湖南多花黄精根腐病病原菌的分离与鉴定
梁忠厚 李静纳

摘要:【目的】明確多花黄精根腐病在湖南地区的发生规律及其病原菌种类,为湖南多花黄精根腐病的综合防治及GAP(中药材生产质量管理规范)种植提供科学依据。【方法】以湖南地区具有典型根腐病症状的多花黄精植株为材料,采用传统的平板分离法对其病原菌进行分离纯化,通过rDNA-ITS序列分析及形态学观测鉴定病原菌的种类和分类地位,并依据Koch?s法则验证病原菌的致病性。【结果】湖南地区的多花黄精根腐病以生长旺盛期(5—7月)发生最严重,平均发病率为12%,严重时可达20%;发病部位主要是多花黄精根部。从患典型根腐病多花黄精根茎的病健交界处分离获得4株分离菌株(G1~G4),菌株G1、G2和G4经PCR扩增分别获得511、573和573 bp的目的条带,菌株G3因PCR扩增条带混杂,导致测序失败。菌株G1与F. foetens(GenBank登录号NR_159865.1)的rDNA-ITS序列相似性为99.22%,菌株G2和G4与F. hostae(GenBank登录号NR_171109.1)的rDNA-ITS序列相似性为99.81%。菌株G1在PDA培养基上生长较快,培养7 d后其菌落直径为6.7 cm,气生菌丝致密,呈毡状,白色至粉紫色;菌株G2和G4在PDA培养基上培养7 d后其菌落直径为6.2 cm,气生菌丝呈放射状,白色至淡黄色。将菌株G1、G2和G4分别接种至健康多花黄精植株的根茎上,接种10 d后均表现出强致病性,呈典型的根腐病发病症状。其中,菌株G1接种10 d后多花黄精根茎开始变褐腐烂,且伴有明显的腥味;菌株G2和G4接种10 d后多花黄精根茎初期变褐色,后期病斑腐烂部分的根茎出现凹陷。【结论】不同地理位置、栽培方式及黄精品种,均有可能导致黄精根腐病的发生情况及其病原菌存在差异,在一定程度上增加了黄精根腐病防治工作的难度,且给后期的产品加工带来安全隐患。F. foetens和F. hostae是引起湖南多花黄精根腐病的致病菌。
关键词: 多花黄精;根腐病;病原菌;Fusarium foetens;Fusarium hostae
中图分类号: S435.672 文献标志码: A 文章编号:2095-1191(2021)07-1923-08
Isolation and identification of the pathogenic fungi of Polygonatum cyrtonema Hua root rot in Hunan Province
LIANG Zhong-hou, LI Jing-na
(Undergrowth Medicinal Plant Application Technology Hunan Engineering Research Center/Landscape Department, Hunan Polytechnic of Environment and Biology, Hengyang, Hunan 421005, China)
Abstract:【Objective】Clarify the regularity of outbreak and pathogenic fungi of Polygonatum cyrtonema Hua root rot in Hunan, and provide a scientific basis for the comprehensive control of P. cyrtonema root rot and GAP(Good Agricultural Practice for Chinese Crude Drugs) planting. 【Method】Samples with typical symptoms of root rot were collected in Hunan, the pathogen of P. cyrtonema Hua root rot was isolated and purified by plate isolation and purification. The classification status of the pathogen was determined based on their morphological characteristics and rDNA-ITS sequence, and Koch?s rule was used to prove the pathogenicity of fungal strain isolated from P. cyrtonema Hua. 【Result】The P. cyrtonema Hua root rot in Hunan was the most serious at growth stage(May-July), with the average incidence of 12% and 20% when serious; the incidence site was mainly root. Four strains(G1-G4) were isolated from the healthy junction of P. cyrtonema Hua rhizome suffering from typical root rot. The isolated and purified strains G1, G2 and G4 were amplified by PCR to obtain DNA fragments with a length of 511, 573 and 573 bp bands, respectively. Due to PCR amplification bands mixed, strain G3 sequencing was failed. According to BLAST homology comparison of pathogen rDNA-ITS sequence, the sequence of strain G1 shared the high identity of 99.22% with Fusarium foetens(GenBank accession number NR_159865.1),while the sequence of strains G2 and G4 had 99.81% similarity with F. hostae(GenBank accession number NR_171109.1). Strain G1 grew rapidly on PDA medium, and after 7 d of culture, colony diameter was 6.7 cm, aerial mycelium was dense, felt shape, white to pink purple; and after 7 d of culture, colony diameters of strains G2 and G4 were 6.2 cm, aerial mycelium was radial, white to pale yellow. Isolates G1, G2 and G4 were infected onto the rhizomes of healthy P. cyrtonema Hua plants, and both were strongly pathogenic and showed typical symptoms of root rot onset. P. cyrtonema Hua root infected by G1 began to decompose and brownish 10 d after infection with obvious fishy smell; 10 d after strains G2 and G4 infection, P. cyrtonema Hua root turned brown, then the tubers in the rotten part of the diseased spot appear depressed. 【Conclusion】Different geographical locations, cultivation methods and P. cyrtonema Hua varieties can lead to differences in the occurrence of P. cyrtonema Hua root rot and its pathogens, which increases the difficulty of the prevention and control of P. cyrtonema Hua root rot to a certain extent, and brings safety risks to the processing of later products. F. foetens and F. hostae are the pathogens that cause P. cyrtonema Hua root rot in Hunan.
Key words: Polygonatum cyrtonema Hua; root rot disease; pathogen; Fusarium foetens; Fusarium hostae
Foundation item: Innovation Ability Demonstration Project of Hunan Development and Reform Commission(XFGTZ〔2021〕319);Forestry Science and Technology Project of Hunan(XLK201959,XCZHZ〔2019〕2,XCZHZ〔2020〕42)
0 引言
【研究意义】多花黄精(Polygonatum cyrtonema Hua)又名鸡头参、老虎姜,隶属于百合科(Liliaceae)黄精属(Polygonatum),为多年生草本植物,与黄精(P. sibiricum Red.)、滇黄精(P. kingianum Coll. et Hemsl.)一同收录于《中国药典》,是中药材黄精的基源植物(肖韵铮等,2020)。多花黄精的根茎中富含多种人体必需的微量元素,具有降血糖、抗肿瘤、提高人体免疫力及延缓机体衰老等功效(杨德等,2020;陈宇等,2021),在新药研制及保健品开发方面具有极高的商业价值。长期以来,多花黄精的药材供应主要以采集野生资源为主,随着市场需求量的逐渐增加,人们开始进行大规模人工栽植。湖南作为我国多花黄精的主要产区之一,目前种植面积达4667 ha(梁忠厚等,2020),但人工栽培條件下多花黄精原有生物的生态平衡被打破,病虫害日益猖獗,严重影响多花黄精的生长和产量(周先治等,2017),成为制约湖南省中药材GAP(中药材生产质量管理规范)实施及现代化产业发展的主要障碍,同时给中药材产品加工带来诸多安全隐患。根腐病是由真菌引起的植物土传病害,是制约多花黄精产量和品质的主要病害之一(韩凤等,2020)。因此,了解根腐病的发病规律及其病原菌种类,对开展多花黄精根腐病综合防治及促进黄精产业健康发展具有重要意义。【前人研究进展】尖孢镰刀菌(Fusarium oxysporum)是多种植物根腐病的主要病原菌,为典型的土传病害真菌(肖荣凤等,2020;姚健等,2020;纪莉景等,2021;唐贵婷等,2021)。已有研究表明,尖孢镰刀菌和腐皮镰刀菌(F. solani)是引起黄精属根腐病的主要病原菌(吴依婷等,2018;杨林毅等,2019;韩凤等,2020)。F. foetens是尖孢镰刀菌的姊妹群,其形态与尖孢镰刀菌相似,产孢细胞着生于分生孢子座上,为单瓶梗,较短,大型分生孢子,具隔膜;区别在于F. foetens具明显的刺激气味,而尖孢镰刀菌无气味(Schroers et al.,2004)。据报道,F. foetens是引起秋海棠枯萎和茎腐病的主要病原菌,植株感染F. foetens后,其茎基部腐烂,叶脉发黄并枯萎,萎蔫的植株表面覆盖有白色霉层(Schroers et al.,2004;Tian et al.,2010;Saurat et al.,2013)。F. hostae与芬芳镰刀菌(F. redolens)的形态类似,大型分生孢子呈镰刀状,具有3~5个分隔,区别在于F. hostae能在25和30 ℃的PDA培养基上缓慢生长,且致病性较芬芳镰刀菌强(Geiser et al.,2001)。F. hostae是引起小麦冠腐和根腐病的主要病原菌,小麦感染F. hostae后其地下根茎基部和地上冠部变褐腐烂坏死(Gebremariam et al.,2016,2018;?zer et al.,2019);芬芳镰刀菌则是引起大麦(Ye?in et al.,2017)和玉竹(曹亮等,2018)根腐病的主要病原菌,可造成植株根部腐烂,严重影响作物的产量和质量。镰刀属(Fusarium)真菌种类多,寄主范围广泛,加上病原菌的形态特征差异非常微小,且易受环境影响,因此仅根据传统分类学的形态特征(菌落、分生孢子和厚垣孢子等)及病害发生情况很难将镰刀菌属真菌鉴定到种。【本研究切入点】随着分子生物学技术的快速发展,核糖体DNA内转录间隔区(rDNA-ITS)序列已广泛应用于物种鉴定(陈玉玺等,2008;马原松等,2012;白映禄等,2019);近年来,关于镰刀菌属真菌引起作物根腐病的报道日益增多,但至今尚无基于ITS序列鉴定湖南多花黄精根腐病病原菌种类的研究报道。【拟解决的关键问题】以患根腐病的湖南多花黄精病株为研究对象,通过病原菌分离纯化、rDNA-ITS序列分析及形态学鉴定,并结合病原真菌的致病性测定,深入了解多花黄精根腐病在湖南地区的发生规律及其病原菌种类,以期为湖南多花黄精根腐病的综合防治及GAP种植提供科学依据。
1 材料与方法
1. 1 试验材料
2020年7月在湖南省境内采集患典型根腐病多花黄精病株的根茎,放入无菌采集袋中并编号,冰盒保存运回实验室进行病原菌分离纯化。健康多花黄精植株由湖南环境生物职业技术学院湖南林下经济科研示范基地提供,为无菌萌发获得的2年生植株,置于26 ℃恒温箱中培养,以保证植株处于健康状态,供致病性测定用。
1. 2 试验方法
1. 2. 1 病原菌分离纯化 在患典型根腐病多花黄精根茎的病健交界处,用无菌刀片切取1.0 cm3大小的组织块,置于75%酒精中浸泡30 s后以1% NaClO浸泡4 min,无菌水冲洗3~4次,消毒滤纸吸干水分,将组织块转移至PDA培养基上25 ℃恒温培养,待产孢纯化后将菌株移接至PDA培养基上,4 ℃保存备用(方中达,1998;Hayden et al.,2004;曹瑱艳等,2020)。
1. 2. 2 病原菌分子鉴定 将分离纯化得到的菌株接种至PDA培养基上进行扩大培养,采用改良的CTAB法(Hayden et al.,2004;刘丹等,2017)提取基因组DNA。选用真菌ITS序列的通用引物(ITS1:5'-TCCGTAGGTGAACCTGCGG-3'和ITS4:5'-TCCTC CGCTTATTGATATGC-3')进行PCR扩增。PCR扩增产物测序委托北京六合华大基因科技有限公司完成,将测序结果提交至NCBI的GenBank进行BLAST比对分析,检索出相似性较高的ITS序列,以确定分离菌株的分类地位;再利用ClustalW对目标序列与从GenBank检索到的相关真菌ITS序列进行比对(何英云等,2020),下载同源的真菌ITS序列,利用MEGA 6.1中的邻接法(Neighbor-Joining,NJ)构建系统发育进化树并分析其亲缘关系。
1. 2. 3 病原菌形态学鉴定 将分离纯化得到的菌株接种至PDA培养基上,25 ℃恒温培养7~10 d。通过光学显微镜观察菌落的形态和颜色,并挑取菌丝置于体视显微镜下观察记录分生孢子的形态、大小及有无隔膜等。参照王拱辰等(1996)的《常见镰刀菌鉴定指南》、Leslie和Summerell(2006)的分类系统进行分离菌株的形态学鉴定。
1. 2. 4 致病性测定 参照蘸根法,将2年生的多花黄精健康植株置于1×106个/mL的病原菌孢子悬液中浸泡30 min,移入有湿润滤纸保湿的500 mL密封塑料杯中,以接种无菌水的多花黄精健康植株为对照,分别置于26 ℃培养箱中培养,每处理10株。接种10 d后,记录多花黄精植株的感染发病情况。依据Koch?s法则,在植株病斑处再次分离纯化病原菌,并与原接种的分离菌株进行比较。
2 结果与分析
2. 1 多花黄精根腐病的发生及田间病害症状
湖南地区的多花黄精根腐病以生长旺盛期(5—7月)发生最严重,平均发病率为12%,严重时可达20%。发病部位主要是多花黄精根部。发病初期,多花黄精植株的地上叶片无明显症状(图1-A),地下根部有水渍状褐色坏死斑(图1-B),具腥味,湿度大时根茎表面可见白色霉层(图1-C),腐烂病株极易从土中拔起;后期严重时根内部腐烂,仅残留纤维状维管束,病部呈褐色或红褐色,地上叶片由外向里逐渐变黄,最后整株枯死(图1-D)。
2. 2 病原菌rDNA-ITS序列分析结果
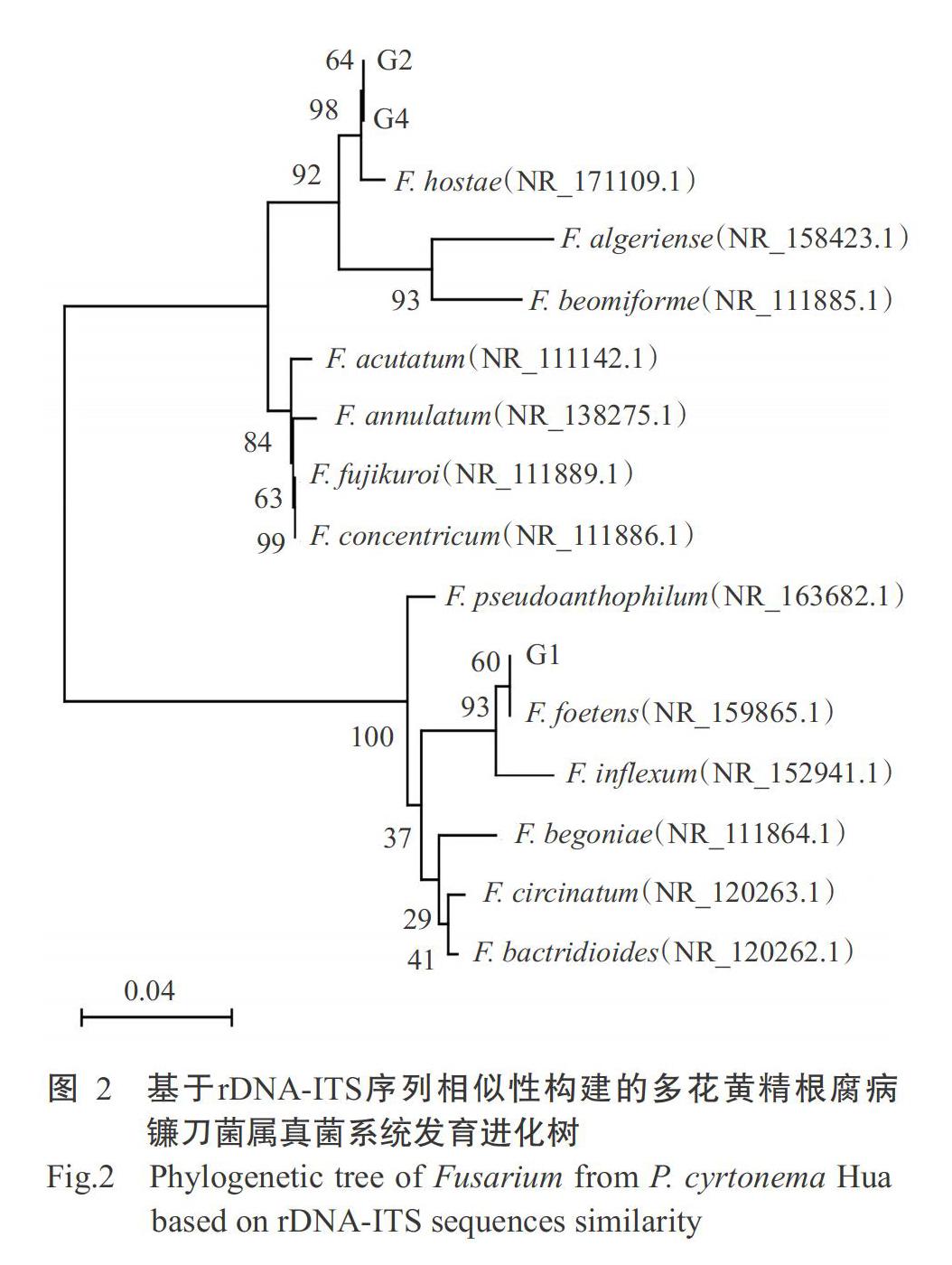
从患典型根腐病多花黄精根茎的病健交界处取样进行病原菌分离纯化,结果获得4株不同的分离菌株(G1~G4)。以ITS1和ITS4为引物对分离获得的菌株进行PCR扩增,经1.0%琼脂糖凝胶电泳检测,结果从菌株G1、G2和G4分别扩增获得511、573和573 bp的目的条带,菌株G3因PCR扩增条带混杂,导致测序失败。将菌株G1、G2和G4的rDNA-ITS序列提交至NCBI的GenBank进行BLAST比对分析,结果表明,菌株G1与F. foetens(GenBank登录号NR_159865.1)的相似性为99.22%,菌株G2和G4与F. hostae(GenBank登录号NR_171109.1)的相似性为99.81%。基于rDNA-ITS序列相似性,利用MEGA 6.1中的邻接法(NJ)构建系统发育进化树,结果(图2)也显示,菌株G1与F. foetens聚为一支,菌株G2和G4与F. hostae聚为一支,进一步证实从湖南多花黄精根腐病植株分离获得的菌株G1为F. foetens,菌株G2和G4为F. hostae。
2. 3 病原菌的形态特征
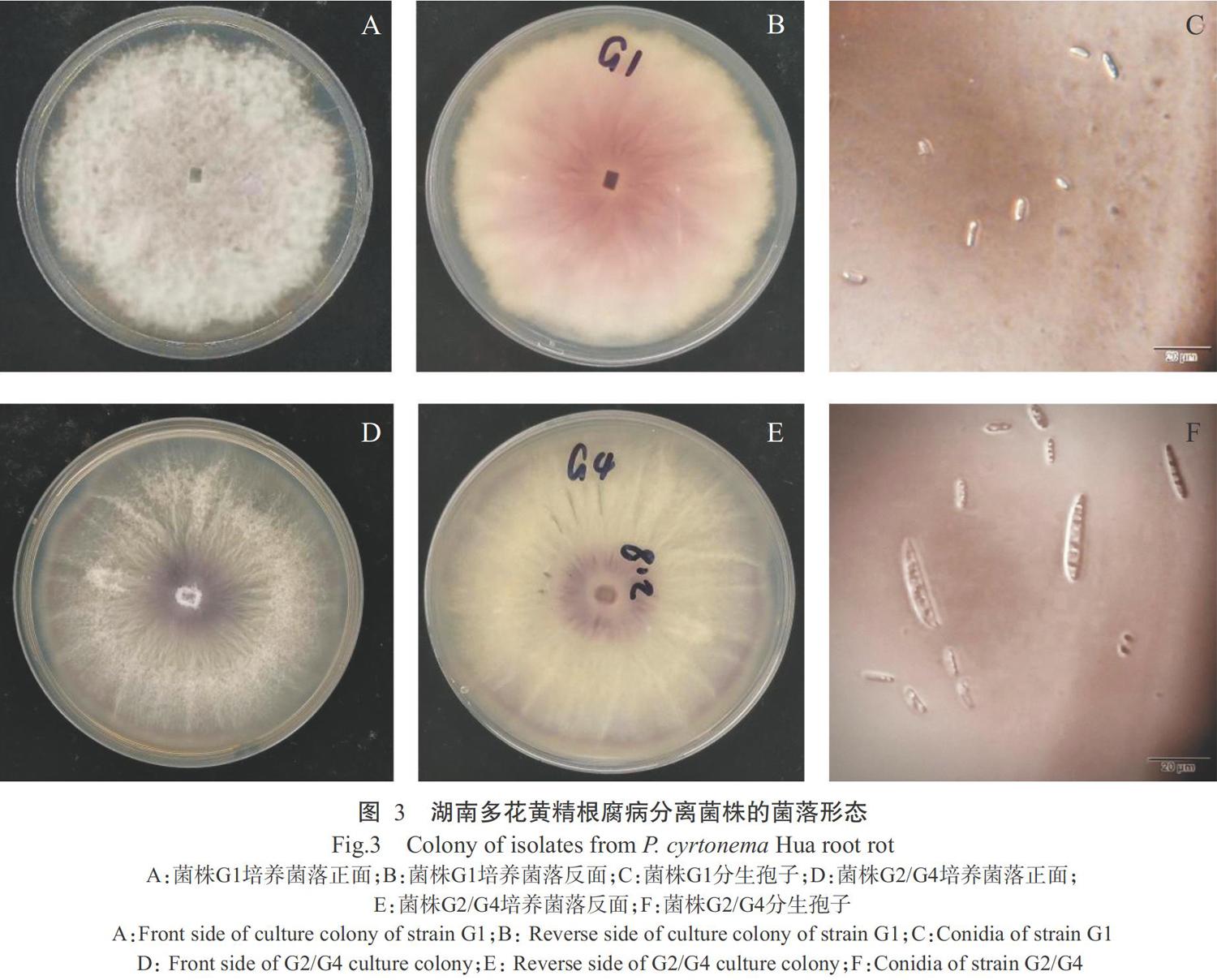
分子鉴定为F. foetens的菌株G1:在PDA培养基上生长较快,培养7 d后其菌落直径为6.7 cm,气生菌丝致密,呈毡状(图3-A),白色至粉紫色(图3-B)。大型分生孢子呈柱形,基部较钝,具隔膜,大小为20.0~33.0 μm×3.2~4.3 μm;小型分生孢子呈卵形或肾形,单胞,单生或串生,大小为2.1~7.8 μm×1.4~3.6 μm(图3-C)。
分子鉴定为F. hostae的菌株G2/G4:在PDA培养基上培养7 d后其菌落直径为6.2 cm,气生菌丝呈放射状(图3-D),白色至淡黄色(图3-E)。大型分生孢子呈镰刀状,有3~5个分隔,端部略弯曲,大小为25.0~38.0 μm×3.3~4.5 μm;小型分生孢子呈卵圆形,部分有轻微弯曲,大小为7.0~10.0 μm×3.0~4.0 μm(图3-F)。
2. 4 病原菌致病性测定结果
通过蘸根法将菌株G1、G2和G4分别接种至健康多花黄精植株的根茎上,接种10 d后观察发现所有多花黄精植株均表现出强致病性,呈典型的根腐病发病症状。其中,菌株G1接种10 d后,多花黄精根茎开始变褐腐烂(图4-A),且伴有明显的腥味;菌株G2和G4接种10 d后,多花黄精根茎初期变褐色,后期病斑腐烂部分的根茎出现凹陷(图4-B和图4-C);而接种无菌水的多花黄精植株未表现出任何症状(图4-D)。依据Koch?s法则,对接种后发病的多花黄精植株取样再次进行病原菌分离纯化,结果均分离得到与原接种菌株性状相同的菌株。由此确定,F. foetens和F. hostae是湖南多花黄精根腐病的致病菌。
3 讨论
本研究选取湖南地区具有典型根腐病症状的多花黄精植株,采用传统的平板分离法对其病原菌进行分离纯化,通过rDNA-ITS序列分析、形态学鉴定及致病性测定,明确引起湖南多花黄精根腐病的致病菌为F. foetens和F. hostae,是继尖孢镰刀菌(F. oxysporum)和腐皮镰刀菌(F. solani)引起多花黄精根腐病(韩凤等,2020)的2种新病原菌。多花黄精植株感染尖孢镰刀菌和腐皮镰刀菌后,其地上部叶片由下而上逐渐枯萎,地下根茎出现水渍状褐色坏死斑,根茎表面可见白色菌丝。F. foetens和F. hostae引起的多花黄精根腐病症状与这2种病原菌引起的病症相似,其区别在于多花黄精感染F. foetens后,除了根茎出现明显的褐色病斑外,还伴有明显的腥味。尖孢镰刀菌和腐皮镰刀菌还能引起黄精和滇黄精发生根腐病。黄精感染尖孢镰刀菌后,导致其根茎腐烂而绝收(吴依婷等,2018);滇黄精感染腐皮镰刀菌初期,其根茎上出现不规则的黑色病斑,随着病斑逐渐扩大,最终导致整个根茎腐烂,茎杆枯萎(杨林毅等,2019)。可见,不同地理位置、栽培方式及黄精品种,均有可能导致黄精根腐病的发生情况及其病原菌存在差异,在一定程度上增加了黄精根腐病防治工作的难度,且给后期的产品加工带来安全隐患。湖南多花黄精根腐病的发生是否与当地气候及其品种有关,还有待进一步研究确认。
本研究通过rDNA-ITS序列分析并构建系统发育进化树,结果证实分离纯化获得的多花黄精根腐病病原菌株G1为F. foetens,菌株G2和G4为F. hostae。基于rDNA-ITS序列分析的分子鉴定已广泛应用于物种鉴定(陈玉玺等,2008;马原松等,2012;白映禄等,2019),但也存在一定局限性(Crouch et al.,2009),单一的rDNA-ITS序列可能会导致病原菌鉴定结果出现偏差(Moriwaki et al.,2002)。因此,下一步需对采集的多花黄精根腐病病原菌进行多基因序列联合鉴定,以准确鉴定病原菌的种类。致病性测定结果表明,F. foetens和F. hostae均为多花黄精根腐病的致病菌。韩凤等(2020)研究表明,多花黄精根腐病可能是多種致病菌复合侵染的结果。湖南地区的多花黄精根腐病以生长旺盛期(5—7月)发生最严重,此时正值南方梅雨季节,高温高湿的自然条件非常有利于病原菌的流行传播。吴依婷等(2018)从黄精根茎中分离出对黄精根腐病具有明显抑制作用的内生细菌——枯草芽孢杆菌(Bacillus subtilis);迟惠荣(2019)从多花黄精根茎中分离获得11株内生细菌,经鉴定分别隶属于3个属,其中芽孢杆菌属(Bacillus)为多花黄精内生细菌的优势菌属。本研究从患典型根腐病的湖南多花黄精根茎中仅分离获得2种病原菌(F. foetens和F. hostae),并未发现其他病原菌。湖南多花黄精根腐病是否存在其他病原菌,且这些病原菌是否存在协同侵染,以及多花黄精根茎中是否含有抵御多花黄精根腐病的内生菌,均有待进一步探究。
4 结论
不同地理位置、栽培方式及黄精品种,均有可能导致黄精根腐病的发生情况及其病原菌存在差异,在一定程度上增加了黄精根腐病防治工作的难度,且给后期的产品加工带来安全隐患。F. foetens和F. hostae是引起湖南多花黄精根腐病的致病菌。
參考文献:
白映禄,薛玉丽,常芸,尚素琴. 2019. 基于rDNA ITS1序列的甘肃省叶螨属Tetranychus和全爪螨属Panonychus种群的系统关系[J]. 甘肃农业大学学报,54(5):128-134. doi:10. 13432/j.cnki.jgsau.2019.05.016. [Bai Y L,Xue Y L,Chang Y,Shang S Q. 2019. Phylogenetic relationship between spider mite genus Tetranychus and Panonychus based on ribosomal DNA ITS1 sequence in Gansu Pro-vince[J]. Journal of Gansu Agricultural University,54(5):128-134.]
曹亮,徐瑞,谢进,朱校奇,周佳民,宋荣,黄艳宁,彭斯文. 2018. 玉竹根腐病防治杀菌剂筛选[J]. 中药材,41(5):1031-1034. doi:10.13863/j.issn1001-4454.2018.05.004. [Cao L,Xu R,Xie J,Zhu X Q,Zhou J M,Song R,Huang Y N,Peng S W. 2018. Screening of fungicides for controlling root rot of Polygonatum odoratum[J]. Journal of Chinese Medicinal Materials,41(5):1031-1034.]
曹瑱艳,杨怡华,申屠旭萍,俞晓平. 2020. 浙江省铁皮石斛根腐病病原真菌的鉴定[J]. 植物保护学报,47(1):178-186. doi:10.13802/j.cnki.zwbhxb.2020.2019040. [Cao Z Y,Yang Y H,Shentu X P,Yu X P. 2020. Identification of the pathogenic fungi of root rot of traditional medicinal Dendrobium officinale in Zhejiang Province[J]. Acta Phytophylacica Sinica,47(1):178-186.]
陈宇,周芸湄,李丹,彭成. 2021. 黄精的现代药理作用研究进展[J]. 中药材,44(1):240-244. doi:10.13863/j.issn1001-4454. 2021.01.046. [Chen Y,Zhou Y M,Li D,Peng C. 2021. Research Progress on modern pharmacological action of Polygonatum cyrtonema Hua[J]. Journal of Chinese Medicinal Materials,44(1):240-244.]
陈玉玺,张利平,吕志堂. 2008. 10株镰刀菌rDNA内转录间隔区(ITS)序列分析[J]. 安徽农业科学,36(12):4886-4887. doi:10.13989/j.cnki.0517-6611.2008.12.018. [Chen Y X,Zhang L P,Lü Z T. 2008. Analysis of the internal transcribed spacer(ITS) sequences in rDNA of 10 strains of Fusarium spp.[J]. Journal of Anhui Agricultural Scien-ces,36(12):4886-4887.]
迟惠荣. 2019. 多花黄精叶枯病病原菌鉴定及贝莱斯芽胞杆菌防病促生效果研究[D]. 杭州:浙江大学. [Chi H R. 2019. The study on the pathogen identification of the leaf blight of Polygonatum cyrtonema Hua and the plant growth promotion and biocontrol effect of Bacillus veleaensis[D]. Hangzhou:Zhejiang University.]
方中达. 1998. 植病研究法[M]. 第3版. 北京:中国农业出版社. [Fang Z D. 1998. Plant disease research method[M]. The 3rd Edition. Beijing:China Agriculture Publishing House.]
韩凤,李巧玲,韩如刚,章文伟,余中莲,林茂祥,杨娟. 2020. 渝产多花黄精根腐病病原菌的分离与鉴定[J]. 分子植物育种,18(11):3693-3698. doi:10.13271/j.mpb.018.003693. [Han F,Li Q L,Han R G,Zhang W W,Yu Z L,Lin M X,Yang J. 2020. Isolation and identification of pathogen of Polygonatum cyrtonema Hua root rot disease in Chong-qing[J]. Molecular Plant Breeding,18(11):3693-3698.]
何英云,陈兴全,张永科,张祖兵,徐万寿,黄美智,张传利,龙继明,马丽宣,田洋. 2020. 云南西双版纳辣木果腐病病原鉴定[J]. 南方农业学报,51(9):2160-2166. doi:10.3969/ j.issn.2095-1191.2020.09.014. [He Y Y,Chen X Q,Zhang Y K,Zhang Z B,Xu W S,Huang M Z,Zhang C L,Long J M,Ma L X,Tian Y. 2020. Seed pod rot pathogen identification of Moringa oleifera in Xishuangbanna,Yunnan[J]. Journal of Southern Agriculture,51(9):2160-2166.]
纪莉景,肖颖,李耀发,贾海民,王亚娇,吴玉星,孔令晓. 2021. 引起河北省保定市白术根腐病的病原镰刀菌种类鉴定[J]. 植物病理学报,51(2):282-286. doi:10.13926/j.cnki.apps.000354. [Ji L J,Xiao Y,Li Y F,Jia H M,Wang Y J,Wu Y X,Kong L X. 2021. Identification of pathogenic Fusarium species causing Atractylodes macrocaphal root rot in Baoding,Hebei Province[J]. Acta Phytopathologica Sinica,51(2):282-286.]
梁忠厚,李有清,邹青,刘慧娟. 2020. 湖南省黄精产业发展现状与对策[J]. 湖南生态科学学报,7(3):35-42. doi:10. 3969/j. issn.2095-7300.2020.03.006. [Liang Z H,Li Y Q,Zou Q,Liu H J. 2020. Development status and countermeasures of Polygonati rhizome industry in Hunan Pro-vince[J]. Journal of Hunan Ecological Science,7(3):35-42.]
劉丹,李焕宇,付婷婷,张云,吕天佑,李远,李敏权,徐秉良. 2017. 基于正交试验设计优化真菌DNA提取的CTAB法[J]. 甘肃农业大学学报,52(2):139-145. doi:10.13432/j.cnki.jgsau.2017.02.022. [Liu D,Li H Y,Fu T T,Zhang Y,Lü T Y,Li Y,Li M Q,Xu B L. 2017. Optimization of CTAB methods for extraction of fungal DNA based on the orthogonal experiment[J]. Journal of Gansu Agricultural University,52(2):139-145.]
马原松,裴冬丽,王文静,李成伟. 2012. 河南省几种白粉菌的ITS序列分析[J]. 河南农业科学,41(9):87-90. doi:10. 3969/j.issn. 1004-3268.2012.09.022. [Ma Y S,Pei D L,Wang W J,Li C W. 2012. ITS sequence analysis of se-veral powdery mildew from Henan Province[J]. Journal of Henan Agricultural Sciences,41(9):87-90.]
唐贵婷,蒋欢,苏宇,何焕然,赵冰峰,吴朝君,张勇,王旭祎. 2021. 重庆南苍术根腐病病原鉴定[J]. 植物病理学报,51(4):641-645. doi:10.13926/j.cnki.apps.000542. [Tang G T,Jiang H,Su Y,He H R,Zhao B F,Wu C J,Zhang Y,Wang X Y. 2021. Identification of pathogen causing Atractylodes lancea root rot in Chongqing municipality[J]. Acta Phytopathologica Sinica,51(4):641-645.]
王拱辰,郑重,叶琪明,章初龙. 1996. 常见镰刀菌鉴定指南[M]. 北京:中国农业科技出版社. [Wang G C,Zheng Z,Ye Q M,Zhang C L. 1996. Guidelines for identification of common Fusarium[M]. Beijing:China Agricultural Science and Technology Press.]
吴依婷,姚传威,邓波侠,张霞,李胜华,邹娟,付明. 2018. 黄精根腐病分离菌及其拮抗内生细菌的鉴定[J]. 浙江农业学报,30(12):2087-2093. doi:10.3969/j.issn.1004-1524. 2018.12.14. [Wu Y T,Yao C W,Deng B X,Zhang X,Li S H,Zou J,Fu M. 2018. Identification of isolated fungus from root rot of Polygonatum sibiricum and its antagonistic endophytic bacteria[J]. Acta Agriculturae Zhejiangensis,30(12):2087-2093.]
肖榮凤,陈燕萍,陈梅春,阮传清,朱育菁,刘波. 2020. 太子参根腐病病原菌的鉴定及防治药剂筛选[J]. 植物保护学报,47(6):1333-1342. doi:10.13802/j.cnki.zwbhxb.2020. 2019211. [Xiao R F,Chen Y P,Chen M C,Ruan C Q,Zhu Y J,Liu B. 2020. Pathogen identification of root rot of Pseudostellaria heterophylla plant and fungicide scree-ning for its efficient control[J]. Acta Phytophylacica Sinica,47(6):1333-1342.]
肖韵铮,韩世明,秦昭,李春奇. 2020. 滇黄精转录组测序及类黄酮合成相关基因的分析[J]. 河南农业大学学报,54(6):931-940. doi:10.16445/j.cnki.1000-2340.2020.06.004. [Xiao Y Z,Han S M,Qin Z,Li C Q. 2020. Analysis of transcriptome sequencing and related genes of flavonoids biosynthesis from Polygonatum kingianum[J]. Journal of Henan Agricultural University,54(6):931-940.]
杨德,薛淑静,卢琪,陈晓春,李露. 2020. 黄精药理作用研究进展及产品开发[J]. 湖北农业科学,59(21):5-9. doi:10. 14088/j. cnki.issn0439-8114.2020.21.001. [Yang D,Xue S J,Lu Q,Chen X C,Li L. 2020. Research progress and product development of Polygonatum sibiricum[J]. Hubei Agricultural Sciences,59(21):5-9.]
杨林毅,陈泽历,赖清玉,陈潞,孙雁,唐朝辉,赵明富,文国松. 2019. 滇黄精腐皮镰刀菌的分离鉴定[J]. 湖北农业科学,58(3):65-67. doi:10.14088/j.cnki.issn0439-8114. 2019.03.018. [Yang L Y,Chen Z L,Lai Q Y,Chen L,Sun Y,Tang Z H,Zhao M F,Wen G S. 2019. Isolation and identification of Fusarium solani from Polygonatum kingianum[J]. Hubei Agricultural Sciences,58(3):65-67.]
姚健,刘玉珍,李建华,王京,孙晓伟,李肖宇,法鹏飞,危月辉. 2020. 许昌烟草根腐病的分子鉴定及致病性分析[J]. 江西农业学报,32(3):99-103. doi:10.19386/j.cnki.jxnyxb.2020.03.18. [Yao J,Liu Y Z,Li J H,Wang J,Sun X W,Li X Y,Fa P F,Wei Y H. 2020. Molecular identification and pathogenicity analysis of tobacco fusarium root rot disease in Xuchang[J]. Acta Agriculturae Jiangxi,32(3):99-103.]
周先治,苏海兰,陈阳,高晖,唐建阳,单寄坪. 2017. 多花黄精主要病害发生规律调查[J]. 福建农业科技,(10):25-27. doi:10.13651/j.cnki.fjnykj.2017.10.008. [Zhou X Z,Su H L,Chen Y,Gao H,Tang J Y,Shan J P. 2017. Occurrence regularity of major diseases of Polygonatum sibiricum[J]. Fujian Agricultural Science and Technology,(10):25-27.]
Crouch J A,Clarke B B,Hillman B I. 2009. What is the value of ITS sequence data in Colletotrichum systematics and species diagnosis? A case study using the falcate-spored graminicolous Colletotrichum group[J]. Mycologia,101(5):648-656. doi:10.3852/08-231.
Gebremariam E S,Karakaya A,Orakci G E,Dababat A A,Sharma-Poudyal D,Paulitz T C. 2016. First report of Fusarium hostaes causing crown rot on wheat (Triticum spp.) in Turkey[J]. Plant Disease,99(9):15042007092 4008. doi:10.1094/PDIS-01-15-0128-PDN.
Gebremariam E S,Sharma-Poudyal D,Paulitz T C,Orakci G E,Karakaya A,Dababat A A. 2018. Identity and pathogenicity of Fusarium species associated with crown rot on wheat(Triticum spp.) in Turkey[J]. European Journal of Plant Pathology,150:387-399. doi:10.1007/s10658-017-1285-7.
Geiser D M,Juba J H,Wang B,Jeffers S N. 2001. Fusarium hostae sp. nov.,a relative of F. redolens with a Gibberella teleomorph[J]. Mycologia,93(4):670-678. doi:10.1080/00275514.2001.12063198.
Hayden K J,Rizzo D,Tse J,Garbelotto M. 2004. Detection and quantification of Phytophthora ramorum from California forests using a real-time polymerase chain reaction assay[J]. Phytopathology,94(10):1075-1083. doi:10.1094/ PHYTO.2004.94.10. 1075.
Leslie J F,Summerell B A. 2006. The Fusarium laboratory manual[M]. Iowa:Blackwell Publishing.
Moriwaki J,Tsukiboshi T,Sato T. 2002. Grouping of Colletotrichum species in Japan based on rDNA sequences[J]. Journal of General Plant Pathology,69:424. doi:10.1007/s10327-003-0092-5.
Saurat C,Fourrier C,Wilson V,Casset C,Ioos R. 2013. First report of begonia elatior wilt disease caused by Fusarium foetens in France[J]. Plant Disease,97(1):144. doi:10. 1094/PDIS-07-12-0659-PDN.
Schroers H J,Baayen R P,Meffert J P,de Gruyter J,Hooftman M,O'Donnell K. 2004. Fusarium foetens,a new species pathogenic to begonia elatior hybrids(Begonia×Hiemalis) and the sister taxon of the Fusarium oxysporum species complex[J]. Mycologia,96(2):393-406.
Tian X L,Dixon M,Zheng Y. 2010. First report of Hiemalis begonias wilt disease caused by Fusarium foetens in Ca-nada[J]. Plant Disease,94(10):1261. doi:10.1094/PDIS-06-10-0402.
?zer G,?mren M,Bayraktar H,Paulitz T C,Muminjanov H,Dababat A A. 2019. First report of Fusarium hostae cau-sing crown rot on wheat in Azerbaijan[J]. Plant Disease,103(12):3278. doi:10.1094/PDIS-05-19-1035-PDN.
Ye?in N Z,?nal F,Tekiner N,Dolar F S. 2017. First report of Fusarium redolens causing root and crown rot of ba-rley(Hordeum vulgare) in Turkey[J]. The Journal of Tur-kish Phytopathology,46(3):101-105.
(責任编辑 兰宗宝)

