Secondary release of the peripheral nerve with autologous fat derivates benefits for functional and sensory recovery
Natalia E. Krzesniak , Anna Sarnowska, Anna Figiel-Dabrowska, Katarzyna OsiakKrystyna Domanska-Janik, Bartłomiej H. Noszczyk
Abstract The reconstruction of nerve continuity after traumatic nerve injury is the gold standard in hand surgery. Immediate, tension-free, end-to-end nerve suture ensures the best prognosis. The recovery is mostly promising; however, in a few cases, insufficient outcomes in motor or sensory function are observed. Intra- and extra-fascicular scarring accompanies the nerve regeneration process and limits final outcomes. Secondary nerve release in those cases is recommended. Unfortunately, scarring recurrence cannot be eliminated after secondary revision and neurolysis. The supportive influences of mesenchymal stem cells in the process of nerve regeneration were observed in many preclinical studies. However, a limited number of studies in humans have analyzed the clinical usage of mesenchymal stem cells in peripheral nerve reconstruction and revisions. The objective of this study was to evaluate the effects of undifferentiated adipose-derived stromal/stem cell injection during a last-chance surgery (neurolysis, nerve release) on a previously reconstructed nerve.Three patients (one female, two males; mean age 59 ± 4.5 years at the time of injury),who experienced failure of reconstructions of median and ulnar nerves, were included in this study. During the revision surgery, nerve fascicles were released, and adiposederived stromal/stem cells were administered through microinjections along the fascicles and around the adjacent tissues after external neurolysis. During 36 months of followup, patients noticed gradual signs of sensory and in consequence functional recovery.No adverse effects were observed. Simultaneous nerve release with adipose-derived stromal/stem cells support is a promising method in patients who need secondary nerve release after nerve reconstruction. This method can constitute an alternative procedure in patients experiencing recovery failure and allow improvement in cases of limited nerve regeneration. The study protocol was approved by the Institutional Review Board (IRB) at the Centre of Postgraduate Medical Education (No. 62/PB/2016) on September 14, 2016.
Key Words: nerve release; neurolysis; stem cells; adipose-derived stem cells; scar;threshold; sensation; pain; hypersensitivity; forearm
Introduction
The reconstruction of nerve continuity after traumatic nerve injury is the gold standard in hand surgery. However, in very few cases, when the initial conditions of reconstruction are limited, i.e., the surgery was delayed, the age of the patient was advanced (older than 50 years), a tension-free suture was impossible or the extensive scarring process disturbed the postoperative course, the observed results are far from that which are expected. Patients complain for persistent numbness of hand, sensation deterioration, severe limitation of function due to pain and hypersensitivity (Onne, 1962;Rosen, 1996; Rosen et al., 2000) (Table 1). In those patients,only partial recovery, or even no signs of sensory restoration,and poor functional recovery can be suspected.
Nerve regeneration is an age-dependent process, and older patients usually obtain worse results than younger patients(Fornander et al., 2010; Mafi et al., 2012). This fact was reported by many researchers and is probably caused by the age-dependent reduction in repair and regenerative processes as well as the time-dependent decrease in number of active axons (Abbas et al., 2016; Hembd et al., 2017; Roh et al.,2019).
Approximately a year after surgery, if poor recovery is observed in both clinical examination and with electromyography stimulation, secondary exploration can be considered. The decision to perform secondary revision is determined carefully after clinical examination as well as a pros-and-cons assessment (Green et al., 2005). The aim of re-exploration is the release of the reconstructed nerve from immobilizing scar tissue (historically called neurolysis). Healing via the formation of a scar is a natural mechanism in adult mammalians, and its intensification differs individually. Scar formation proceeds along with axon growth and regeneration(Sunderland, 1991; Elliot, 2014). Nerve repair is accompanied by a fibrotic response, which may include the outermost layer of the nerve called the paraneurium as well as the inner nerve sheath: the epineurium, perineurium or even nerve fascicles may be involved in the formation of scar tissue(Millesi et al., 1993; Mazal and Millesi, 2005). Chronic scarring induces ischemic stress conditions, axon degeneration, and finally limited nerve functions. Pain and function disorders are the main symptoms of scarring neuritis, also called scar neuropathy (Tos et al., 2015).

Table 1 | Patients’ demographics, history of injury, and clinical evaluation before and after nerve release and ADSC administration
The abundant scar can imprison regenerating axons, acting similar to a tight clamp that markedly limits or even stops the nerve conduction. The clinical examination shows that after a period of gradual improvement following surgical reconstruction, the signs of regeneration slow down or even stop. In the reconstructed area, the arrested and immobilized Tinel’s sign can be observed. During the revision surgery, traction neuropathy (fibrosis around the nerve) and neuroma-in-continuity (tuberous distensions of the previously reconstructed nerve) are found (Tos et al., 2015). These patients are considered for nerve release (neurolysis) during re-exploration. However, a recurrent course that is scar free after the secondary surgery and nerve release cannot be guaranteed.
Due to the lack of an effective method to protect the reconstructed nerve against the recurrence of extra- and intra-neural scarring, we proposed the usage of adiposederived stem cells (ADSCs) during the secondary nerve release(neurolysis). The anti-scarring potential of ADSCs alone or fat graft containing ADSCs was observed in a few models.Experimental models:in vitroandin vivostudies in BALB/c mice (Li et al., 2016), fibrin nerve conduits seeded with ADSCs in sciatic nerve reconstruction in rats (Di Summa et al., 2018), perineural scar modification in sciatic nerve in rats after fat graft (Dumanian et al., 1999), inhibition of perineural adherence by human ADSCs in athymic mice (Cherubino et al., 2017), and clinical reports as: painful end-neuromas of the upper limb in humans (Vaienti et al., 2013), saphenous nerve entrapment neuropathy caused by scar tissue (Ulloa and Banda, 2017) and scar prevention in secondary carpal tunnel(Krzesniak et al., 2013) have been already published. The objective of this study was to observe the long-term followup results in three consecutive patients (after unsuccessful median and ulnar nerve repair in past) who were treated by nerve release with ADSC implantation. In addition to the promoting effects of ADSCs (delivery of growth factors) on nerve regeneration, the inhibition of neural scar recurrence can be hypothesized.
Subjects and Methods
Subjects
Three patients after sharp nerve injuries (knife, window glass)with unsatisfactory median and ulnar nerve reconstruction at the level of middle forearm were included in this 36-month observational study (Table 1). In majority of cases (except median nerve in patient No. 3), patients had delayed nerve reconstruction and they reported no sensory function and impaired casual functionality. Patients were evaluated before and after the treatment by clinical examination,electromyography (EMG), the DASH (Disabilities of the Arm, Shoulder and Hand survey (Hudak et al., 1996) for casual duties and the Visual Analogue Scale (VAS) for pain(Badalamente et al., 2013). Chanson et al. (1977) performed sensory, motor, pain, and functional evaluation using the British Medical Research Council classification system in outpatient controls (Table 2). Every patient completed the diagram indicating a percentage of sensation in the affected hand compared to that in the opposite healthy hand after treatment (Figure 1). Thresholds of sensation were recorded during EMG control sessions. This study was approved by the Institutional Review Board (IRB) at the Centre of Postgraduate Medical Education (No. 62/PB/2016) on September 14, 2016 and patients were included after providing informed consent.
Pre-operative examination
Three patients that had undergone failed nerve reconstruction(two cases of secondary sutures of the median nerve, one case of primary suture of median nerve and nerve graft to the ulnar nerve) were included in the study (Table 1). Two patients (No. 1 and No. 2) suffered from severe pain and cold intolerance and described the impression of having a strange or alien hand. No. 3 patient with median and ulnar nerve injury complained mostly of the lack of sensation.The motor function in hands of No. 1 and No. 2 patients was preserved but obviously limited by pain (F1). Patients No. 1 and No. 2 complained of constant pain during both relaxation and manual function, which markedly influenced their casual activities. They also suffered from cold intolerance (sympathetic reinnervation disorders) and tactile hypersensitivity. Patient No. 3 was free of pain but complained of severe sensation loss in the entire hand. All patients could perform their casual manual activities but complained of difficulties with function and precise manipulation.
One-step isolation of ADSCs and surgical treatment
All patients underwent a surgical revision with nerve release and scar removal. Intraoperatively, adipose tissue was harvested from the lower abdomen, and autologous ADSCs were isolated in a one-step procedure. After standard infiltration of the lower abdomen with Klein solution (lidocaine and epinephrine in Ringer’s solution), fat was harvested by syringe liposuction with a cannulas using low pressure. ADSC isolation was conducted according to the manufacturer’s instructions for collagenase VI (Collagenase NB6 Grade Cat#17458, Serva Bio-Techne, Warsaw, Poland). First, the tissue was transferred from the syringe to 50-mL sterile tubes. Thenthe mixture of collagenase VI with collagenase activity of 0.2 PZ U/mL in PBS was prepared. Subsequently, the adiposetissue with the enzymes was incubated at 37°C in an orbital shaker with a rocking speed of approximately 60 r/min for 30–60 minutes (the time of incubation depends on the breakdown of the extracellular matrix; approximately 45 minutes). The next step was separation of the stromal vascular fraction by centrifugation of the enzyme-treated tissue for 20 minutes at 200 ×g. Then the fraction of supernatant was removed.The pellets were washed twice with PBS supplemented with 1% antibiotics (Penicillin-Streptomycin, Cat# 15070-063, Gibco, Thermo Fisher Scientific, Warsaw, Poland) and centrifuged at 200 ×gfor 2 minutes. From each isolation, 1 mL of lipoaspirate was sent to the cell culture laboratory for further cell analysis (e.g., the number of isolated cells/mL, cell viability, marker expression or cell phenotype; Figure 2) (Lech et al., 2016; Moniuszko et al., 2018; Kuzma-Kozakiewicz et al.,2018).

Table 2 |British Medical Research Council classification for sensory,motor, pain and function
At the same time, the treated nerve was identified and released. Scar tissue was removed, and nerve fibers were exposed. The prepared solutions of ADSCs (9.6 × 105cells/300 μL or 3.2 × 106/mL) were administered via microinjection(volume 300 μL) with a 30 G needle along the released nerve fascicles, above and below the reconstructed area and around the adjacent tissue, which stayed in contact with the nerves.The wounds were closed, and no splint was used. Patients were followed up during the postoperative period of 36 months. All patients routinely visited the outpatient clinic and completed physiotherapy. Signs of sensory, motor and functional recovery were evaluated by clinical examination and electromyography. The end point of the study was 36 months of follow-up in all cases.
Patient No. 1
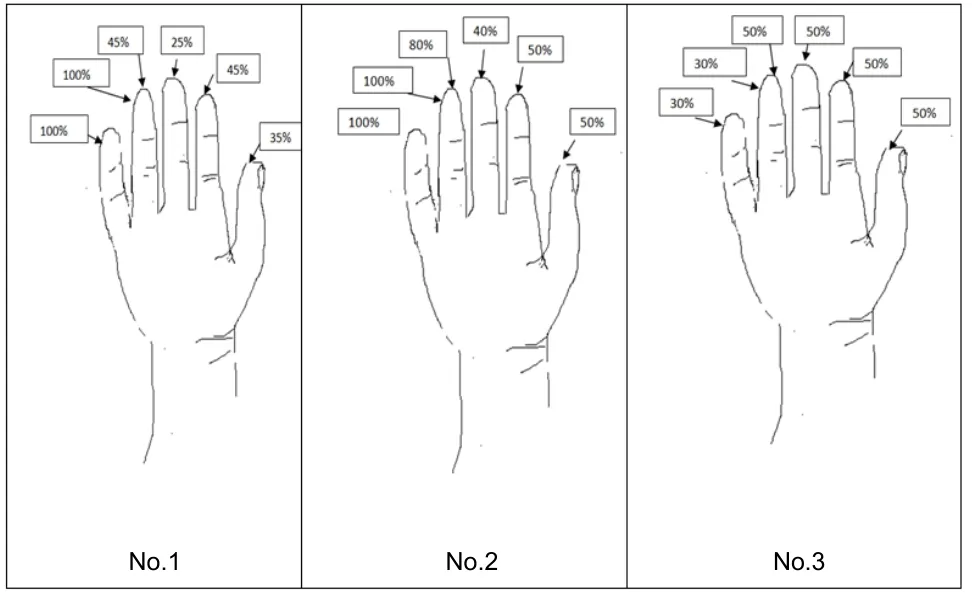
Figure 1| Diagram of sensation in pulp area after nerve release and intraoperative
Patient No. 1 was a 55-year-old male who suffered from a median nerve injury by a knife at the level of the middle forearm. Reconstruction of the superficial flexor tendons and median nerve was performed one year later with a failed nerve recovery result (Table 1). The patient experienced a pain at rest in the hand (VAS 9–10) and entire loss of sensation (S0 almost completely insensate) in fingers I–IV,severe cold sensitivity, and deeply impaired hand function(F1 pain and limited function in British Medical Research Council classification modified by Chanson et al., 1977) (Table 2). Three years later, surgical revision, nerve release and decompression, and scar removal with ADSC administration around the nerve were performed. The patient reported no evident signs of recovery during the first 7 months. After 7 months, he noticed a gradual return of sensation in the region of the midpalm (metacarpal area) and then in all fingers. Thirty-six months after revision, recovery of 30–45%sensation in fingers I–IV was achieved (compared with that of the healthy hand) (Figure 1), and no cold sensitivity or no pain at rest was experienced; the patient rarely experienced pain after extremely hard work (frequency; once a month, severity:slight, 1 point in VAS grading scale). The patient declared that motor function of the treated hand markedly improved. The DASH Disability/Symptom Score decreased from 74% to 26%(from 119 points to 62 points, from extreme difficulties to moderate problems), with a 48% reduction in problems in casual activity. Two-point discrimination (2PD) improved from S0 to S4 (6 mm). The patient observed a gradual improvement in function during the 2.5 years after surgical treatment (From F1 to F5) and after that time he no longer noted any further changes (Table 3).
Patient No. 2
A 64-year-old female had extensive injury of the middle forearm 4 years before treatment. Median nerve reconstruction was performed a half year after the injury.Due to an unsatisfactory result, she underwent a neurolysis 1.5 years later with no improvement. She was included in the group due to complete sensation loss in fingers I–IV (S0),severe permanent pain in the hand at rest, and severe cold sensitivity. Immediately after revision with neurolysis (nerve decompression, massive scar removal, ADSC administration),she experienced recovery of sensation in one finger (IV on the radial side), and during the subsequent 36 months, she noticed gradual recovery of sensitivity in all fingers I–IV: she obtained approximately 50% sensation compared to that in the healthy hand. The DASH Disability Symptom Score decreased from 60%to 32% (from 99 points to 67 points). Signs of gradual recovery were observed for 2 years. She also showed improvement in mobility of the hand due to scar release and global manual function enhancement (from F1 to F5).
Patient No. 3
A 58-year-old male experienced severe trauma of the middle forearm with median and ulnar nerve injuries and underwent immediate reconstruction of all tendons and the median nerve. The reconstruction of the ulnar nerve with a nerve graftwas delayed for 1 year. Three years after injury, the patient demonstrated complete sensation loss in fingers I–V (S0). In contrast to other patients, no signs of cold intolerance or pain in the hand were recorded. After both nerves were released with ADSC administration, the patient recovered 50% of the sensation in the median nerve dermatomes and 30% of thesensation in the ulnar nerve dermatomes. However, the DASH Disability/Symptom Score increased from 61 to 87 points (30%to 47.5%). The patient confirmed that he did not put enough effort into physiotherapy and physical exercise after surgical revision compared to that during the primary treatment. He complained that he had expected better results. However,the recovery of sensation of 50% for the median nerve dermatomes and 30% for the ulnar nerve dermatomes compared to that of the healthy side were obtained.

Table 3 |Summary of results before and after intraoperative adipose-derived stem cell administration and nerve release
Summary
Two of the three patients noticed improvement in both functionality and sensation in the hand. Both of them indicated that surgery was beneficial. Patients No. 1 and No.2 who previously suffered from severe pain, finally became painless at rest and during casual duties. Both of them confirmed that their manual efficiency markedly improved;however, a few small problems occurred rarely when the hand was overworked. Additionally, hypersensitivity of the hand and cold intolerance disappeared. They reported improved tolerance of lower temperatures without the constant need for gloves usage. All patients reported that they no longer experienced accidental burns, about which they had previously complained. Patient No. 3, whose symptoms were milder in the beginning (no pain and cold intolerance), obtained less obvious effects. Despite the fact that his sensation improved to 50% for the median nerve dermatomes and 30% for the ulnar nerve dermatomes, he did not experience functional improvement.
Interestingly, in all, the observed recovery in sensation was gradual. Patient No. 1 noticed significant sensation improvement in the middle palm not earlier than 7 months after revision, and from that time, sensation started to recover gradually in all the affected fingers. Patient No. 2 noticed improvement in one finger (IV, on the radial side)immediately after surgery, which could be a direct result of neurolysis. However, in fingers I–III, the recovery was gradual and proceeded slowly over the following 2 years. Sensation recovery in patient No. 3 proceeded for approximately 2 years and discontinued after that time.
It is worth noting that in all electromyography studies, very good recovery in motor fascicles was observed early after the treatment. All latencies and indexes of conduction velocities(CVI) decreased in time, while conduction velocity (CV) of the nerves increased slowly during every single control study(Table 4). The motor branch of the median nerve (anterior interosseus nerve, AIN) was intact in all patients; however, the motor fibers of the median nerve below the middle forearm serviced the thenar muscles via the recurrent branch. The recovery of motor function of the ulnar nerve, which contains a large volume of motor fascicles to the intrinsic and lumbrical muscles, is much more unpredictable; however, successfulresults were observed in the motor function of the ulnar nerve in the electromyography control. Table 4 presents changes in electromyography after revision.

Figure 2|ADSC phenotype.
In contrast to the early improvement in the motor fascicles observed in electromyography, we did not clearly demonstrate sensory recovery in the EMG during the first free years.However, patients were satisfied with the evident subjective sensation recovery. For a better understanding of the recovery process, we used repeatable examination of both the sensory threshold in EMG (Table 5) and subjective graphic evaluation of sensation in all patients (Figure 1). Starting from very high pathologic values of the sensory threshold, all values were significantly reduced during 3 years of follow-up, and in patients No. 1 and No. 3, the values approached normal ranges of sensation. However, only in the control study of patient No. 2 (with still an uncorrected threshold level) was the recovery of sensory nerve conduction (SNC) in the EMG evaluation observed. SNC returned no sooner than after 36 months of follow-up.
At the same time, patients were asked to compare the percent of sensation in the treated hand with that in the opposite healthy hand using a graphic evaluation. Before the treatment,all patients declared 0–5% of sensation on the fingertips,while 30–50% improvement was observed after neurolysis with stem cell administration (Figure 1). These results were appreciated by the patients, and along with sensation improvement, manual dexterity of the hand also increased(DASH survey, British Medical Research Council classification –No. 1, 2).
Discussion
The most significant improvement in nerve conduction occurs during the first year after nerve repair (Chassard et al., 1993;Rosen, 1996; Rosen et al., 2000). In patients who experience failure of nerve regeneration, traction neuropathy (fibrosis around the nerve) or neuroma-in-continuity can be suspected(Elliot, 2014; Tos et al., 2015). The decision to pursue additional treatment in individuals with poor outcomes is prudently considered. Secondary revision encompasses external neurolysis, which separates the paraneurium and epineurium from the surrounding tissue, and internal neurolysis, which separates the perineurium and nerve fascicles from intraneural scar tissue (Millesi et al., 1993; Mazal and Millesi, 2005). When extensive paraneural, epineural, and perineural scar formation appears after nerve reconstruction,growing axon cones of the reconstructed nerve are inhibited or even stopped. A limited number of axons can finally reach their distal destination, and the functional regeneration is indeed affected. Even when proper axonal growth through the injury site is present, gradual progressive clamping (similar to compressive neuropathy) can be observed.
There are defined limiting factors, which undoubtedly worsen the conditions of nerve reconstruction. The age of patients is an important prognostic factor for general regenerative ability(Fornander et al., 2010; Mafi et al., 2012). Complete sensory recovery (however, no higher than 80%) can be achievedonly in patients younger than 20 years of age (Lungeborg 2001; Wiberg et al., 2003; Chemnitz et al., 2013). In older individuals, the potential of recovery decreases in a manner inversely proportional to age. In patients older than 40 years of age, proper 2PD on the finger pulp, defined as equal or less than 6 mm, will not be achieved in most cases after digital nerve reconstruction (Chassard et al., 1993; Jerosch-Herold,2000).

Table 4 |Changes in electromyography before and after nerve release and adipose-derived stem cell administration
Few concepts partly explain the age-dependent deterioration of nerve repair. First, the number of axons in the nerve fascicles decreases with age, and results of functional reconstruction in the elderly are less satisfying (Ruijs et al.,2005; Paprottka et al., 2013; Bulut et al., 2016; Hembd et al., 2017; Midha and Grochmal, 2019). Additionally, the regenerative abilities of the central and peripheral nervous systems gradually decline with age (Fornander et al., 2010;Geoffroy et al., 2017). It is also known from animal studies that overactivated inflammatory markers are present at the injury site in older subjects. The hyperinflammatory state is accompanied by increased macrophage infiltration, and increased levels of monocyte chemoattractant protein 1(MCP1), and CC chemokine ligand 11 (CCL11) were observed in intact nerves. The presence of chronic inflammation worsens the conditions of recovery after injury (Büttner et al.,2018).
The possible mechanism of Schwann cell senescence was also debated. With age, Schwann cells lose their capacity to dedifferentiate and differentiate after nerve repair.As a consequence, some of these cells remain in the undifferentiated state. Schwann cells experience a reduced ability to clear axon and myelin debris in aging mammalians,and finally, they do not produce a sufficient amount of myelin during the post-injury period, thus impeding regeneration(Shen et al., 2008; Painter et al., 2014; Wei et al., 2019).Moreover, with a time of denervation, Schwann cells lose theircapacity to support nerve regeneration (Wilcox et al., 2020).Additionally, age-related neuromuscular junction instability results in more extensive loss of the motor endplates in the distal target region (Apel et al., 2009).

Table 5 |Electromyography evaluation of sensory threshold
All patients in our studied group initially revealed poor conditions before the first reconstruction. All of them were advanced in age (older than 50 years of age) at the time of injury. They waited for nerve reconstruction beyond 6 months(except for the median nerve in patient No. 3), and in all cases,delayed nerve repair was unsuccessful (hand sensation at level S0, 0–5% compared to the opposite hand). The level of injury in all cases was the middle forearm. All of these reasons worsened the final outcome of reconstruction and forced the patient to search for additional help. Patients complained of a lack of sensation, and two of them (except patient No.3) complained of constant pain in the hand and severe cold intolerance. The threshold of sensation remained at the level of significant pathology.
In patients with failure of nerve regeneration, the dilemma of operative treatment is an issue: should the revision involve nerve release (neurolysis) or secondary reconstruction (with cable nerve grafts)? Keeping in mind that the initial conditions(e.g., age, level of injury or time since the accident) may not improve, the expected results could be similar or even worse(cable grafts could cause more extensive axon loss) (Tseng,2015), and additional time for recovery would be necessary.For these reasons, nerve release (neurolysis) is recommended in most cases.
The standard external neurolysis procedure, defined as paraneuriotomy or epineuriotomy (Millesi et al., 1993), was previously proposed as the recommended procedure and may be beneficial (opposite to the internal neurolysis procedure)in selected cases (Mazal and Millesi, 2005). However, there is still no possibility to prevent scarring recurrence (Atkins et al.,2006; Ngeow, 2010; Kokkalis et al., 2016; Lemke et al., 2017;Wang et al., 2019). For this reason, in patients referred for the neurolysis procedure, the additional usage of autologous ADSC was considered. We expected that the regenerative properties of the ADSC would allow for favorable nerve remodeling, stimulate repair, and inhibit abundant scarring.
Mesenchymal stem cells demonstrated their regenerative abilities in numerous preclinical studies concerning nerve injuries. The growth factor delivery (adjuvant properties)remains the most fundamental ADSC activity. Except for the potential to differentiate toward the mesodermal lineage(Zuk et al., 2001; Strem et al., 2005), ADSCs were reported to possess the ability to differentiate into Schwann cell-like phenotype cells, which enhanced neurite outgrowthin vitro(Kingham et al., 2007). Di Summa et al. (2010) suggested that adipose-derived mesenchymal cells placed in artificial conduits are as effective as bone marrow cells during the process of sciatic nerve regeneration in the rat model. Additionally, the stromal vascular fraction, which was used to fill vein grafts,created a more permissive environment for nerve growth than empty grafts (Özkan et al., 2016). Braga-Silva et al. (2008)investigated silicon tube-filled with autologous bone marrow mononuclear cells harvested from the iliac crest used in the repair of human median and ulnar nerves. After 1 year,patients with tubes filled with mononuclear cells showed better recovery (decreased pain and recovery of motor and sensory functions) than the control group.
Conduits are often used in preclinical studies and some clinical studies (Table 6); however, their usage in practice is limited.Secondary nerve suture or an autologous nerve graft still remain the most predictable and most often applied methods of secondary nerve reconstruction in humans. Thus, the idea of stem cell administration along with conduits (Di Summa et al., 2010; Liu et al., 2011; Klein et al., 2016; Masgutov et al.,2016; Shimizu et al., 2018), scaffolds, 3D matrices or direct injections into the nerve epineurium and fascicles as well as into the adjacent tissue were investigated (Li et al., 2013;Tabakow et al., 2014; Ikumi et al., 2018).
In the studied group, we performed the revision surgery with nerve release and direct administration of the prepared stem cell solution by microinjection. The stem cells suspended in liquid were injected around the fascicles along the reconstructed part of the nerve as well as above and below the intact nerve. We expected that their regenerative activity would improve the results of the nerve release. Additionally,we expected that they could inhibit abundant re-scarring. Such potential for a reduction of fibrotic tissue formation (Dumanian et al., 1999; Vaienti et al., 2013; Krzesniak and Noszczyk, 2015;Li et al., 2016; Cherubino et al., 2017; Ulloa and Banda, 2017;Di Summa et al., 2018) could be highly appreciated in revision surgery cases. ADSCs from autologous fat tissue are easily accessible for harvesting and direct isolation (contrary to a limited number of accessible bone marrow cells or Schwann cells, which demand laboratory extension). Their origin is autologous; thus, they are nonimmunogenic and prepared for the individual patient during surgery (one-step procedure)without the need of expansion.
Hypothetically, we can assume several mechanisms of ADSC activity. First, mesenchymal ADSCs have the ability to secrete multipotent, neurothrophic, and neuroprotective factors(Kingham et al., 2007). They were reported to deliver some nerve trophic factors, such as glial-derived neurotrophic factor, brain-derived neurotrophic factor, insulin-like growth factor-I, nerve growth factor, and angiopoietin 1, which are relevant for nerve regeneration (Salgado et al., 2010; Zhang and Rosen, 2018). It is anticipated that ADSCs could attract and stimulate neurons as well as activate Schwann cells from the undamaged part of the nerve to more efficient de- and redifferentiation (Hill et al., 2006; Wei et al., 2010; Song et al.,2018).
Second, the suspected factors associated with nerve fibrosis include ischemic conditions in scarred surrounding tissues.ADSCs have proangiogenic abilities, which enhance more efficient neovascularization and improve local vascular support to the nerve structures (Grinsell and Keating, 2014; Zhao et al., 2017).
Third, stem cells possess anti-inflammatory potential(Zhang and Rosen, 2018). As mentioned previously, chronic inflammation can affect nerve regeneration (Büttner et al.,2018). The anti-inflammatory activity of stem cells could modulate the local inflammatory process or suppress it. The abilities of ADSCs include activation of the M2 macrophage phenotype as well as stimulation of the immunosuppressive activity of interleukin-10 (Le Blanc et al., 2012; Franchi et al., 2015). Moreover, pro-inflammatory cytokines, such as interleukin-1 and interleikin-6, are inhibited by ADSCs (Zhang et al., 2017; Song et al., 2018).
Although the trans-differentiation of ADSCs into Schwann celllike cells (phenotype) still remains hypothetical (Santiago et al., 2009; Wei et al., 2010), Di Summa et al. (2010) showed that even differentiated ADSCs enhance sciatic nerve repair through conduits similar to differentiated bone marrow mesenchymal stem cells. Schwann cells are crucially important in nerve regeneration due to their role in the degradation and remodeling of myelin. These cells remove the debris associated with the death of cells and secrete neurothrophic factors, as well as guide new regenerating axons. Even the slightest damage of the nerve (neurapraxia), which is associated solely with the loss of myelin without fascicle injury,results in the retention of nerve conduction. In favorable conditions, myelin is reconstructed and spontaneously regenerated within 12 weeks.
The care related to the target place is a concern. After nerve injury, the resting potential with permanent impulse generation from the central nervous system ceases. Muscleatrophy and gradual neuro-muscular plate degeneration worsen the conditions after delayed nerve reconstruction. In animal models, ADSCs were shown to attenuate denervationinduced muscular atrophy (Wu et al., 2015).
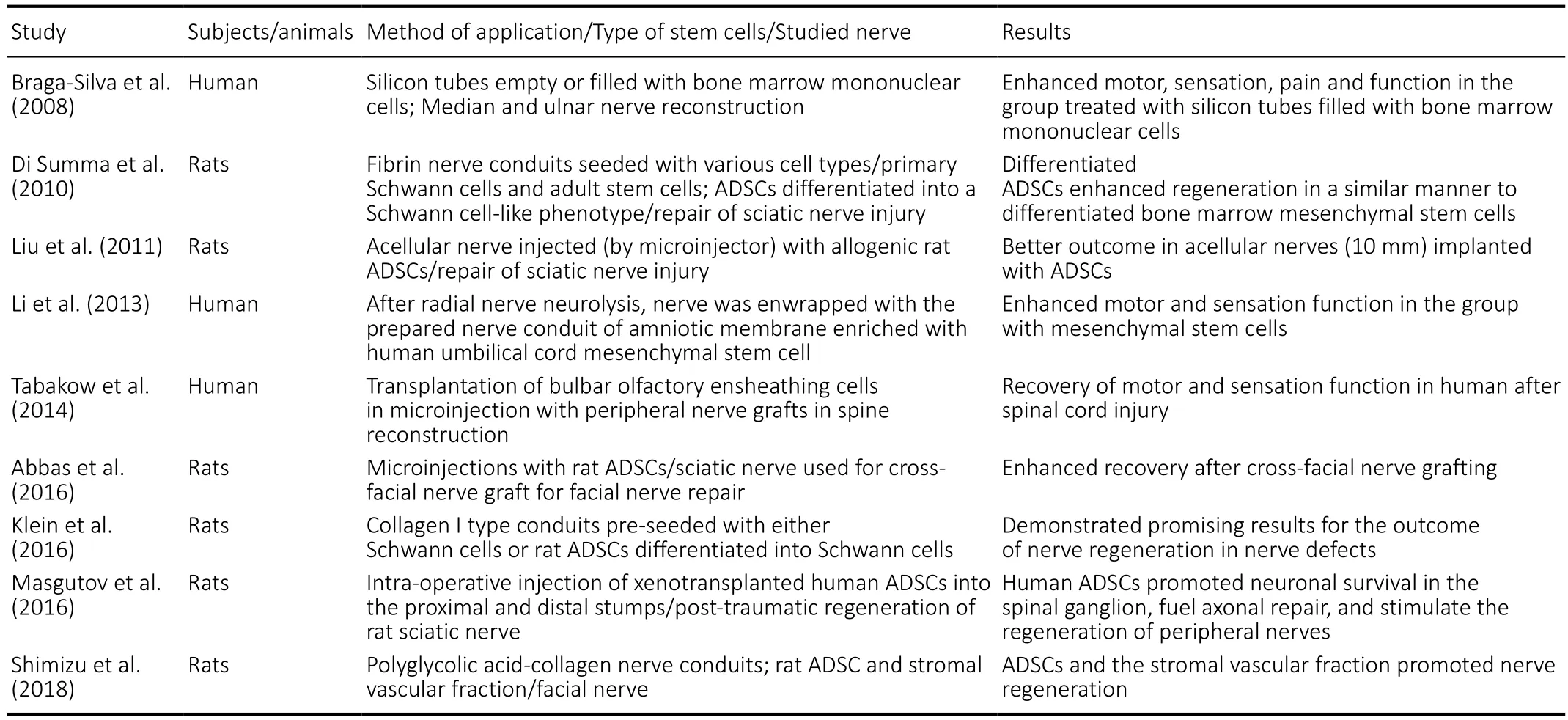
Table 6 |Literature review for usage of ADSCs in nerve regeneration studies
Most of these favorable contributions of ADSCs were observed and confirmed in animal models (Table 6). However,they cannot be directly proven in humans due to ethical limitations. Based on the knowledge from preclinical studies,we can hypothesize that some improved recovery could have merit related to ADCS implantation in humans. The studied group contained patients with unsuccessful repair and poor outcomes of previous treatment who required nerve release(neurolysis) as a last chance option. Throughout the intense follow-up period, we performed clinical examinations. All patients were regularly controlled during the 36-month postsurgical period. All patients were assessed by using the DASH manual survey and VAS scale and were evaluated according to the British Medical Research Council classification modified by Chanson et al. in 1977.
In our study, 2PD did not correlate strictly with sensation recovery. Previous studies (Chassard et al., 1993; Rosen, 1996;Rosen et al., 2000; Fonseca et al., 2018) have shown that 2PD is not an ideal method for the functional evaluation of outcomes after nerve repair. Jerosch-Herold (2000) showed that after reconstruction, patients obtained 2PD values of approximately 20 mm during the 2 years of follow-up (children obtained better results of approximately 15 mm, which is still not perfect). Rosen observed similar 2PD prolongation(16 mm) with proper recognition tests for shape and object evaluation (Chassard et al., 1993; Rosen, 1996; Rosen et al.,2000). Mackinnon and Tung observed good 2PD results after early nerve grafts and transfers (Novak et al., 1992; Moore et al., 2014). Chassard et al. (1993) emphasized a strong relationship between sensory recovery and the functional result in multivariate analysis.
All patients in the studied group had gradual improvement in hand sensation after treatment (they improved from S0 to S3 or S4 levels) in median and ulnar nerve dermatomes.Sensory recovery promoted the improvement in motor skills and casual functionality, which was observed in the DASH survey. Based on diagrams of sensation before and after the treatment, all patients reported that sensation improved from 0–5% to 30–50% compared to that in the healthy hand.Together with improved sensation, the dexterity of the treated hand and casual efficiency increased. These results seem to be encouraging for further studies.
Contrary to evident clinical improvement in sensation, EMG studies did not confirm SNC for an extended period of time following the procedure. The symptoms of EMG improvement were not found earlier than 3 years of follow-up, and improvement was found in only one patient. The explanatory hypothesis assumes that the electrical impulse of the nerve is conducted by jumping between Ranvier nodes, which are myelin-deficient regions along the length of the myelinated fibers. The primary structure of uninjured never fibers is very different from that of reconstructed ones, which shows uneven, nonsynchronous, partial and messy myelinization of sensory fibers. Those myelin deviations and disturbances in impulse conduction would expose the “silence” recordings in subsequent electromyography evaluations; however after 3 years, sensory potentials appeared in one patient. Similarly,the results were observed in other patients in our department as well as were confirmed by other authors (Wiberg et al.,2003; Tseng et al., 2015).
Gradual motor recovery was observed in EMG studies from the beginning, when latency and conduction velocity changed from significant injury to mild injury values in every single EMG control. However, the velocity of nerve conduction through the repaired part of the nerve remained at the slower level. Before the treatment, all patients complained of relevant hand manual problems, which affected their casual activity, work, and functionality. After the treatment,two of the patients proved that general efficiency increased;however, they still had some mild manual problems, especially with very small objects in the absence of sight control (patients No. 1 and No.2). After self-dependent training, patient No. 1 presented with very good manual function, with precise small object sensation. This result is consistent with that of previous studies, which reported that sensory relearning after nerve repair is extremely important for the achievement of better final outcomes (Lundborg and Rosen, 2001).
A remaining question is whether our results are only caused by neurolysis itself. The fact that all patients observed gradual recovery in time after surgery denies that thesis.The results of treatment increased gradually within the time frame of approximately 2–2.5 years, after which no further improvements were noted. Only patient No. 2 had significant recovery in a single finger immediately after neurolysis, which could be a direct effect of nerve release. However, sensation of the rest of the hand improved slowly from month to month.All patients recorded gradual improvement, which required time for sensation and motor functionality recovery and could be considered a result of both nerve release and ADSC administration.
Before the treatment, the most important problem for patients No. 1 and No. 2 was a constant pain in the hand,which made their casual life very difficult. The pain was caused by unsuccessful recovery of sympathetic reinnervation after previous treatment. On the VAS scale, patients No. 1 and No.2 reported constant pain as severe as a score of 9–10. Both of them noted that after treatment, an approximate 90–100%decrease in pain occurred (the VAS scale score decreased from 10 to 0-1). The second problem was excessive cold sensitivity.After nerve release with ADSC administration, they confirmed that the tolerance to cold temperature improved: cold/hot discrimination started to protect patients from accidental burns.
The usage of autologous fat grafts in painful neuroma management, after scar tissue release, and protection against the recurrent scarring was already mentioned (Vaienti et al.,2013; Krzesniak and Noszczyk, 2015; Ulloa and Banda, 2017).However, we are not aware of any report of the impact of ADSCs on reconstructed nerves that required secondary revisions in humans. Unsuccessful cases of delayed nerve repair rarely occur in clinical practice, but there is no effective and clinically efficient treatment that could be offered to those patients. Nerve release (neurolysis) is a recommended procedure that allows for the decompression of nerve fascicles. The procedure improves nerve conduction but does not modulate rescarring and does not guard against secondary relapse.
The weakness of the study is the small number of patients in the studied group and a lack of control group. However,patient (No. 2) underwent neurolysis a year before our treatment, with no result and hence she can be considered as a control group. Due to the innovatory character of the study and the limited number of patients who suffer from a failed result of reconstruction and finally require nerve release/neurolysis, we initially performed an observational study with a long and intense follow-up period. The study had a pilot character and allowed the confirmation of the safety of the procedure and preliminary favorable results. A study protocol with the inclusion of a larger and blinded group should be planned for better data analysis.
In summary, based on clinical observation, we can confirm that the performed treatment was safe for all patients, and any adverse events were noted. All patients recovered 30–50% sensation in the median nerve regions compared to that of the healthy extremity, which could be considered a success compared to a complete lack of sensation experienced earlier.The recovery was gradual, and improvement occurred during the period of 2–2.5 years after treatment. Functional recovery was accompanied by sensory improvement. Better results were observed in patients who continued manual training and self-learning with sensation exercises.
Author contributions:NEK designed and conducted the study in cooperation with AS, AFD, and KO. NEK prepared the paper. BHN and KDj supervised the entire study course and edited the paper. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there is no conflict of interest regarding the publication of this paper.
Financial support:None.
Institutional review board statement:The study protocol was approved by the Institutional Review Board (IRB) at the Centre of Postgraduate Medical Education (No. 62/PB/2016) on September 14, 2016.
Declaration of patient consent:The authors certify that they have obtained all appropriate consent forms from the patients. In the forms,the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical journals developed by the International Committee of Medical journal Editors.
Biostatistics statement:No statistical methods of this study need to be reviewed by the biostatistician.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Alberto Ballestin, jesús Usón Minimally Invasive Surgery Center Microsurgery Unit, Spain.
- 中国神经再生研究(英文版)的其它文章
- Therapeutic effectiveness of a single exercise session combined with WalkAide functional electrical stimulation in post-stroke patients: a crossover design study
- Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy
- Surgical intervention combined with weight-bearing walking training improves neurological recoveries in 320 patients with clinically complete spinal cord injury:a prospective self-controlled study
- Recognition of moyamoya disease and its hemorrhagic risk using deep learning algorithms: sourced from retrospective studies
- D-serine reduces memory impairment and neuronal damage induced by chronic lead exposure
- An integrative multivariate approach for predicting functional recovery using magnetic resonance imaging parameters in a translational pig ischemic stroke model