Axonal remodeling of the corticospinal tract during neurological recovery after stroke
Zhongwu Liu, Hongqi Xin, Michael Chopp,
Abstract Stroke remains the leading cause of long-term disability. Hemiparesis is one of the most common post-stroke motor deficits and is largely attributed to loss or disruption of the motor signals from the affected motor cortex. As the only direct descending motor pathway, the corticospinal tract (CST) is the primary pathway to innervate spinal motor neurons, and thus, forms the neuroanatomical basis to control the peripheral muscles for voluntary movements. Here, we review evidence from both experimental animals and stroke patients, regarding CST axonal damage, functional contribution of CST axonal integrity and remodeling to neurological recovery, and therapeutic approaches aimed to enhance CST axonal remodeling after stroke. The new insights gleaned from preclinical and clinical studies may encourage the development of more rational therapeutics with a strategy targeted to promote axonal rewiring for corticospinal innervation, which will significantly impact the current clinical needs of subacute and chronic stroke treatment.
Key Words: axonal degeneration; axonal integrity; axonal remodeling; corticospinal tract;motor performance; neurological recovery; stroke; therapeutic strategy
Introduction
In adults, stroke remains the leading cause of serious, longterm disability worldwide. In patients surviving stroke, 40%are left with moderate functional deficits and 15–30% have severe permanent disability. Long-term disability after stroke not only significantly affects quality of daily life, but also has serious emotional, social and economic consequences on stroke survivors and their families. Hence, it is extremely important to restore the functional performance poststroke. Unfortunately, although there is experimental and clinical evidence regarding spontaneous post-stroke recovery and neurological improvement resulting from rehabilitative approaches, improvement tends to be modest and the mechanisms of brain repair and functional recovery remain poorly understood. Consequently, there are urgent needs to develop more effective therapies to remodel the motor system and thereby promote neurological recovery after stroke.
For decades, the primary goal to develop therapeutic approaches for stroke has concentrated on neuroprotection,namely attempting to salvage the injured tissue and reduce the volume of cerebral infarction. Enormous effort has been expended to the development of neuroprotective agents in order to rescue neurons from irreversible ischemic injury;however, all these endeavors have failed to show efficacy in clinical trials of stroke (Schmidt-Pogoda et al., 2020). Therefore,a major paradigm shift in treatment of stroke is the focus on neurorestoration, which targets the intact or compromised tissue of the central nervous system (CNS), to promote neuronal remodeling to compensate for the damaged tissue,and thereby to enhance functional improvement following the acute phase after stroke.
Post-stroke hemiparesis results from the cortical motor signal loss or interruption to the spinal motor neurons. Thus,restoration of corticospinal innervation provides a neurological basis of motor control. The corticospinal tract (CST) consists of long axons of the pyramidal neurons originating from the sensorimotor cortex, to innervate the spinal motor neurons directly or indirectly. The CST is the primary transmission pathway of descending motor signals from the brain,to control the peripheral muscles for precise voluntary movements. Therefore, there is an evolving preclinical and clinical literature which investigates the functional contribution of the CST axonal integrity and remodeling in the brain and spinal cord to neurological recovery after stroke.We have performed a PubMed literature search of articles published in the period December 1969 – May 2020 on CST in stroke.
Corticospinal Tract Axonal Damage and Degeneration following Stroke
As described more than one and a half centuries ago as Wallerian degeneration in peripheral nerves, injury to the axon or the cell body of the neuron is followed by disintegration of the axon and its myelin sheath below the lesion. In the CNS,Daniel and Strich found that after spinal cord injury in baboon,myelin sheath recedes at the nodes of Ranvier from the axon,the axon then degenerates, and most axonal debris is entirely removed by 1 month (Daniel and Strich, 1969).
Middle cerebral artery occlusion (MCAo) is a widely employed model for ischemic stroke in rodents, and yields a large cortical and subcortical infarction in a unilateral hemisphere.Thus, this stroke model provides an opportunity to investigate axonal morphological change after stroke using anterograde and retrograde fiber tracers, such as DiI, WGA-conjugated HRP, and biotinylated dextran amine, or transgenic fluorescent labeling in the CST. At 1–2 weeks after MCAo, degenerating CST axons in the spinal dorsal funicular appear swollen with fragmented fibers labeled with intracortical injection of DiI in rats or in transgenic yellow fluorescent protein (YFP)mice (https://figshare.com/s/7c8db9a19520792534aa). CST axonal degeneration is also evident 1 week post-MCAo by immunohistological straining in rats (Dang et al., 2016).
In stroke patients, magnetic resonance imaging (MRI)techniques with prolonged T1 and T2 relaxation times were employed to detect the ischemic infarcts and the adjacent areas of Wallerian degeneration. In the CST, active and chronic Wallerian degeneration appeared as a well-defined band of hypointense signals on T2-weighted images at 4 weeks, and the signal became permanently hyperintense after 10–14 weeks following onset of clinical symptoms of stroke (Kuhn et al., 1989). Diffusion tensor imaging (DTI) portrays restricted diffusion of water moleculesin vivo, and provides images of neural tracts in the brain. Thus, DTI has been utilized to monitor the structural degeneration of CST axons in an area remote from the cerebral infarct, and thereby provides insight into the processes of structural evolution post-stroke(Werring et al., 2000). Using DTI and signal intensity changes on conventional MR imaging in the CST in patients with middle cerebral artery ischemic stroke, axonal degeneration can be revealed in the pons at 30 days, but not at 3 days(Puig et al., 2010). In another study of adult stroke patients,early Wallerian degeneration of the CST was detected with decreased apparent diffusion coefficient of the MRI signal in a time-dependent fashion, which was maximal at 7 days,and associated with poor motor performance (DeVetten et al., 2010). However, in these studies, post-stroke Wallerian degeneration was only detected in descending motor tracts at the level of the internal capsule and the brainstem. A later study investigated axonal degeneration in the lateral cervical spinal cord, showing that relative fractional anisotropy (FA)is reduced in the lateral tracts on the stroke-impaired side compared with the unaffected side in chronic ischemic stroke patients as compared with controls (Lindberg et al., 2011).
Contribution of Corticospinal Tract Axonal Integrity and Remodeling to Neurological Recovery after Stroke
Mechanisms influencing motor recovery in hemiparetic stroke are important; however, they are poorly understood in detail.Contingent upon the location or the degree of damage,patients may spontaneously recover from functional deficits with time, however, incompletely in most cases, and recovery can be improved by rehabilitative training (Rijntjes, 2006).Early recovery after stroke may be a result of dispersion of brain edema, absorption of infarct tissue, or restoration of the blood flow in the ischemic penumbra. Following the initial 14 days, functional improvement is likely attributable neuronal remodeling and substantial structural plasticity of the remaining tissue.
To directly monitor the CST axonal morphological change after stroke, MCAo was performed in transgenic CST-YFP mice. A significant correlation was evident between the behavioral outcome and the CST axonal density in the stroke-impaired side of the spinal gray matter (Liu et al., 2009). To directly verify the functional contribution of CST axonal remodeling to motor recovery after stroke, bilateral pyramidotomy was performed at the medulla level in mice subjected to MCAo to eliminate the CST axons in the spinal cord. The data showed that voluntary motor performance is dependent on CST integrity, and highly correlated to density of synapsis formed on CST axons in the stroke-impaired spinal gray matter during the later phase after stroke (Liu et al., 2013). To identify the cerebral cortical origin of these axons in the stroke-impaired side of the spinal cord, anterograde neural tracing studies performed in rodents subjected to MCAo demonstrated that CST axons originating from the contralesional hemisphere re-cross the midline in the spinal cord into the denervated side (Wiessner et al., 2003), while axons originating from the ipsilesional hindlimb area extend into the cervical cord, which originally terminate in the lumbar cord when the infarct is located in the forelimb cortical area (Starkey et al., 2012).
In stroke patients, the majority of neuroimaging studies were performed to demonstrate the relationship between CST integrity and functional outcome of arm/leg, due to the significance of hand use and walking for quality of daily life.Studies showed that preservation of the pyramidal tract is a major prognostic determinant of hand motor recovery following acute brain ischemia (Binkofski et al., 1996), as the extent of CST involvement is directly associated to the severity of functional deficits, and inversely related to the degree of neurological recovery (Konishi et al., 2005). In chronic stroke patients, the potential for recovery depends on CST functional integrity, which is assessed by motor evoked responses to transcranial magnetic stimulation (TMS) in the stroke-affected arm and asymmetry in FA of the internal capsules (Stinear et al., 2007). In addition, considering the different severity of stroke, the relationship between functional connectivity of the CST motor pathway and hand motor performance was compared in two groups of stroke patients with severe or mild impairement. The results demonstrated that upper limb functional recovery is mainly dependent on the degree of CST injury (Rosso et al., 2013). Importantly, the degree of CST lesion, but not the infarct size, highly contribute to functional motor deficit after a stroke (Zhu et al., 2010).Furthermore, poor motor outcome in stroke patients was associated with a decline of FA values and well-recovered patients had an FA index ratio of ipsilesional to contralesional side above 0.85 (Moller et al., 2007). High correlation was demonstrated between the degree of CST damage and motor functional deficits in ischemic stroke patients at each of acute(3–7 days), subacute (30 days), and chronic (90 days) phases(Stinear et al., 2007). Hence, the integrity of CST obtained in the early stage may serve as a good predictive parameter for the potential late long-term motor recovery in patients surviving from ischemic stroke (Feng et al., 2015; Buch et al., 2016; Guggisberg et al., 2017; Lin et al., 2019), as well as intracerebral hemorrhagic stroke (Venkatasubramanian et al.,2013). Similarly, DTI and TMS techniques also demonstrate a strong correlation of CST damage to knee extensor weakness and activation (Madhavan et al., 2011), as well as walking impairment (Jayaram et al., 2012) in chronic stroke survivors,while the CST integrity can predict walking recovery in subacute stroke patients (Soulard et al., 2020).
Since cell membranes and organelles in the gray matter of the CNS restrict water diffusion, TDI produces a non-Gaussian diffusion in the spinal cord. Therefore, another MR technique,diffusion kurtosis imaging, which assesses the non-Gaussian distribution of the diffusion constants (Jensen et al., 2005),was employed to investigate the CST integrity in the cervical spinal cord in chronic stroke survivors, and demonstrated that the reduction of FA values in C3–4 and C5–6 in the strokeaffected lateral CST bundles is significantly correlated with the degree of upper motor impairment (Panara et al., 2019),indicating an advantage for direct measurement of CST axonal integrity in the spinal cord, which may more precisely predict motor recovery after stroke.
Therapeutic Approaches Aimed to Enhance Corticospinal Tract Axonal Remodeling and thereby Promote Stroke Recovery
Axonal regeneration is highly inhibited in the CNS following stroke and other neural injury in the adult mammal, which severely limits functional recovery. The underlying mechanisms for the failure of axonal regeneration in the white matter, may,in-part, be attributed to the imbalance of facilitative/inhibitory factors, namely insufficient neurotrophins, and/or presence and production of growth-inhibitory molecules (Yiu and He,2006), including Nogo, myelin-associated glycoprotein and oligodendrocyte-myelin glycoprotein (Xie and Zheng, 2008),leading to deficient axonal outgrowth, and ineffective rewiring of the denervated tissue to restore the lost functions. Thus,development of axonal remodeling targeted therapies is based on strategies to increase the capacity of axonal outgrowth,and/or neutralize the inhibitory molecules.
Since the most commonly used rodent ischemic stroke model,MCAo, produces a large cortical and subcortical infarction in a unilateral hemisphere, many preclinical studies focused on collateral sprouting of the uninjured CST axons originating from the contralesional cerebral hemisphere. Strategies employed to promote axonal outgrowth include inosine infusion into the cisterna magna (Chen et al., 2002) or into the contralesional lateral ventricle (Zai et al., 2009); bumetanide infusion into the contralesional lateral ventricle (Mu et al.,2017); intravenous transplantation of bone marrow stromal cells (Liu et al., 2008); subcutaneous injection of amphetamine sulfate (Papadopoulos et al., 2009); intranasal delivery of tPA (Chen et al., 2018; Pu et al., 2019); and intramuscular injection of adeno-associated viral vector (AAV)-1 encoding neurotrophin-3 (Duricki et al., 2016) or neurotrophin-3 protein(Duricki et al., 2019) into the affected triceps brachii muscles.Approaches employed to reduce axonal outgrowth inhibitory factors include monoclonal anti-Nogo-A antibody 7B12 infusion into the contralesional side of the lateral ventricle(Wiessner et al., 2003), 11C7 intracerebroventricular infusion(Tsai et al., 2011), or 11C7 into the subarachnoid space of the lumbar cord (Wahl et al., 2014); Nogo receptor antagonist NgR(310)Ecto-Fc infusion into the contralesional lateral ventricle (Lee et al., 2004), silencing Nogo receptor expression by adenovirus-mediated RNA interference (Wang et al.,2010), and digestion of chondroitin sulphate proteoglycans with chondroitinase ABC injected into the cervical cord(Soleman et al., 2012). In addition, therapeutic effects physiotherapeutic approaches were also examined in rodents,such as rehabilitative training (Okabe et al., 2016; Wiersma et al., 2017); constraint-induced movement therapy (Liu et al., 2019; Okabe et al., 2019); and optogenetic stimulation to the contralesional cortex (Wahl et al., 2017). In concert, all the approaches listed above demonstrated that CST axons originating from the contralesional cortex extend and re-cross the midline into the stroke-impaired side of the spinal gray matter to rewire the denervated spinal neurons, and as such,axonal plasticity is enhanced by these therapeutic approaches.
Since the contribution of neuronal activity in the contralesional cerebral hemisphere to stroke recovery is controversial (Dodd et al., 2017), the restorative role of the contralesional CST remains under debate (Alawieh et al.,2017). Using a trans-synaptic neural tracer, pseudorabies virus encoding monomeric red fluorescent protein, to retrogradely trace the neural pathways from the stroke-affected forelimb muscles in CST-YFP mice, showed that neuronal reorganization occurs in both ipsilesional and contralesional cortices, and it is much higher in the ipsilesional hemisphere (Liu et al., 2009;Chen et al., 2018), suggesting that motor recovery after stroke may be in large part be attributed to ipsilesional CST axonal remodeling. Recent evidence also supports that neurological recovery is mainly dependent on ipsilesional CST axonal integrity in stroke patients (Okabe et al., 2018; Yarossi et al.,2019) and in experimental animals (Hu et al., 2019). Among such data are, administration of transcranial direct current stimulation over bilateral motor cortices in chronic patients with unilateral hemispheric stroke significantly improved functional recovery of the affected arm, and increased motor fibers in the ipsilesional, but not in the contralesional CST, as determined by FA values using DTI (Zheng and Schlaug, 2015),as well as reduced ipsilesional CST degeneration in subacute stroke patients (Nicolo et al., 2018). Similarly, patients treated with high frequency repetitive TMS showed significant improvement of functional recovery, which correlated with increased ipsilesional FA value (Guo et al., 2016). Additionally,in combination with functional electrical stimulation, braincomputer interfaces increased functional connectivity between motor areas in the stroke-affected hemisphere(Biasiucci et al., 2018), which further indicates the importance of ipsilesional neuronal plasticity during functional recovery after stroke.
In contrast to the white matter, the gray matter of spinal cord is permissive for neurite elongation (Savio and Schwab,1989). A previous study demonstrated that neither MCAo nor a neurorestorative treatment using bone marrow stromal cells affect the number of CST axons originating from the contralesional hemisphere at the upper cervical level, indicating that denervated spinal motor neurons may be rewired with CST axons that survived the injury, which sprout and elongate within the spinal gray matter to form new synaptic connections and thereby restore innervation from the motor cortex (Liu et al., 2008). Thus, strategy of targeting the axonal remodeling in the stroke-affected side of spinal gray matter to enhance the neuronal rewiring between the motor cortex and spinal cord, which can circumvent the restriction of axonal regeneration in the injured white matter, could be of major interest in the development of new therapeutic strategies for stroke. Of treatments noted above either aimed to increase the capability of neuronal outgrowth,or reduce the inhibitory factors for axonal outgrowth, none of them can specifically target the CST axons in the strokeimpaired spinal gray matter to restore the cortical innervation on the denervated spinal motor neurons, the final common pathway of motor control. AAV vectors are broadly used for gene therapy in both preclinical and clinical studies (Li and Samulski, 2020). Recently, a novel strategy aimed to facilitate axonal remodeling in the denervated spinal gray matter was examined in CST-YFP mice subjected to MCAo (Gan et al.,2020). Recombinant AAV-5 encoding tPA gene was injected into muscles of the stroke-affected forelimb. To avoid tPA expression in the injected muscles, tPA expression was controlled by a neuron specific Synapsin promoter. Thus, after injection into the stroke-affected muscles, the AAV vectors were retrogradely transported along the axons innervating these muscles back to the spinal motor neurons. Here, tPA overexpressed in the denervated spinal motor neurons was used as a neurorestorative agent to promote neurite remodeling and synaptic plasticity but not for thrombolysis.The data showed that intramuscular AAV-tPA administration increased CST axonal density in the stroke-impaired side of the spinal gray matter as measured by the transgenic YFP labeling in the CST axons, as well as enhanced synaptic corticospinal innervation as confirmed by trans-synaptic pseudorabies virus tracing to the bilateral motor cortices, which were significantly correlated with behavioral outcomes of the stroke-impaired forelimb (Gan et al., 2020). The data and insight from this study may encourage development of strategies for stroke treatment, which not only focus on the injured brain, but also target the remote tissues, particularly the motor tracts in the spinal cord.
This review primarily focusses on remodeling of the CST.However, other descending neural pathways in the spinal cord may also be involved in axonal plasticity and functional recovery after stroke. The red nucleus, for example, is a prominent structure in the motor system of mammals.Corticorubral tract axons originate from the ipsilateral primary and supplementary motor cortex, and terminate on both parvocellular and magnocellular divisions of the RN. RN neurons in the magnocellular division form the rubrospinal tract, a mainly crossed pathway, and participate in the coordination of movements across joints, such as skilled forelimb movements, locomotion and motor responses to pain. Since the corticospinal and rubrospinal axons possess very similar branching patterns in the spinal cord, the corticorubro-spinal pathway appears to be a backup to the CST which may enhance the behavioral recovery after CST lesion.In rodents, ipsilateral corticorubral projects were increased after stroke, and such axonal plasticity was further enhanced by treatment of monoclonal anti-Nogo-A antibody IN-1 injected into the ipsilesional side of the cerebral parenchyma(Papadopoulos et al., 2002). In addition, the cortico-reticulospinal tract, consisting of the corticoreticular pathway and the reticulospinal tract, is known to be an important neural tract for walking ability. Compensation of the corticoreticular pathway is related to walking ability in patients with chronic stroke (Jang et al., 2013). Thus, axonal plasticity in other descending pathways may be synchronous with CST axons,and the therapeutic effect may be amplified by enhancing axonal remodeling in such pathways.
Rehabilitative approaches, including constraint-induced movement therapy (Barzel et al., 2015; Dos Anjos et al.,2020), and electrical stimulation (Levy et al., 2016; Page et al., 2020), are broadly used in clinical practice to promote stroke recovery, however, efficacious therapies to enhance neurorestorative processes, particularly plasticity of the corticospinal innervation are still underdeveloped. The strategy of increasing the corticospinal neuronal rewiring to promote functional recovery after stroke provides a neuroanatomical basis for neurological recovery. Therapeutic approaches and agents could be developed under this strategy via promoting neuronal capacity for axonal outgrowth, either in the ipsilesional or the contralesional cortex; reducing inhibitory factors of regeneration in the neural tracts; and enhancing axonal connectivity specifically in the denervated spinal gray matter (Figure 1). Such efficacious therapies would greatly impact a large population of stroke patients with a significant unmet medical need of successful stroke treatment beyond the acute stage.
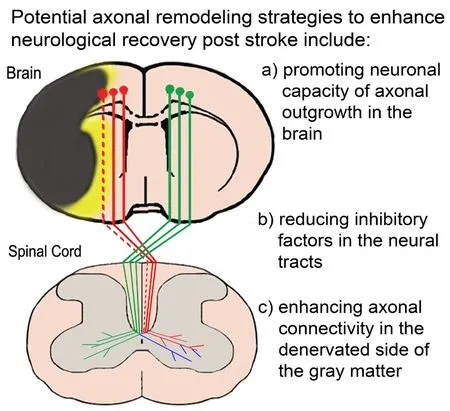
Figure 1 | A diagram showing corticospinal tract (CST) axonal degeneration and remodeling after stroke.
Author contributions:Manuscript conception, design, and revising: ZL,HX and MC; manuscript writing: ZL. All authors read and approved the final version of this manuscript.
Conflicts of interest:None declared.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study
- Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy
- Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury:a prospective self-controlled study
- Recognition of moyamoya disease and its hemorrhagic risk using deep learning algorithms: sourced from retrospective studies
- D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure
- An integrative multivariate approach for predicting functional recovery using magnetic resonance imaging parameters in a translational pig ischemic stroke model