Anti-viral activity of Staphylococcus aureus lysates against herpes simplex virus type-I infection: an in vitro and in vivo study
Tian-Lan Lin, Chao Cheng, Wei-Ting Zeng, Fang Duan, Yin-Hui Pei, Xiu-Ping Liu,Fu Shang, Kai-Li Wu
Zhongshan Ophthalmic Center, State Key Laboratory of Ophthalmology, Sun Yat-sen University, Guangzhou 510060,Guangdong Province, China
Abstract
● KEYWORDS: herpes simplex virus type-1; Staphylococcus aureus; infection; cornea epithelial cells; keratitis
INTRODUCTION
Herpes simplex virus type 1 (HSV1) is a prevalent human pathogen that infects over 3.72 billion individuals worldwide[1]. The virus causes a broad range of diseases,including labial herpes, ocular keratitis, genital disease, and encephalitis. Ocular infection with HSV1 and its associated sequelae account for the majority of corneal infections and can result in vision impairment or blindness[2]. HSV1 has a complex interaction with host responses. It establishes latent infection in neuronal cell bodies and becomes reactivated when triggered by internal or external factors[3].
HSV1 is a linear double-stranded DNA virus and is a member of the α-herpes virus subfamily[4], characterized by a short replication cycle, high cytopathogenicity, and distinct neurotropism[5-8]. HSV1 replication is temporally regulated by immediate early (IE), early (E), and late (L) genes, usually occurring approximately 1-2h post-infection[9]. The main anti-HSV drugs are nucleoside analogs[10], such as acyclovir, which inhibits HSV DNA replication. A sufficiently safe and efficient vaccine is not currently available[11].
The development of new anti-HSV drugs is urgently needed because the extensive use of nucleoside analogs has led to drug resistance and side effects[10,12]. It has recently been noted that some natural products have anti-HSV activity,including compounds extracted from various plants, such as neem (AzadirachtaindicaL.) bark[13],Cornus canadensis[14],Houttuynia cordata[15-17],Phyllanthus urinaria[18],Prunella vulgaris[19],Euphorbia denticulataLam[20],Baicalein[21],andMallotus peltatus[22].In addition, carrageenan-rich enzymatic extracted from Solieria filiformis[23], Brazilian propolis[24], and abalone hemocyanin[25]have anti-HSV1 effects. Importantly,some studies have reported that compounds derived from prokaryotes could block the replication of HSV. Tiwariet al[26]provide evidence that a novel 11-kDa sugar-binding antiviral protein, cyanovirin-N (CV-N), originally isolated from the cyanobacteriumNostoc ellipsosporum, acts as a potent inhibitor of HSV1 entry in natural target cells. Furthermore,human immunodeficiency virus (HIV) and HSV infections are inhibited by vaginallactobacilli[27].Antiviral effects ofLactobacillus crispatusagainst HSV-2 have been reported in mammalian cell lines[28]. Accordingly, prokaryotes, especially bacteria, are promising sources of potential antiviral drugs.Staphylococcus aureus(S. aureus), a gram-positive bacterium,is a human commensal or a potentially lethal opportunistic pathogen[29-30]. It is the leading cause of keratoconjunctivitis[31].Lysates ofS. aureusand other bacteria have been used empirically in Europe to prevent respiratory infections in children for the past 100y[32]; however, research has focused on their immunoregulatory properties and not on their impact on viruses. Purified components ofS. aureusable to inhibit viral infection have been identified over the last few decades.For example, staphylococcal protein A (SPA) substantially increases anti-porcine circovirus type 2 (PCV2) monoclonal antibody bioactivity[33]. Lipoteichoic acid (LTA) is a potential innate immune stimulant that can be used against infectious laryngotracheitis virus (ILTV) in chickens[34]. Additionally,α-hemolysin fromS. aureus(α-toxin) modulates the skin host response to HSV1 infection[35]. However, the effects of components ofS. aureuson HSV1 infection are largely unknown. In this study, to address the gap in knowledge regarding the effects ofS. aureuson HSV1 infection, the effects ofS. aureuslysates (SALs) on HSV1 infection were evaluated in cultured cells and on the ocular surface of mice.
SUBJECTS AND METHODS
Ethical Approval All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. All procedures were conducted in accordance with the Association for Research in Vision and Ophthalmology(ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines of the Ophthalmic Animal Laboratory of the Zhongshan Ophthalmic Center. The research protocol was approved by the Animal Care Committee of the Zhongshan Ophthalmic Center at Sun Yat-sen University(Approval #: 2017-102).
Culture of Cells, Viruses, and Bacteria Transformed human corneal epithelial (HCE) cells (RCB2280; RIKEN Cell Bank,Tokyo, Japan) were cultured in Dulbecco’s modified Eagle medium (DMEM)/F-12 (Gibco, Grand Island, NY, USA)containing 10% fetal bovine serum (FBS; Gibco), 10 ng/mL human epidermal growth factor (hEGF; Sigma-Aldrich, St.Louis, MO, USA), 5 μg/mL insulin (Sigma-Aldrich), and 1% penicillin/streptomycin (Gibco) at 37℃ in a humidified atmosphere containing 5% CO2. Both Vero cells (African green monkey kidney cells, CCL-81; American Type Culture Collection, ATCC, Manassas, VA, USA) and HeLa cells(Henrietta Lacks strain of cancer cells; ATCC CCL-2) were grown in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS and 1% penicillin/streptomycin in an incubator at 37℃ with 5% CO2and saturated humidity. BV2 microglial cells (ATCC CRL-2467TM) were maintained in high-glucose DMEM (Invitrogen) with 5% FBS.
The HSV1 F strain[36](HSV1f) and green fluorescent protein(GFP)-expressing HSV1 strain (HSV-1 strain H129 with knocked-in GFP, HSV1g, with a cloned-in GFP gene, a gift from professor Minhua Luo, Wuhan Institute of Virology,Chinese Academy of Sciences) were propagated and titrated on Vero cells. A virus titer of 105PFU/mL [multiplicity of infection (MOI)=1] was determined by a plaque assay, and viral particles with a MOI of 1.0 or 0.1 were used in all experiments[36].
The bacterial culture was performed as described previously[37].S. aureus(ATCC 25923) was obtained as a lyophilized powder and was stored at 4℃ until use. A wild-typeS. aureusstrain was isolated from a patient with blepharitis by individual skin swabs using cotton sticks. Pure cultures ofE. coli(ECL, ATCC 25922) andStaphylococcus epidermidis(SEL, ATCC 29887)were grown overnight at 37℃ on LB media. All bacteria were initially subcultured on chocolate agar plates (bioMérieux,Craponne, France) for 24h at 37℃. Bacterial colonies were routinely identified in a clinical laboratory in the Zhongshan Ophthalmic Center.
Preparation of Bacterial Lysates A bacterial colony was picked and suspended in sodium chloride broth, 7.5% (HKM,Ltd., Guangdong Province, China) for 24h to increase the bacterial yield. The cultures were centrifuged at 10 000×gfor 10min and the supernatant was discarded. The pellet containing the bacteria was washed three times with pyrogenfree phosphate-buffered saline (PBS; Gibco). The pellet was resuspended in PBS, vortexed thoroughly, diluted to the proper concentration, and dispensed into EP tubes. TZP beads (0.4-0.6 mm; Hualian Hi-Tech Ltd., Suzhou, China) were added and the bacteria were lysed using a tissue grinder (Scientz,Ningbo, China) for 0.5min×65 Hz at 3-min intervals on ice.The bacterial lysates were centrifuged (12 000×g, 4℃) for 15min. The supernatants for SAL, SEL, or ECL were filtered with 0.22-μm filters (Merck Millipore, Tullagreen, Ireland)and stored at -80℃ for further use. The protein concentrations were measured using the BCA protein assay kit (Beyotime,Shanghai, China), as described previously, to quantify active substances in various lysates[38].
HSV1 Infection in Cultured Cells HCE, Vero, HeLa, or BV2 cells were infected with HSV1 (HSV1f or HSV1g) at different MOIs following a previously described method[39]. Pre- and post-virus infection, SAL was added at various concentrations to the culture medium, and the cells were cultured for 24h.GFP fluorescence of HSV1g in cells was examined under a fluorescence microscope and images were obtained.Plaque formation by HSV1f was examined as described previously[36]. Vero cells were grown in 12-well plates to 70%-80% confluence; cells were treated with SAL and infected with HSV1f as described above. After 1h, the cells were overlaid with 1 mL of a 1:1 mixture of low-melting-temperature agarose (NuSieve GTG Agarose; Lonzo, Morristown, NJ,USA) and 2×DMEM/high glucose to permit only cell-to-cell spread of the virus. At 48h, the agarose was removed carefully.The plates were stained with crystal violet for 20min and images were obtained. Finally, plaque sizes were measured.
Cell Viability Assay Cell viability was assessed by a cell counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan)according to the manufacturer’s instructions. Briefly, HCE or Vero cells were seeded in 96-well culture plates at a density of 7000 cells per well and cultured overnight. Then, cells were incubated with various concentrations of SAL (0, 0.01,0.05, 0.1, 0.3, and 0.5 mg/mL) for 24h. After treatment, cells were washed twice with PBS. Then 100 μL of cell viability detection solution (10 μL of CCK-8 reaction solution and 90 μL of serum-free medium) was added to each well, and the cells were incubated for 2h at 37℃ in the dark. Absorbance was measured at 450 nm using SynergyH1 (BioTek, Winooski, VT,USA).
Treatment of HSV1-infected Cells SAL at the indicated concentrations was added to the medium of various cultured cells with or without HSV1 infection. Next, the effects of several purified components ofS. aureus,i.e., LTA-sa[34](0.02 mg/mL;InvivoGen, San Diego, CA, USA), peptidoglycan-sa (PGNsa)[40](0.04 mg/mL; InvivoGen), SPA[41](0.04 mg/mL; Sigma-Aldrich), and α-toxin[35](40 ng/mL; Sigma-Aldrich), on HSV1g infection in cultured cells were evaluated. Additionally,SAL was subjected to the following pretreatments: 1) heated at 100℃ for 30min; 2) precooled, acetone-precipitated, and resuspended in PBS; and 3) ultrafiltration with a molecular weight cut-off of 100 kDa (Merck Millipore), separating SAL into a <100 kDa fraction and a fraction that did not permeate the ultrafiltration membrane. For validation, all pretreated SALs were separated by SDS-PAGE and subjected to Coomassie blue R-250 staining using a commercial kit following the manufacturer’s instructions (Beyotime,Shanghai, China). The effects of lysates of ATCC25923 (SAL),the wild-type strain ofS. aureus(W-SAL), SEL, and ECL on HSV1g infection in target cells were compared.
Effects of SAL on a Mouse Model of HSV1f Keratitis Twenty-four BALB/c female mice, aged 6-8wk were purchased from the Animal Supply Center of Sun Yat-sen University (Guangzhou, China). The mice were quarantined and acclimatized for a week before the experiments. The mice were free of clinically observable ocular surface disease before the study.
The mice were randomly assigned to three groups (eight mice per group), and the right eye of each mouse was chosen for the experiment. Animal models of herpes simplex keratitis were established based on our previously described method[42].SAL eye drops (1 mg/mL in 10 μL of PBS) or PBS drops only were applied topically four times the day before infection and continued for 3d post-infection. Corneal changes were examined daily using a slit-lamp microscope (Sun Kingdom Medical Instruments, Chongqing, China) and images were obtained using a digital camera. Alterations in epithelial lesions were evaluated as increased (ineffective), unchanged,or decreased (effective).
Statistical Analysis All statistical analyses were conducted with ANOVA and post-hoc tests. Fluorescence was quantified using Image J (http://rsb.info.nih.gov/ij/). Values ofP<0.05 were considered statistically significant.
RESULTS
Anti-HSV1 Infection Effects of SALin VitroTo test the effects of SAL on HSV1 infection, we treated HCE cells with SAL pre- or post-HSV1g infection. As shown in Figure 1, the inhibitory effects of SAL on HSV1 infection were observed in HCE cells treated with SAL both pre- and post-HSV1g infection. When HCE cells were treated with SAL for 12h before infection, substantial reductions in the cytopathic effect(CPE) and GFP expression were observed. Similarly, the addition of SAL to the media after HSV1g infection resulted in the inhibition of viral infection. Thus, SAL treatment postinfection was used in subsequent experiments.Figure 2 summarizes the inhibitory effects of SAL on HSV1 infection in different cell types. When 0.04 mg/mL SAL was added to the culture media, the CPE and HSV1g GFP fluorescence decreased significantly in HCE, Vero, HeLa, and BV2 cell lines (Figure 2A, 2C). In HCE cells, as determined by a plaque assay, HSV1f induced the formation of a large number of plaques and treatment with SAL significantly decreased the plaque sizes and numbers (Figure 2B, 2D).

Figure 1 Comparison of the inhibitory effects of SAL on HSV1g infection when administered pre- or post-infection HCE cells were infected with HSV1g (HSV-1-H129 with GFP knock-in) at an MOI of 1.0. SAL (0.04 mg/mL) was added to the medium 12h before viral infection (upper panel) or 1h post-infection (lower panel); phase-contrast images and GFP fluorescence were obtained by microscopy under the same magnification (scale bar=50 μm). Both pre- and post-virus-infection, the addition of SAL to the culture medium resulted in substantial decreases in cell lesions and in HSV1g GFP expression.
Dose- and Time-dependent Antiviral Activity of SAL HSV1g infection was inhibited by SAL in a dose-dependent manner during the entire culture period (Figure 3). In contrast to the continuous increase in GFP expression in HSV1g-infected HCE cells, the addition of SAL decreased GFP fluorescence at 12 and 24h (Figure 3A, 3C) and at 36 and 48h (data not shown).Consistent with the results of the plaque assay for HSV1f,when HSV1g-infected HCE cells were cultured with various concentrations of SAL, GFP fluorescence decreased in a dosedependent manner (Figure 3B, 3D). HSV1g replication was inhibited by greater than 90% by 0.06 mg/mL SAL.
Effect of SAL on the Viability of HCE and Vero cells To determine the safe concentration range of SAL in cells, HCE or Vero cells were treated with different doses of SAL (0,0.01, 0.05, 0.1, 0.3, and 0.5 mg/mL) for 24h, and cell viability was evaluated by a CCK-8 assay. As shown in Figure 4, SAL concentrations ranging from 0.01 to 0.1 mg/mL did not reduce cell viability. However, when the concentrations of SAL were ≥0.3 mg/mL, the viability of HCE and Vero cells was significantly lower than that of untreated control cells.
Antiviral Effects of Pretreated SAL HSV1g-affected HCE were treated by SAL that was prepared by various methods(Figure 5). When SAL was heat-treated, its inhibitory effect on viral infection was lost, indicating that the active component was heat-sensitive. When SAL was precipitated by cooled acetone and resuspended in PBS, it retained the ability to reduce GFP fluorescence in HSV1g-infected cells, indicating that the active components of SAL could be precipitated by cold acetone. After ultrafiltration, the SAL fraction that did not permeate the ultrafiltration membrane retained the inhibitory activity against GFP expression in HSV1g-infected cells.However, components of <100 kDa did not show obvious inhibitory effects on GFP expression. In addition, pretreated SALs were subjected to SDS-PAGE to examine changes in protein components (Figure 5C). We found that heated-SAL and the low-molecular-weight fraction obtained by ultrafiltration (<100 kDa) displayed distinct band patterns compared with those for the other three protein samples.
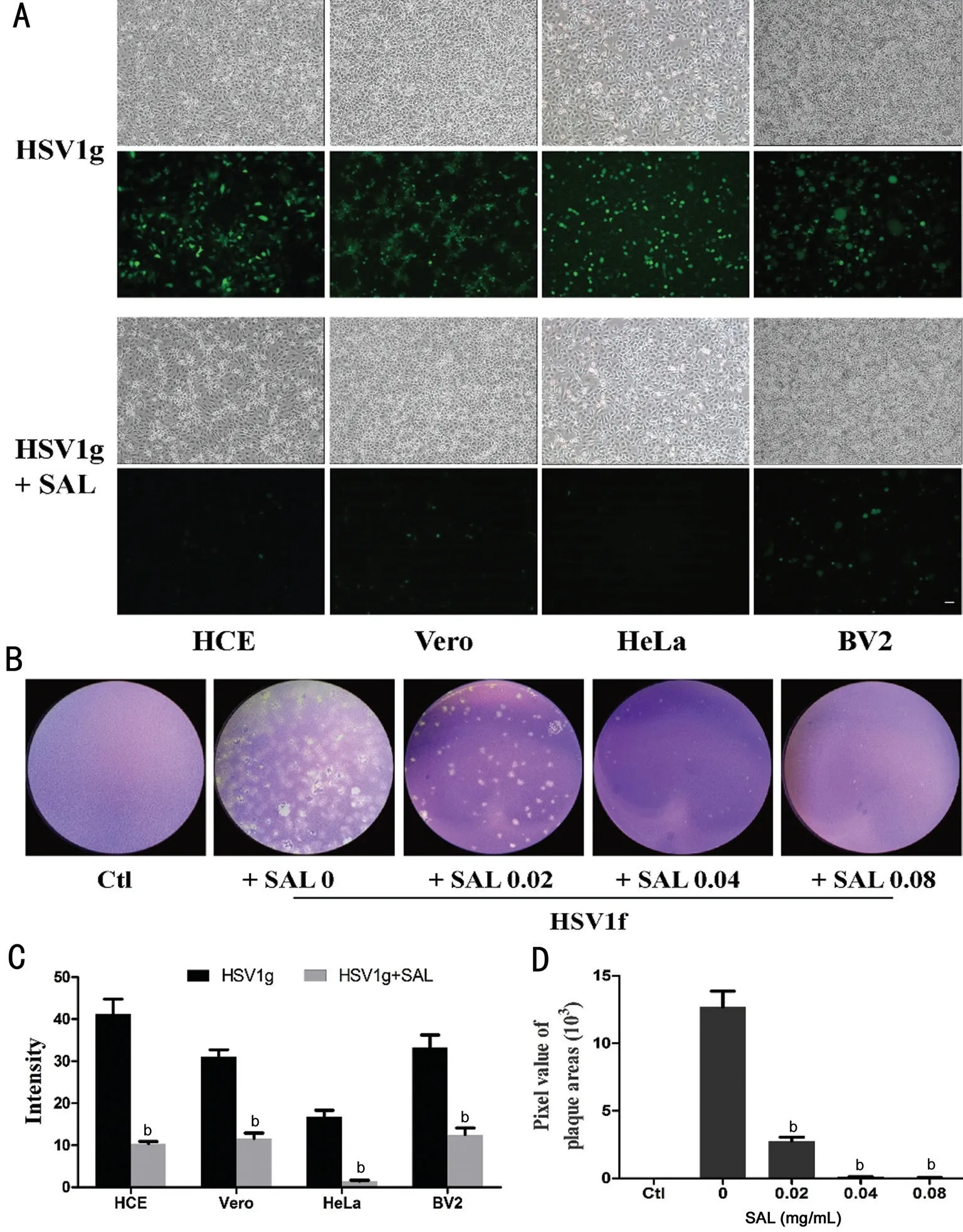
Figure 2 Comparison of the inhibitory effects of SAL on different cells infected with HSV1, as determined by GFP fluorescence (A, C)and plaque assays (B, D) A: SAL at 0.04 mg/mL was added to HSV1g-infected HCE, Vero, HeLa, or BV2 cells for 24h. Phase-contrast images were obtained and GFP fluorescence was evaluated by microscopy under the same magnification (scale bar=50 µm). B: For a plaque test, HCE cells were infected with HSV1f (MOI 0.01) for 1h, followed by the addition of SAL (0, 0.02, 0.04, and 0.08 mg/mL) for 24h. The control group(Ctl) consisted of cells without viral infection and SAL treatment. C: Sum of fluorescence intensities in A. bP<0.01 vs without SAL. D: Average plaque areas calculated from B. bP<0.01 vs HSV1f-infected cells without SAL. Each experiment was repeated three or more times.
Effects of Purified Components ofS. aureuson HSV1 Infection Figure 6 presents a comparison of the effects of SAL and different purified components ofS. aureus. The addition of SAL obviously inhibited GFP expression in HSV1g-infected cells. Upon the addition of LTA, the fluorescence signal was weakened. However, target cells were sensitive to LTA,with significant cell death observed by phase microscopy. In contrast to SAL, α-toxin resulted in strong GFP expression in cells. The addition of SPA or PGN did not significantly alter GFP fluorescence in HSV1g-infected cells; however,there were slightly increases in fluorescence intensities. Taken together, these results suggest that LTA, α-toxin, SPA, and PGN were not the main active components in SAL responsible for the inhibition of viral infection.
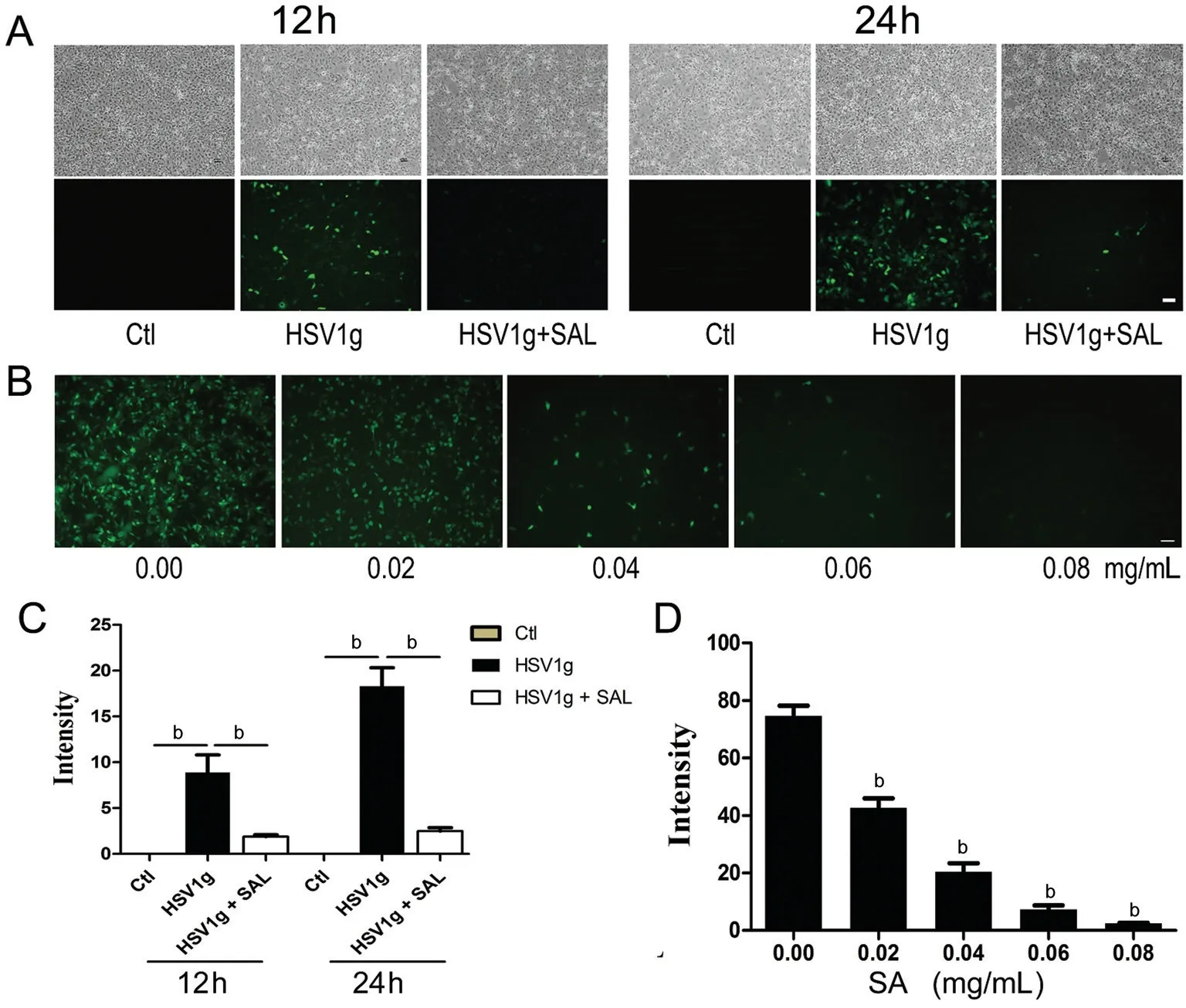
Figure 3 SAL inhibits HSV1g infection in a dose-dependent manner and HCE cell lines were infected with HSV1g A: Comparison of the inhibitory effects of SAL at 12 and 24h. The control group (Ctl) consisted of cells without virus infection and with SAL treatment. B: HSV1g-infected HCE cells were incubated with 0, 0.02, 0.04, and 0.08 mg/mL SAL for 24h. Images of GFP fluorescence were obtained. C: Comparison of GFP fluorescence intensities in A. bP<0.01. D: Average fluorescence intensities calculated from B. bP<0.01 vs HSV1g infected cells without SAL. All photograms were obtained at the same magnification (scale bar=50 µm). Each experiment was repeated at least three times.

Figure 4 Effects of SAL on the viability of HCE and Vero cells HCE (A) and Vero cells (B) were treated with the indicated concentrations of SAL,and cell viability was determined by the CCK-8 assay (aP<0.05, bP<0.01 compared with the control group). Each experiment was repeated three times.
Comparison Between SAL and Lysates of Different Bacteria Lysates of a gram-negative bacterium (ECL), grampositive bacterium (SEL), and W-SAL were added at 0.05 mg/mL to the culture media to determine their effects on HSV1 infection(Figure 7). Compared with levels in untreated cells, SAL and W-SAL clearly inhibited GFP expression in cells infected with HSV1g. SEL displayed moderate inhibitory effects on GFP expression. In contrast, ECL increased GFP expression in target cells. These results suggested thatS. aureushas unique anti-HSV1 substances.
Anti-HSV1 Effects of SALin VivoWe attempted to use SAL to treat HSV1f-infected corneas via eye drops. Disruptions of the corneal epithelium were observed in animals when examined by slit-lamp microscopy and were stained with fluorescein sodium (FLS) post-infection (Figure 8). SAL eye drops or PBS drops were applied topically pre- (24h) and post-infection (up to 72h) every 4h to BALB/c mice with HSV1 keratitis. Corneal damage was obviously reduced by SAL compared with that in untreated animals on Day 3 post-infection(5 of 7 SAL-treated eyesvs1 out of 8 control eyes; Table 1).
DISCUSSION

Figure 6 Comparison of the effects of purified components of S. aureus on cells infected with HSV1g A: SAL (0.04 mg/mL), LTA (0.02 mg/mL),PGN (0.04 mg/mL), SPA (0.04 mg/mL), and α-Toxin (40 ng/mL) from S. aureus were added to HeLa cells. Images of GFP fluorescence were obtained. B: Fluorescence intensities in A were calculated from triplicate experiments. aP<0.05, bP<0.01 vs the HSV1g group.
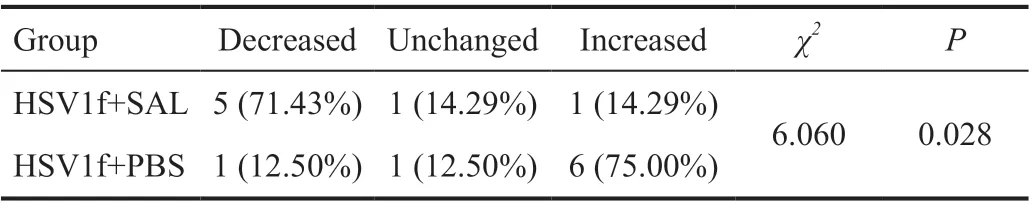
Table 1 SAL effects on corneal ulcers of mouse HSV1 keratitis
This study focused on the combined effects of bacterial lysates and HSV1 in infection models. Immunoregulatory and immunostimulatory effects of bacterial lysates in respiratory infections and asthma have been reported[32]. For example,Broncho-Vaxom (OM85-BV), a mixture of extracts from eight strains of gram-positive (includingS. aureus) and gram-negative bacteria, promotes anti-infection immune responses[43]. Antigens have been obtained from mass cultures of appropriate bacterial strains by mechanical cell disruption(polyvalent mechanical bacterial lysate) or chemical proteolysis(polyvalent chemical bacterial lysate)[44]. Additionally, bacterial lysates have a long history of use for the prevention of respiratory infections in children[32]. In the present study, we discovered that SAL could inhibit HSV1 infection, providing a basis for the development of novel anti-HSV1 agents. To our knowledge, this is the first evaluation of the interaction between SAL and HSV infection. However, the precise active components remain to be identified. In addition, although the antiviral components are effective in our mouse models of herpes simplex keratitis,S. aureusproduces more than 20 toxic proteins[45]. These toxic substances may cause anaphylaxis,toxic reactions, or other adverse effects topically as well as systemically. Thus, SAL cannot be appliedin vivountil its active components are characterized.

Figure 7 Comparison of the effects of different bacterial lysates on HSV1g replication Lysates at 0.05 mg/mL were added to the culture media for 24h. A: Images of GFP fluorescence were obtained; B: Fluorescence intensities in A were evaluated quantitatively using Image J. Lysates were as follows: SAL: S. aureus ATCC25923; W-SAL: Wild-type S. aureus; SEL: S. epidermis; ECL: E. coli. bP<0.01 vs the HSV1g group (n=3).
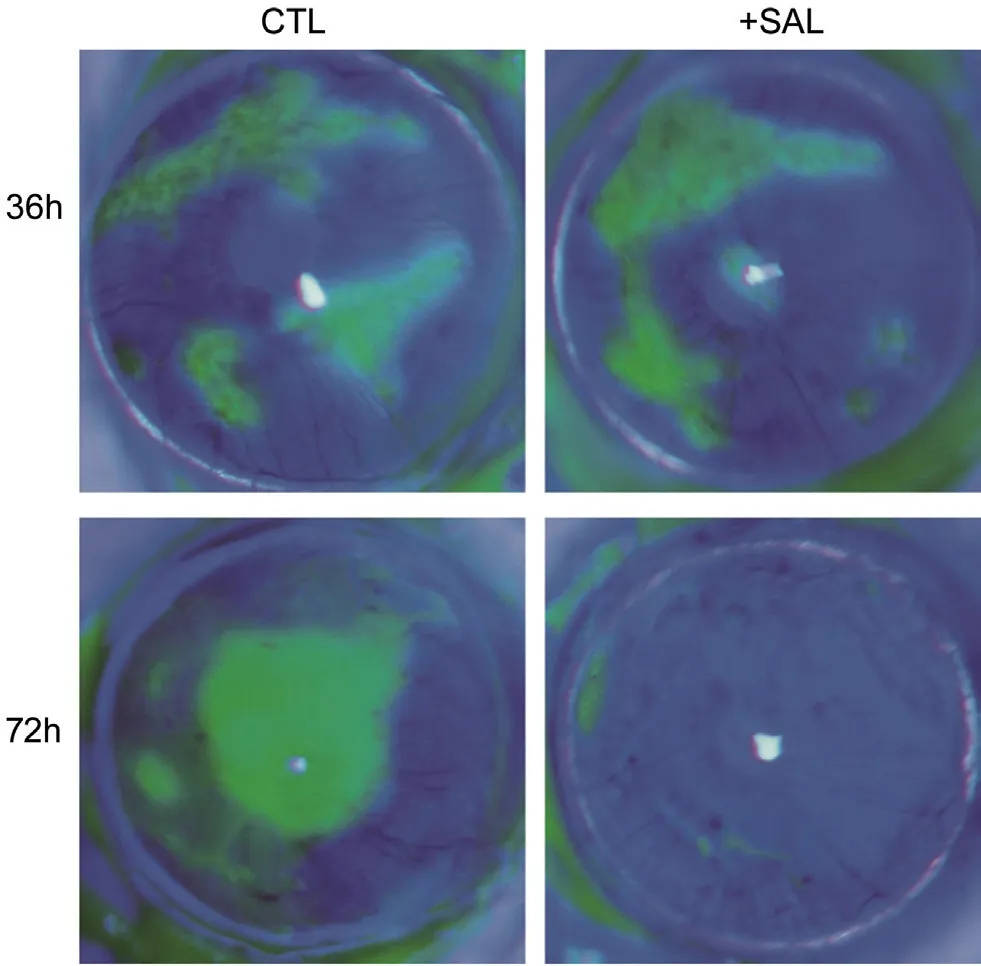
Figure 8 SAL inhibits HSV1f (F-strain) infection in mouse corneas Photographs of corneas stained with fluorescein sodium are shown.BALB/c mice were used to create an HSV keratitis model. SAL eye drops were applied topically 4 times daily on pre-infection and up to 3d post-infection. Mice were evaluated on day 3 post-infection.
The incidence and severity of infection caused by HSV1 have increased over the past decade and have become an important global public health issue[46]. Owing to the emergence of drugresistant strains and drug toxicity[47], the development of novel anti-HSV1 agents, especially those with different mechanisms of action, is desperately needed. Our results demonstrate that SAL has anti-HSV1 activity, without significant effects on cell viability. The topical application of SAL eye drops significantly reduced corneal lesions in mice with HSV1 keratitis. The inhibitory effects of SAL on viral infection were dose-dependent. The antiviral components ofS. aureusremain to be identified; however, we were able to rule out the roles of several known components (e.g., LTA-sa, α-toxin, PGN-sa, and SPA). We speculate that the active components are proteins or glycoproteins. However, further studies are needed to determine the exact nature of the active components.
Nucleoside analogs[48], small molecules (phenolics, polyphenols,and terpenes) derived from plants[13], and small RNAs[36]have been reported to inhibit HSV infection. Numerous studies have reported the antiviral activity of antimicrobial proteins/peptides/cytokine, including VEGF-A by CD4-T[49], and targeting with CRISPR-Cas9[50]. In the current study, we demonstrated that the active substance(s) was heat-sensitive,could be precipitated with acetone, and has a molecular weight of >100 kDa. These results suggested that the anti-HSV1 substance is a soluble protein.
The viral life cycle can be divided into the following major steps: entry into the host cell, expression of viral genes,replication, virion assembly, and egress of the new generation of viral particles[51]. In permissive cell lines, this cycle takes approximately 18-20h. In our experiments, SAL had inhibitory effects on HSV1 infection in HSV1g-infected HCE cells when administered both pre- and post-infection, with a more obvious effect post-HSV1g infection. These findings suggest that SAL influences the adhesion and replication process of viral particles but may not directly affect host cells. The mechanism underlying the effects of SAL remains to be determined.In conclusion, we investigated the effects of SAL on HSV1 infection in various cultured cells and in a mouse model of HSV1 keratitis. Our results demonstrate that SAL inhibits HSV1 infectionin vitroandin vivo. The identification and characterization of the active component of SAL merits further research and is expected to aid in the development of therapeutic agents against HSV infection.
ACKNOWLEDGEMENTSWe thank Prof. Min-Hua Luo (Wuhan Institute of Virology,Chinese Academy, Chinese Academy of Sciences) for the gift of HSV1 strain-H129 knocked-in GFP gene, and Prof.Huangxuan Shen (Zhongshan Ophthalmic Center, Sun Yatsen University) for the gifts of HeLa cells (ATCC CCL-2) and BV2 microglial cells (ATCC®CRL-2467TM).
Foundations:Supported by the National Natural Science Foundation of China (No.81770896; No.81970848); the Guangzhou Science Technology and Innovation Commission(No.201607020011).
Conflicts of Interest:Lin TL, None; Cheng C, None; Zeng WT, None; Duan F, None; Pei YH, None; Liu XP, None;Shang F, None; Wu KL, None.
 International Journal of Ophthalmology2021年10期
International Journal of Ophthalmology2021年10期
- International Journal of Ophthalmology的其它文章
- lmpact of intraocular pressure fluctuations on progression of normal tension glaucoma
- Effective treatment for secondary angle-closure glaucoma caused by traumatic lens subluxation:phacoemulsification with capsular-tension-ring implantation combined with ophthalmic endoscopecontrolled goniosynechialysis
- Efficacy and safety of newly developed preservativefree latanoprost 0.005% eye drops versus preserved latanoprost 0.005% in open angle glaucoma and ocular hypertension: 12-week results of a randomized,multicenter, controlled phase III trial
- Progressive restrictive strabismus in an infant
- Association of peripheral anterior synechia, intraocular pressure, and glaucomatous optic neuropathy in primary angle-closure diseases
- Protective effect of LIF-huMSCs on the retina of diabetic model rats
