Guizhi-Fuling formula inhibits ovarian cancer progression by targeting STAT3 signaling network
Qihong Ma, Shi Dong, Yuanyuan Shi, Tiangong Lu
1 School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China.
2 Shenzhen Research Institute, Beijing University of Chinese Medicine, Shenzhen, China.
Abstract Objective:Ovarian cancer (OC) is the most lethal gynecological malignancy.Frequent peritoneal dissemination is the main cause of low survival rate.Guizhi-Fuling formula (GZFL) is a classical traditional Chinese herbal formula, and has been clinically used for treating ovarian cancer with good outcome.However, its therapeutic mechanism for treating OC has not been clearly elucidated.Methods: Network pharmacology analysis was used to predict potential molecular mechanisms of GZFL in treating OC.In vitro and in vivo analysis, including STAT3 KO/WT cells proliferation assay, scratch assay and antitumor efficacy study were performed to assess the biological activity of GZFL on targeting STAT3 in OC cells.Results:We generated a “GZFL target - OC - STAT3” gene interaction network, and predicted that GZFL is tightly associated with IL6/JAK/STAT3 signal pathway and cancer metastasis.Our preliminary data showed that GZFL inhibited OC cell proliferation in a STAT3 dependent manner.It suppressed cell migration and downregulated p-STAT3 expression.In a tumor bearing mouse model, GZFL displayed a safety profile.Conclusion: GZFL inhibits OC progression by targeting STAT3 signaling network.Our newly proposed pharmacological mechanisms of Guizhi-Fuling formula will provide a new insight for its clinical use in treating OC.
Keywords:Guizhi-Fuling formula, Ovarian cancer, STAT3, TCM, Cancer metastasis
Background
Ovarian cancer (OC) is the fifth leading cause of cancer death in women and the most lethal gynecological malignancy in the world [1].According to the latest data from National Cancer Center, ovarian cancer devastates more than 53,000 women in China every year, and the number is still increasing [2].About 70% cases are diagnosed at stage III or IV with peritoneal metastasis and results in a 38.9% 5-year survival rate [3].Conventional therapies, including cytoreductive surgery and chemotherapy, initially improve therapeutic outcomes, but often result in drug resistance that leads to relapse [4, 5].In recurrent tumor, targeted therapies such as poly (ADP-ribose) polymerase inhibitors, anti-angiogenic agents, and immunological therapies show limited efficacy; combination therapies are under evaluation [6].Thus, there is an urgent need to develop more effective and safe alternative treatments to improve patient outcomes.
Guizhi-Fuling Wan (Keishi-bukuryo-gan in Japanese and Gyejibokryeong-hwan in Korean) is a classical traditional Chinese herbal formula, and was originated in “Essential Prescriptions from the Golden Cabinet “(Jin Gui Yao Lüe).Its modern dosage form, Guizhi-Fuling capsule, has been approved by China Food and Drug Administration (CFDA) for clinically treatment of blood stasis syndromes in gynecological diseases.Numerous clinical and pharmacological studies on various gynecological diseases, such as climacteric syndrome [7], inflammation [8], endometriosis [9] and primary dysmenorrhea [10], have shown that Guizhi-Fuling formula (GZFL) exhibits significant therapeutic effect with few clinical side effects.Recent evidence suggests that GZFL has antitumor effects in various cancer types [11, 12].In OC, GZFL sensitized cells towards cisplatin through inhibiting MTDH-PTEN and PI3K/AKT/mTOR pathway [13, 14].Chinese clinicians have demonstrated that GZFL improves curative effect of chemotherapy in patients with advanced OC and reliefs clinical adverse effects [15].However, its therapeutic mechanism for treating OC has not been clearly elucidated.
Signal transducer and activator of transcription 3 (STAT3) participates in a wide variety of physiological processes.STAT3 is highly expressed and activated in a variety of human malignant tumors, making it a popular antitumor target.In ovarian cancer patients, phosphorylation activated STAT3 (p-STAT3) positively correlates with disease aggressiveness and negatively correlates with survival, indicating STAT3 plays a key role in the development and metastasis of OC [16, 17].We previously reported a multi-omic genome-wide analysis onSTAT3KO ovarian cancer cells [18].We have revealed that STAT3 is critical in regulating cell adhesion and migration, and responding to tumor microenvironment signaling.
Herein, we investigated the underlying pharmacological mechanisms of GZFL on OC progression.Network target analysis predicts that GZFL may tightly associate with STAT3 signaling network and cancer metastasis.Importantly, GZFL significantly inhibits cell proliferation in a STAT3 dependent manner, downregulates p-STAT3 expression, and suppresses migration of OC cellsin vitro.It shows a safety profile in a 21-day administration xenograft study.Our study provides new pharmacological mechanism for GZFL and promote its clinical application in treating ovarian cancer.
Materials and Methods
Preparation of Guizhi-Fuling freeze dried powder
Guizhi-Fuling formula is composed of Guizhi (Cinnamomum cassia[L.] J.Presl), Fuling (Poria cocos[Schw.] Wolf.), Baishao (Paeonia lactiflora Pall), Mudanpi (Paeonia suffruticosa Andrews) and Taoren (Prunus persica[L.] Batsch).The five herbs were purchased from Beijing Tongrentang.Guizhi (40g), Fuling (40g), Baishao (40g), Mudanpi (40g) and Taoren (40g) were mixed and immersed water for 30min.The mixtures were boiled twice, 1 h for each time (1:6, w/v).The extracted water solutions were combined and filtered.Then a rotary evaporator was used to concentrate to obtain an extract of 200g.The extract was freeze dried by a vacuum freeze dryer to obtain freeze dried powder (17.7g/200g, w/w, freeze dried powder/crude drug) and stored at - 20 °C until use.The weight of treatment used in in vitro experiment refers to the weight of GZFL freeze dried powder.
Reagents and cell culture
Cisplatin was purchased from Meilunbio, China, stored at room temperature and protected from light.Antibodies for p-STAT3 (Try705, D3A7, #9145), STAT3 (124H6, #9139) were purchased from Cell Signaling Technology (Beverly, USA).Antibodies for GAPDH (60004-1-lg) was purchased from Proteintech (Wuhan, China).
SKOV3 and OVCAR3 cells were purchased from the Cell Resource Center, Peking Union Medical College.All cells were cultured in RPMI-1640 with 10% heat-inactivated FBS.Cells were routinely checked free of mycoplasma contamination by PCR and culture.The identity of both cell lines was authenticated with STR profiling (FBI, CODIS).
Cell proliferation assay
Growth inhibition of GZFL in SKOV3 and OVCAR3 cells was assessed using cell counting Kit-8 (CCK-8) assay following the manufacturer’s protocol (Keygen Biotech, China).Briefly, cells were seeded into 96-well plates at a density of 7×104cells/well.On the following day, cells were treated with or without GZFL (0.001, 0.004, 0.11, 0.33, 1 and 3 mg/ml).After 72h, supernatant in each well was removed and 100 μL of 10% CCK-8 solution was added to each well.The plate was incubated at 37 °C for 1–2 h.Then, the optical density of each well was measured at 490 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).All assays were conducted in triplicate.Percentage of cell growth inhibition was expressed as: (1-A/C) × 100% (A and C were the absorbance values from experimental and control cells, respectively).The concentration of GZFL required to inhibit 50% cell population (IC50) was determined using nonlinear regression analysis.
CRISPR-Cas9 mediated genome editing
TwoSTAT3CRISPR guide sequences (1:ACAATCCGGGCAATCTCCAT and 2:CATTCGACTCTTGCAGGAAG) were designed and purchased from Invitrogen™, ThermoFisher Scientific.Generation of single clone cell lines was performed as described [18].Briefly, cells were seeded into 24-well plates at a density of 5×104cells/well.On the next day, cells were cotransfected withSTAT3gRNAs and Cas9 protein via Lipofectamine transfection reagent (Invitrogen, USA).Control cells were generated by transfecting SKOV3 cells with Cas9 nuclease mRNA only.At 48 h post-transfection, the cells were harvested and a single clone isolation was further performed.The clones were screened by real-time PCR and Western blot to assess STAT3 gene and protein expression levels.STAT3sequence in SKOV3STAT3KO cells was confirmed with a homozygous 1 bp deletion by Sanger sequencing.
Acquisition of components of GZFL by HPLCMS
GZFL freeze-dried powder was diluted to 10 mg/mL and filtered using a 0.22μm membrane filter before HPLC-MS analysis.The UPLC system was coupled with an LTQ-Orbitrap XL (Thermo Fisher Scientific, SanJose, CA, United States) equipped with an electrospray ionization source (ESI) and controlled by Xcalibur software (Version 2.1).Dionex Utimate 3000 UHPLC Plus Focused Ultra High Liquid Chromatography System included binary pumps, an autosampler, a Column thermostat and DAD detector (Thermo Fisher Scientific, SanJose, CA, United States).0.22μm microporous membrane (Tianjin Jinteng Experimental Equipment Co., Ltd., Tianjin, China).Sartorious BT 25S electronic analytical balance (Beijing Sartorius Instrument Co., Ltd., Beijing, China).
The MS conditions were as follows: the capillary temperature was 350 °C, the capillary voltage was set to 25.0 V, the tube lens was set to 110 V.the Spray voltage was 3.8 kV in positive ion mode, 3.2 kV in negative ion mode.In a Fourier transform (FT) cell, full MS scans were acquired in the m/z range of 100–1500.The MS/MS experiments were set as data dependent scans.The data was recorded and processed using Xcalibur, ChemDraw.
Prediction of the Putative Targets of the Identified Constituents in GZFL
The potential protein targets of the identified constituents of GZFL were predicted by TCMSP (www.tcmsp-e.com/) and SwissTargetPrediction (www.swisstargetprediction.ch/) [36, 37].Then, the Universal Protein Resource (UniProt, (www.uniprot.org/) was used to convert the protein targets into validated gene targets.After removing the duplication, a protein name of bioactive ingredients to the gene names.A total of 203 GZFL putative genes targets were obtained.
Acquisition of RNA-seq or Bru-seq of STAT3 KO significantly differentially expressed gene
Significantly differentially expressed gene lists from RNA-seq or Bru-seq ofSTAT3KO significantly differentially expressed gene were obtained from previous published data [18].For RNA-seq, genes were considered significantly differentially expressed with mean FPKM > 0.5 and absolute log2 fold change > 1.5 and FDR adjusted p-value < 0.05.A total of 2126 genes was identified.For Bru-seq, gene changes with absolute fold change > 2 and mean RPKM > 0.5 were considered significant.A total of 1833 genes was identified.
Acquisition of ovarian cancer-related targets
The known OC-related targets were searched from the GeneCards database v5.0 (www.genecards.org/) [38] using “ovarian cancer” as the keywords.A total of 7946 potentially therapeutic gene targets were obtained.
Network construction and analysis
The intersection of GZFL putative target, known OC related targets and RNA-seq or Bru-seq ofSTAT3KO significantly differentially expressed gene was obtained using Venny 2.1.0.The intersection protein targets were input into STRING database (http://string-db.org/, version 11.0) to obtain the inter-protein interactions (PPI) related to STAT3 signaling pathway through which GZFL treating OC.Cytoscape software (version 3.7.2, Boston, MA, USA) was used to calculated and visualized the PPI network.Then based on three topological indicators, “degree”, “combine score” and “closeness” key nodes were evaluated and selected.
Pathway enrichment performance
The DAVID (http://david.abcc.ncifcrf.gov/home.js p/, version 6.8) were used to perform pathway enrichment analysis while the Metascape were used to perform Hallmark category based on the data of Kyoto Encyclopedia of Genes and Genomes database (KEGG, http://www.genome.jp/kegg/, updated on November 1st, 2020).KEGG pathways with enrichment p values less than 0.05 were selected as significant signaling pathways.
Wound healing assay
Cells were seeded in 12-well plates (2 × 105cells/well) in RPMI 1640 supplemented with 10% FBS overnight.After cells were attached, a single scratch was made in each well.Cells were washed with phosphate-buffered saline (PBS) once, IC50or 2×IC50of GZFL (1 and 2 mg/ml) in RPMI 1640 with no FBS were added and incubated for 24 h.Cells were stained with crystal violet solution (2%) for 30 min and washed with water.Stained cells were imaged on a microscope (Olympus Corporation).
Western blot
Western blot assay was performed as described [39].Generally, after GZFL treatments, SKOV3 and OVCAR3 cells (only attached cells were collected) were washed with ice-cold PBS and lysed in cold lysis buffer containing a protease/phosphatase inhibitor cocktail (Beyotime, Shanghai, China) at 4 °C for 30 min and centrifuged (12 000 rpm, 10 min, 4 °C).Total protein concentration was measured with BCA assay (Beyotime, Shanghai, China).30 μg of protein per sample was subjected to SDS-PAGE analysis and transferred to nitrocellulose membranes (BBI, Shanghai, China).Membranes were blocked with 5% defatted milk in TBST buffer, and probed with primary and subsequently secondary antibodies at dilutions according to the manufacturer’s instructions.The protein bands were detected using electrochemiluminescence (ECL) reagent (Tanon, Shanghai, China) and the intensities of the signals were quantitatively analyzed using ImageJ Software.
In vivo tumor xenograft studies
SKOV3 cells (5 × 106cells in 100 mL of PBS/mouse) were implanted subcutaneously into the dorsal flanks of 6- to 8-week-old female BALB/c nude mice (SPF level, weighing 18~20g, vitalriver, Inc.) under aseptic conditions as described [40].This study was approved by the ethics committee of Beijing University of Chinese Medicine.Tumor size was monitored twice a week by caliper measurement using the following equation: tumor volume (mm3) =D × d2/2, where D and d are the longest and shortest diameters, respectively.When tumors were allowed to grow to an average volume of 20 mm3, mice were then randomly grouped (n = 5 per group) for treatment.
GZFL capsule was purchased from Jiangsu Kanion Pharmaceutical Co., Ltd.GZFL low dosage (0.36 g/kg) and high dosage (0.72 g/kg) were administrated intragastrically once a day for 21 days.Dosage was converted from adult daily usage to mice according to measured surface area using Meeh-Rubner formula.Solutions of GZFL were prepared freshly in PBS.The treatment of cisplatin (5 mg/kg) was following a routine of first-line treatment, and was conducted once a week.Stock solutions of cisplatin were prepared freshly in PBS and avoid of light.
Statistical analysis
Data are expressed as mean ± SD of at least 3 independent experiments.Student’s t-test and ANOVA (for post-hoc-analysis) were used for statistical analysis and theP-values were determined using Prism 7 (GraphPad Software, Inc.).Differences were considered statistically significant atP< 0.05.
Results
GZFL inhibits proliferation of ovarian cancer cells
CCK-8 assay was used to assess the antiproliferative activity of GZFL against SKOV3 and OVCAR3 ovarian cancer cells.After 72-hour exposure, GZFL displayed potent anti-proliferative activity.The IC50(the concentration of GZFL required to inhibit 50% cell population) values of GZFL in SKOV3 and OVCAR3 cells are 1.02 and 0.64 mg/ml, respectively (Figure 1).These data confirm that GZFL inhibits ovarian cancer cell proliferation potently.1 and 0.6 mg/ml, respectively, in SKOV3 and OVCAR3 cells were used as IC50concentrations in subsequent assays.
Network pharmacology analysis predicts GZFL’s tightly association with STAT3 signaling network and cancer metastasis
We first submitted GZFL decoction freeze-dried powder to UPLC-ESI-Q-TOF-MS/MS analysis (Figure 2).Comparing the information collected from the literature and standards with spectrogram, we identified 22 compounds as main active ingredients, including Cinnamaldehyde, Quercetin, Paeonoside, Amygdalin, Paeoniflorin (Table 1).

Figure 1.Growth curve of GZFL against SKOV3 and OVCAR3 cells.

Figure 2.Total ion chromatogram monitored in positive and negative ion modes for GZFL extract.
To systematically explore potential molecular mechanisms of GZFL in treating ovarian cancer, we performed network pharmacology analysis of GZFL on ovarian cancer.GZFL putative targets were identified by GZFL active ingredients via
TCMSP and SwissTargetPrediction, and were converted to genes name through uniport.A total of 203 GZFL component putative target genes were obtained (Supplemental Table S1).We crosscompared GZFL predicted gene targets and OCrelated targets retrieved from the GeneCards database, and 168 common targets were obtained (Supplemental Figure S1A).These common targets were considered as potential therapeutic targets for GZFL to treat OC.AKT,IL6andVEGFAare identified as the core targets (Supplemental Figure S1B).
Next, based on our previous identified “STAT3KO differentially expressed genes”4b from Bu-seq and RNA-seq analysis, we generated a “GZFL target - OC - STAT3” gene interaction network.31 and 40 common genes were identified when overlapping GZFL targets with OC-related targets andSTAT3KO significantly differentially expressed genes by Bru-seq and RNA-seq, respectively (Figure 3A).Notably, common genes are highly enriched in STAT3 signaling (such asCCND1, IL6,BIRC5andSTAT1) and cell migration and angiogenesis (such asVEGFA, MMP-1,-2,-9, CLDN4, PLATandSERPINE1) (Supplemental Table S2 and S3).Therefore, we performed pathway enrichment analysis on overlapped genes.In Hallmark category, “TNFA signaling via NFKB”, “IL6 JAK STAT3 signaling” and “Epithelial mesenchymal transition” were most enriched gene sets (Figure 3B).NFKB is reported to epigenetically regulate STAT3 activation via IL6 [19].Moreover, epithelial mesenchymal transition (EMT) plays a key role in all stages of cancer progression, especially cell motility and invasion [20].Taken together, pathway enrichment analysis indicates that GZFL is tightly associated with IL6/JAK/STAT3 signal pathway and cancer metastasis.Interaction network analysis showed that these common genes formed a close signal network and IL6, MMP1, TP53 and VEGFA were identified as core targets in “GZFL target - OC - STAT3” gene interaction (Figure 3C).

Table 1.Identification of chemical constituents from Guizhi-Fuling formula by UPLC-ESI-Q-TOF-MS

Figure 3.Network pharmacology predicts that GZFL affects cell metastasis pathways.

Figure 4.GZFL downregulates STAT3 signaling network.

Figure 5.GZFL displays a safety profile in tumor bearing xenograft model.
GZFL downregulates STAT3 signaling network
To validate GZFL’s effect on STAT3 signaling network, we used CRISPR-Cas9 mediated editing to knock outSTAT3in SKOV3 cells.SKOV3STAT3KO single cell clones were generated (Supplemental Figure S2A) and confirmed with a homozygous 1 bp deletion by Sanger sequencing (Supplemental Figure S2B).
Supplemental Table S1.Guizhi-Fuling component putative target genes

Supplemental Table S2.GZFL-STAT3 KO Bru-seq-OC overlapping gene

Supplemental Table S3.GZFL-STAT3 KO RNA-seq-OC overlapping gene
In order to assess the sensitivity of cells with different STAT3 expression status towards GZFL, we tested the anti-proliferative activity of GZFL against wildtype (WT) andSTAT3knockout (KO) cells using CCK-8 assay.As shown in Figure 4A, WT cells were more sensitive towards GZFL treatment compared toSTAT3KO cells.WT cells were nearly 100% inhibited by GZFL at highest test concentration (3mg/ml), while ~30%STAT3KO cells were still viable.This observation suggests that GZFL inhibits ovarian cancer cell proliferation in a STAT3 dependent manner.We further verified GZFL’s effect on activated STAT3 by western blotting.After 6-hour treatment, expression of phosphorylated STAT3 was significantly inhibited by GZFL in both SKOV3 and OVCAR8 cells at tested concentrations (Figure 4B).
STAT3 has been shown to be necessary for migration and invasion [21].Since GZFL downregulated activated STAT3 in OC cells, we evaluated its impact on cell migration.After 24h exposure to GZFL at IC50, migration of SKOV3 cells were suppressed (Figure 4C).Notably, totally blockage of cell mobility was further observed in 2× IC50GZFLtreated cells.Collectively, GZFL downregulates p-STAT3 and inhibits OC cell migration.
GZFL displays a safety profile in tumor bearing xenograft model
Next, we evaluated the efficacy and safety of GZFL on SKOV3 tumors after subcutaneous inoculation in the flank of BALB/c mice.Cisplatin was used as a positive control in this experiment.The treatment of cisplatin (5 mg/kg, once a week) was followed a routine of firstline OC treatment.Low dosage (0.36 g/kg) of GZFL was converted from adult-to-mice daily usage.Two times of daily usage was set as the high dosage (0.72 g/kg).In a cycle of 21 days of administration, mice treated with GZFL and cisplatin showed reduction in tumor growth, but were not significant comparing to control group (Figure 5A).However, a significant loss of body weight was observed in Cisplatin group mice (P< 0.01) (Figure 5B).Meanwhile, there were no significant difference in body weight of mice when comparing both GZFL groups and the vehicle group.No death was observed in GZFL low dosage group during the study, indicating that GZFL did not exert significant adverse effects in mice at its effective dose.
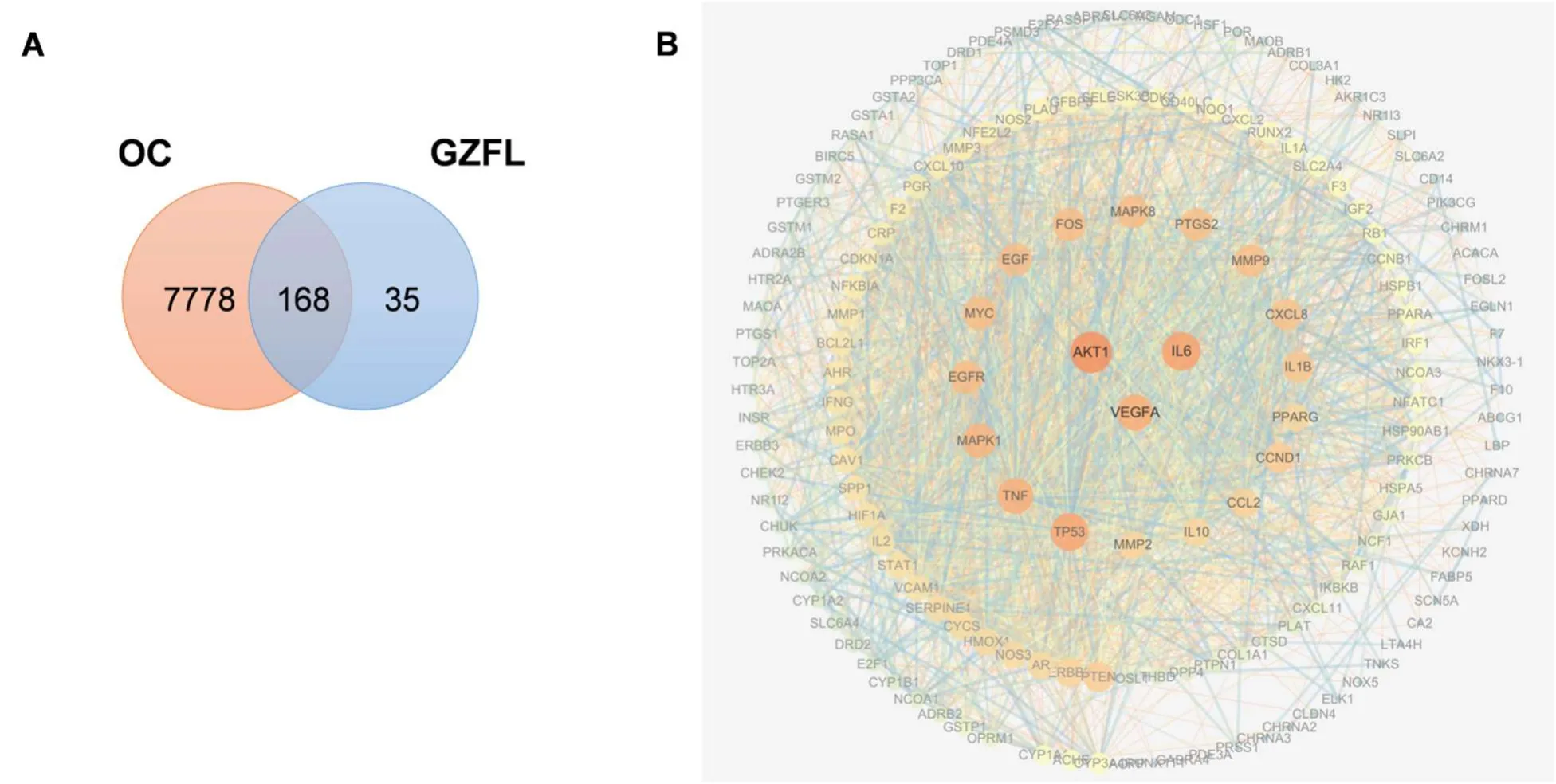
Figure S1.Network pharmacology analysis of GZFL on ovarian cancer.

Figure S2.Generation of STAT3 KO ovarian cancer cell lines using CRISPR-Cas9 genome editing.
Discussion
Traditional Chinese Medicine (TCM) have accumulated valuable clinical experiences through long history development, and are receiving increasing attention worldwide.Numerous herbal formulae are effective and safe and have been used as complementary or alternative medicine in cancer treatment.Ovarian cancer belongs to the category of "accumulation", "cancer" and “blood stasis” in traditional Chinese medicine.Chinese clinicians have confirmed that OC patients are accompanied by qi stagnation, blood stasis and phlegm coagulation in different degrees [22].Guizhi-Fuling formula is a classic prescription for promoting blood circulation and resolving stasis.In this study, we integrate computational and experimental approaches, and report GZFL’s inhibitory effect on OC progression by targeting STAT3 signaling network.
Network pharmacology is a newly developed crossdiscipline method and has been widely used to investigate comprehensive mechanisms of TCM [23].Two recent individual studies reported Guizhi-Fuling’s potential molecular mechanisms against ovarian cancer.Though utilizing different target prediction algorithms, both of work predict that GZFL’s core antitumor targets overlapped on TP53, AKT1, IL6 and VEGFA [24, 25].We confirmed these previous findings as well in this study.In addition, integrating genome-wide multi-omics with network pharmacology analysis, we provides the first evidence that GZFL is tightly associated with STAT3 signaling network.Significantly differentially expressed genes inSTAT3deletion represent genes that either transcriptionally regulated by or tightly associated withSTAT3.Our “GZFL target - OC - STAT3” gene interaction analysis represents a reliable and precise analysis of potential pharmacological mechanisms of GZFL on OC.
In this study, we identified 22 main active ingredients of GZFL.Numerous studies have confirmed several main bioactive constituents of GZFL, including gallic acid, paeoniflorin and cinnamaldehyde, possess inhibitory effect on tumor progression and metastasis [26-28].Many of these components have been reported to interfere with STAT3 signaling network.For example, a recent study demonstrated that Quercetin reduces STAT3 nuclear localization and in turn down-regulates STAT3 targeted genes (MCL1,MMP2,MMP9andVEGF) in melanoma cells [29].Paeoniflorin is reported to suppresses IL6/STAT3 pathway via SOCS3 in dendritic cells [30].Therefore, identification of precise chemical compounds in GZFL that interact with STAT3 will further guide the understanding of GZFL’s pharmacological mechanisms.
Peritoneal metastasis is a major factor governing patient survival and is the main cause of high mortality [31].We observed that GZFL inhibits OC proliferation in a STAT3 dependent manner, and significantly suppresses cell migration in anin vitrowound-healing assay.It is well known that STAT3 plays important roles in cell migration and extracellular matrix [17].Previous study demonstrated that aberrant STAT3 signaling elevates the expression of cyclin D1 and promotes tumor proliferation [32].In tumor cells, hyperactivated STAT3 promotes the expression of immunosuppressive factors such as IL-6, VEGF, and CD44 [33].Consistent with our prediction, two recent studies showed that by down-regulating IL6, VEGF, p-STAT3, CyclinD and CD44, GZFL reduces adhesion of ovarian cancer cells with surrounding matrix and inhibits tumor metastasis and invasion [34, 35].Altogether, we hypophysis that GZFL suppress ovarian cancer cell migration by targeting STAT3 networks and the precise interactions worth further assessment.
Supplementary materials
Supplementary data are available online atTMR Modern Herbal Medicine.
 TMR Modern Herbal Medicine2021年4期
TMR Modern Herbal Medicine2021年4期
- TMR Modern Herbal Medicine的其它文章
- Research progress on mechanism of Chinese material medica in preventing and treating insulin resistance and type 2 diabetes mellitus
- lncreased macrophage inflammation response in pancreatic cancer patients with a diagnosis of Shi-Re
- Experimental study on the effect of Huangqi and Danshen ultramicro gel on vascular healing of wound in rats based on network pharmacology
- The clinical efficacy of traditional Chinese medicine in the auxiliary treatment of grade 1 hypertension: A systematic review and metaanalysis
- Network pharmacology-based analysis on bioactive compounds and mechanisms in Yiqifumai formula in the treatment of heart failure
- Application of Chinese Herbal Medicine in the lnhibition of Salmonella and its virulence
