Notch 1 is involved in the NF-κB-driven microglia-mediated inflammatory response.Use of a γ-secretase inhibitor and Notch1 antisense transgenic technology can inhibit cerebral ischemia and reduce the toxicity of microglia, and these two methods have a synergistic effect (Wei et al., 2011). Other researchers have found that the Notch signaling pathway causes neuronal death after CI/R through the pro-death protein Bim, calcium signaling, and the caspase pathway (Arumugam et al., 2011). Other studies have also found that Notch signaling promotes the activation of eNSCs, increases the number of new precursor cells after brain injury, and improves motor function (Androutsellis-Theotokis et al., 2006).
Activation of the Notch signaling pathway after CI/R induces differentiation of spinal cord eNSCs into astrocytes, amplifies the inflammatory cascade, inhibits eNSC proliferation,and impedes nerve repair. EA can inhibit expression of the Notch signaling pathway and pro-inflammatory cytokines,induce the proliferation and differentiation of eNSCs, inhibit differentiation of eNSCs into astrocytes, and promote the recovery of damaged nerves (Geng et al., 2015). Hippocampal dentate gyrus-derived eNSCs are also regulated by EA.However, in contrast to previous studies, EA promoted the proliferation and differentiation of eNSCs into functional neurons and glial cells in one study by up-regulating Notch 1 and Hairy and enhancer of split-1 (Hes1) expression levels to restore neurological function and improve cognition (Zhao et al., 2015a). Tao et al. (2014) found that EA promoted the proliferation and differentiation of hippocampal eNSCs by promoting the secretion of BDNF and glial cell derived neurotrophic factor through Notch signaling. The different results of this study may be related to the function of the injury site in the model. EA can bidirectionally regulate the positive role of the Notch signaling pathway in nerve injury.Zhao et al. (2015b) found that EA preconditioning significantly increased Notch 1, Notch 4, and Jag1 gene transcription in the striatum before ischemia. After ischemia/reperfusion,the Hes1, NICD, and hypoxia inducible factor-1α (HIF-1α)levels in the ischemic striatum of the EA preconditioning group were significantly elevated. Intraventricular injection of the γ-secretase inhibitor MW167 and HIF-1α antagonist 2ME2 reversed this neuroprotective effect, suggesting that EA pretreatment can induce tolerance to focal cerebral ischemia by activating the typical Notch pathway and the downstream HIF-1α pathway (Wu et al., 2015b; Zhao et al., 2015b). The effects of acupuncture on inflammatory responses in different signaling pathways are summarized in Table 4.
Conclusion and Considerations
The initiation of DAMPs after stroke triggers glial cell activation within a few minutes, activates multiple inflammatory signaling pathways, causes secretion of inflammatory mediators, and further induces circulating leukocytes to infiltrate through the endothelial cell barrier to the injured brain tissue, releasing additional inflammatory mediators and aggravating secondary damage after ischemia.In the 21stcentury, the number of studies on the application ofYin-Yangtheory in modern medicine has increased rapidly. For example, in Nature Immunology, theTai Chi Yin-Yangdiagram drawn on the cover clearly expresses the important role of regulatory thymus-dependent lymphocytes in maintaining the immune balance in the body, and it is said that the “Yin-Yangicon symbolizes the balance between two opposing forces in harmony”. These forces are prevalent in the immune system, including the balance between Treg and reactive cells, and are important in maintaining homeostasis (No authors listed, 2005; Mueller,2013). As “Su Wen” said, “Yin is peaceful, Yang is secret,and spirit is governance”. The theory ofYinandYangholds that the relative dynamic balance ofYinandYangmust be maintained between man and the external natural and social environment to maintain the normal physiological activities of the human body. The theory ofYinandYang,as a theoretical system of unified dynamic balance of the whole body, not only clarifies the origin and essence of life,physiological and pathological changes, and the fundamental law of disease diagnosis and treatment, but also resonates with modern medicine in expounding the law of dynamic change, analyzing the regulation of opposition restriction,and describing the harmonious balance of homeostasis in organisms. The combination of traditionalYin-Yangtheory and modern biological science may indicate that an important thought mode is changing from traditional simplification to integration (Gilca et al., 2013). Acupuncture is an important component of traditional Chinese medicine.Through the conduction of meridians and acupoints,acupuncture methods such as twirling and lifting are used to stimulate specific parts of the body. TheQiand blood are passed through the pulse, which is the relative balance of the humanYinandYang. This function tends to reconcile the balance to achieve the purpose of treating the disease.

Table 4|The effects of acupuncture on signaling pathways
“YinandYang” properties are present in the pathophysiological process of CI/R. Activated glial cells, infiltrating leukocytes,and activated inflammatory pathways not only magnify the inflammatory response of brain tissue, but also trigger anti-inflammatory, neuroprotective, and other pathological damage factors against CI/R to a certain extent, which promotes recovery after stroke. Based on the perspective of various inflammatory signaling pathways, we found that EA can interfere with inflammatory cells, inflammatory mediators,and inflammatory signaling pathways and effectively reduce the inflammatory cascade response after CI/R, promote neuroprotective factors, and inhibit nerve injury factors, thus reducing secondary inflammatory injury and promoting nerve function repair. This intervention mechanism affects multiple targets and pathways, resulting in multi-dimensional cross-talk(Figure 1). Therefore, acupuncture is an effective treatment strategy for ischemic stroke.

Figure 1|Inflammatory signal transmission related to acupuncture interventions after CI/R.
However, because of the variations of standardized operation of acupuncture and its repeatability, the diversity of parameters and acupoints, and individualized treatment characteristics, these studies are difficult to convert into clinical studies. Many types of acupuncture have been studied, including manual acupuncture, EA, and scalp acupuncture. Clinicians adopt a single or combined treatment according to the patient’s condition, specialty characteristics,and research direction. The difference among these three types of acupuncture mainly consists of the site and whether the stimulation is superimposed, but the difference in the clinical biological effect has not been studied. At present,most research is focused on animal experiments, and clinical research is relatively rare. Therefore, research on clinical effects should be strengthened, and well-designed, highquality clinical trials involving multiple centers with large samples and randomized controlled studies should be conducted. Further research and in-depth mechanism studies should be performed to clarify the exact mechanism of the effects of acupuncture on the inflammatory response after CI/R and provide reliable evidence for the clinical application of acupuncture for CI/R in the future. In addition, recent studies have found that multiple inflammatory signaling pathways are involved in focal cerebral infarction in remote locations,which is an important cause of ACI damage and nerve injury.Therefore, it is necessary to further study whether the effects acupuncture can be transmitted along the inflammatory conduction path after cerebral infarction and reduce damage at the distant part of a focal cerebral infarction. It is expected that acupuncture can be used to study the key molecular mechanism of distant damage and nerve repair and to provide new ideas and strategies for the treatment of ACI.
Author contributions:All authors wrote and critically reviewed the manuscript and approved the final manuscript.
Conflicts of interest:None declared.
Financial support:This work was supported by the National Natural Science Foundation of China, Nos. 81072947, 81473470, 81774423,the Natural Science Foundation of Guangdong Province of China, No.2014A030311033 (all to FT).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study
- Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy
- Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury:a prospective self-controlled study
- Recognition of moyamoya disease and its hemorrhagic risk using deep learning algorithms: sourced from retrospective studies
- D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure
- An integrative multivariate approach for predicting functional recovery using magnetic resonance imaging parameters in a translational pig ischemic stroke model