镍钴锰酸锂电极材料改性研究进展*
王贵欣,李晨玥,黄心怡,杨柳依,韦 双,郝虎明,敬娜娜
镍钴锰酸锂电极材料改性研究进展*
王贵欣†,李晨玥,黄心怡,杨柳依,韦 双,郝虎明,敬娜娜
(四川大学 化学工程学院,成都 610065)
镍钴锰酸锂(LiNiCoMn1−−O2,NCM)是一种具有高使用容量的三元正极材料,但存在元素混排、相变、热稳定性差、微裂纹等缺陷,导致电池出现容量衰减和安全问题,影响其广泛应用。针对目前三元材料存在的问题,归纳总结了特殊结构与形貌、掺杂、替代、包覆、修饰、复合等改性方法的最新研究进展,探讨了不同方法对材料电化学性能、循环稳定性和安全性的影响,分析比较了不同方法的优缺点。结合材料、电化学、热和力等多学科知识及本课题组利用负热膨胀材料对能源材料改性的研究成果,提出了原位利用电极循环过程中的热调控形变和界面行为改善材料性能的新思路,为解决电池的热失控和应力等安全问题提供参考。
镍钴锰酸锂;改性;热和形变调控;电化学性能;安全
0 引 言
能量密度和安全性对锂离子电池的应用至关重要,受电极材料和电解液的影响较大。常见的LiFePO4、LiCoO2、LiNiCoM1−−O2(NCM; M = Mn, Al; 0 <,< 1)等正极材料中,三元材料LiNiCoMn1−−O2因可利用容量高和能量密度大而备受关注[1-5]。NCM兼有LiNiO2、LiCoO2、LiMnO2等层状材料的优点,目前朝着高镍、低钴方向发展,市场上的常见类型及不同成分之间的相互影响见图1。NCM具有六边-NaFeO2型层状岩盐结构,空间群为[1-5],过渡金属离子占据3b空位形成二维交替层,与O2−共同组成MO6八面体结构;O2−占据八面体的6c位形成立方密堆阵列;Li+占据剩下的3a空位,位于八面体层之间,在层间可逆地嵌入和脱出。高电压的氧化还原对和紧凑的结构使NCM具有较高的工作电压和比能量[6-8]。NCM材料中,Co为+3价,Mn为+4价,Ni是主要电化学活性物质,为+2、+3价,其含量决定材料的容量,含量增加可提高容量[1-5]。但是,Ni在循环过程中会产生Ni2+,Ni2+与Li+半径接近,会引发Li-Ni混排[5],导致循环性能下降。Co、Ni是同周期相邻元素,Co的加入不改变三元材料层状结构,且Co3+半径(0.055 nm)比Ni3+的(0.056 nm)小,因此Co的加入使材料晶格参数和减少,进而减少各向异性和阳离子混排[9],降低晶胞体积和阻抗,提高电导率[10],但是Co价格比Ni高,会增加成本。Mn是非电化学活性物质,在充电过程中保持+4价不变,主要起稳定结构的作用,抑制层状结构到尖晶石结构的相变[11-13],但Mn含量过高易出现尖晶石相,破坏材料的层状结构[14-16]。Ni、Co和Mn三种元素之间存在协同作用,各有优缺点且相互协调,形成具有优良性能的NCM正极材料。
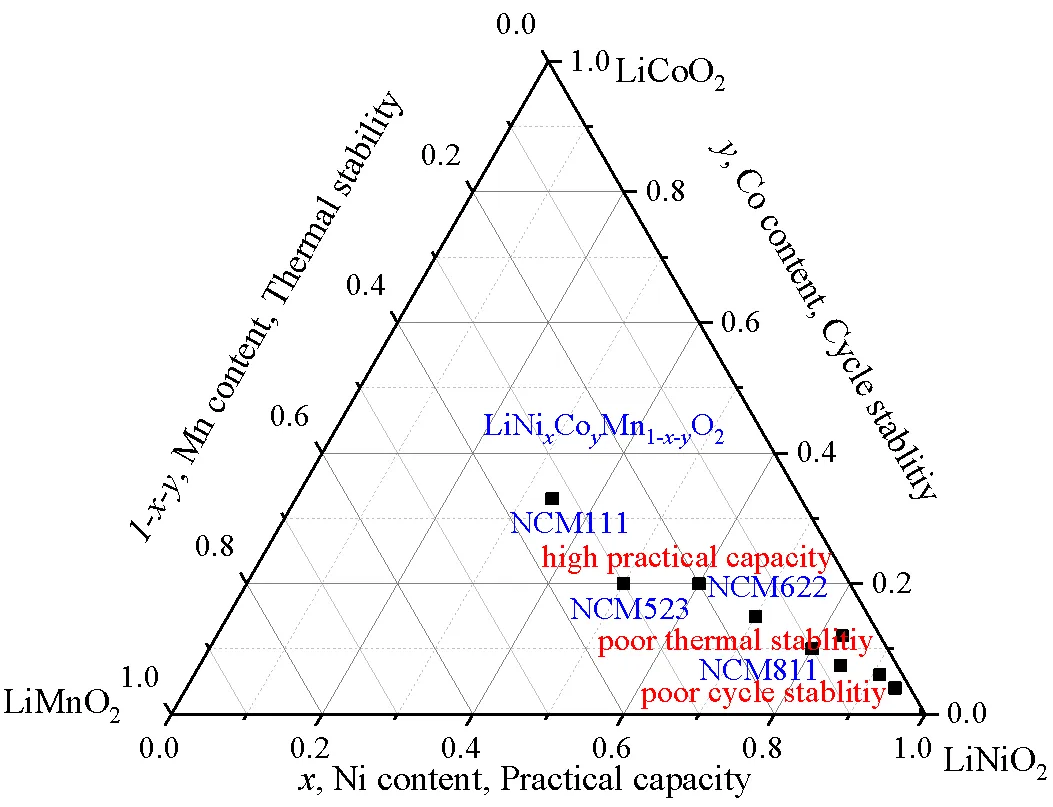
图1 NCM中不同成分间的关系及相互影响示意图
然而,NCM仍存在LiNiO2、LiCoO2、LiMnO2的固有缺陷,在使用过程中存在元素混排、相变、热稳定性差、微裂纹等导致的库仑效率低、容量衰减、高温适应性和安全差等问题,阻碍了其广泛应用,研究者多采用改性提高性能。本文综述了NCM材料改性的最新研究进展,结合多学科知识和实验室工作,展望了NCM的发展方向。全文导图如图2所示,一种改性措施可提高材料不同方面的性能。

图2 NCM改性示意图
1 NCM材料存在的问题
1.1 阳离子混排
NCM的Ni2+未完全氧化成Ni3+,部分Ni3+的位置被Ni2+占据,为了维持电荷平衡,Ni2+占据部分Li+的位置。虽然Ni2+(0.068 nm)与Li+(0.076 nm)的半径小,Ni2+占据Li+的位置时,层间距减小,但是容易产生锂镍混排[5]。另外,充电过程中Ni2+被氧化成Ni3+和Ni4+,失去电子后的离子半径进一步缩小,晶体空间会产生局部塌陷[16],阻碍Li+迁移,增大阻抗,降低循环稳定性。NCM充电时Li+脱出形成Li+空位,放电时Li+却不能完全嵌入,而Ni2+占据Li+空位进一步阻碍了Li+迁移,增加不可逆容量,降低首次库仑效率[17]。
1.2 不可逆相变

1.3 微裂纹
Li+脱嵌使NCM的体积膨胀和收缩达3.9%[22],内部晶界附近出现晶格膨胀和收缩裂纹,生成晶间微裂纹,造成初级粒子分离、电接触损失,被视为阴极容量衰减的一个主要原因[21-23]。另外,NCM单晶晶胞极化过程中相邻层之间存在平面滑移的不均匀运动,与循环晶体和多晶NMC颗粒沿晶间边界开裂完全不同,平面滑移导致材料侧面出现宽大的晶体台阶,从4.2 V充电至4.5 V时,出现更多的平面滑移(约83 nm),产生平面滑移的微裂纹[24]。微裂纹网络为电解液提供了通道与场所,渗透的电解液加速了微裂纹表面的降解,如图3所示[25]。

图3 NCM材料的微裂纹容量衰减机制[25]
1.4 热失控
热失控是目前研究较多的电池安全问题,高电压、高温下,电池极化程度增加,不可逆热增大,诱发热失控[26]。另外,NCM存在H2到H3的相变,引起有害晶格收缩或膨胀[27],微裂纹暴露在电解液中,造成电极材料粉化,并伴随着晶格氧的逸出,晶胞参数的改变和裂纹产生导致更多内部颗粒暴露出来[28],继而又与电解液作用,降低材料热稳定性,释放更多的热量和氧,而且相变温度越低,热分解温度越低[29],因此电解液氧化也是电池热失控的一个重要原因[30]。
2 NCM电极材料的改性
针对上述问题,目前提高NCM材料性能的主要方法是改性,通常包括特殊结构或形貌、掺杂或替代、修饰、复合等方法。
2.1 特殊结构或形貌改性
为解决NCM在循环过程中Li+脱嵌动力学差、释放氧、热失控、胀气、燃烧、爆炸等问题,可设计具有特殊结构(如核壳结构、浓度梯度等)和形貌(如球形、纤维等)的NCM材料[1-5,31-33]。核壳结构以镍为核,以锰为壳,利用非电化学活性物质的稳定性抑制相变与混排,既提高容量,又稳定结构。核壳结构NCM811经过500次循环后容量保持率约86%(比未改性的材料提高了29%),而且能有效降低锂镍混排,减少镍与电解液间的副反应,抑制副反应的示意图如图4[33]。核壳结构虽然可以改善NCM的稳定性,但是核壳间存在明显界面缺陷,高温煅烧后的核体积变化可达9% ~ 10%,而壳体积变化仅2% ~ 3%,体积变化的差异导致核壳结构的核层与壳层空隙逐渐变大,最终产生内应力大、界面分离、过渡金属组分突变等问题,无法保证长期循环稳定性;此外,核壳结构的空隙使Li+和电子的扩散通道消失,Li+传输过程受阻,材料的电导率降低[31-32]。

图4 核壳结构抑制NCM811副反应示意图[33]
为了弥补核壳结构的缺陷并抑制容量衰减,具有浓度梯度(以Ni为核、元素含量沿半径由内向外逐渐变化)的NCM引起了研究者们的重视。在从内到外的梯度层中,Ni含量逐渐减少,Co和Mn含量逐渐增加,避免了核壳结构成分不匹配、体积收缩比差异等问题,改善了电化学性能。全浓度梯度(full concentration gradient, FCG)的NCM颗粒示意图如图5所示[34],材料层状结构完整,从核到壳,Mn、Co含量逐渐升高,Ni含量逐渐降低。准浓度梯度的多壳结构NCM循环500次后也能保持80%的容量[35]。与非梯度NCM相比,FCG材料的Li+扩散和电子转移容易,放电容量高,循环后的容量保持率高,锂镍混排低,热分解温度高,具有优异的结构稳定性、倍率性能和热安全性能。通过微结构调控,发展氧稳定晶面,可以抑制微裂纹和氧的析出[31],有利于提高NCM的性能。

图5 从内到外镍浓度降低、锰浓度增加的全浓度梯度锂过渡金属氧化物颗粒示意图[34]
特殊结构或形貌不需要外加其他物质,能一定程度上提高NCM的循环稳定性和热稳定性,但是存在结构、浓度等精准调控难的问题。
2.2 掺杂或替代改性
用其他元素离子对NCM进行体相掺杂(包括阳离子掺杂、阴离子掺杂和多种离子共掺杂)[36-45]或取代Ni2+和Li+[16,46-47],阻碍Ni2+进入锂位,提高材料电子电导率,降低混排(离子混排程度用(003)/(104)和/值评价,当(003)/(104)> 1.2、/> 4.9时,混排程度低[3]),稳定晶体结构。NCM的常见掺杂阳离子元素有Mg[36,38-39]、Al[36-37]、Ti[36]、Ta[36]、W[40]、Zr[41]、Nb[45]等。Mg2+半径(0.072 nm)大于Ni2+的半径(0.068 nm),增大NCM的/值,易形成有序层状结构,循环350次后仍有81%的容量保持率,高于改性前的67%,提高了循环稳定性[39]。1%的W能提高不同NCM的循环稳定性,经过1 000次循环,比容量衰减非常小[40];梯度掺杂Zr4+的NCM材料,在不降低容量的情况下,提高了倍率和循环性能,外层掺杂的Zr4+会同时占据镍位和锂位,在表面形成快离子导体,过渡层的Zr4+因Zr-O之间较强的化学键,提高了晶体结构的稳定性,在脱/嵌锂过程中起支撑作用,降低阳离子混排[41]。
相对于阳离子掺杂的多样性,阴离子掺杂目前主要以Si[36]、B[42]、F[43-44]元素为主。B掺杂能改变Li[Ni0.90Co0.05Mn0.05]O2(NCM90)的表面能和次级粒子内部的微观结构(图6),缓解NCM90在锂化/脱锂过程中的应变,提高循环性能[42]。当F−取代O2−时,临近F−的O2−将得到更少的负电荷,临近F−的Ni2+将获得更多的正电荷,有利于提高Li+输运的阳离子混合值,增大反位浓度,适量的反电势可以提高NCM的动力学特性和颗粒界面稳定性[43]。掺杂1% F−的NCM具有最佳倍率性能,抑制了容量快速衰减,提高了循环性能,在3.0 ~ 4.5 V循环300次后,容量保持率达81.1%,高于原先的35.1%[44]。
当单一元素掺杂不能达到预期效果时,人们尝试多元素共掺杂或共替代的方法。Al/Mg共取代的NCM材料中,Al3+以固溶体形式溶于过渡金属层,Mg2+占据Li+位使得Ni2+在锂层的含量大幅降低,稳定了晶体结构,降低了混排程度[46];Fe/Al元素共取代改性将NCM的首次放电容量提高了22%[47]; F/Mg元素共掺杂的NCM首次库仑效率高达98.6%,100次循环后容量保持率为96.3%,循环性能优异[48]。
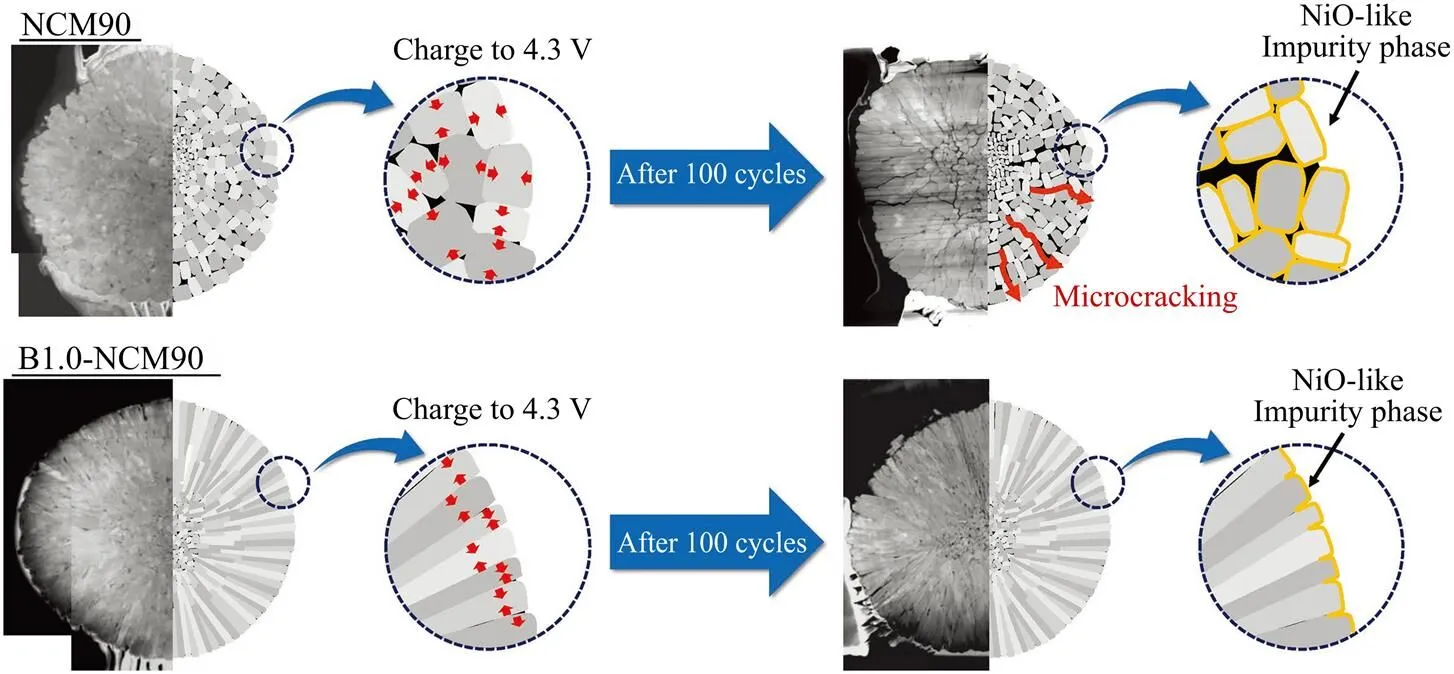
图6 硼掺杂对NCM90结构的影响[42]
掺杂能有效提高NCM的循环性能,但由于部分掺杂元素不具备电化学活性、元素分布难调控,电池首充比容量随着掺杂量的增加会有所下降,因此根据不同元素调配适合NCM材料的掺入量、掺杂方式及元素分布是研究的重点。
2.3 修饰改性
作为抑制NCM不可逆相变和表面副反应的一种有效方法,将其他物质部分或全部覆盖在NCM表面,阻止电解液与材料接触及微裂纹产生,目前应用较广[49-50]。修饰改性方法包括包覆、涂层、接枝等,常用氧化物(如ZrO2、WO3、SiO2等)、金属(如Ag、Zr等)、磷酸盐(如AlPO4、LiFePO4等)、导电聚合物和氟化物等作为改性材料,需要依据不同改性材料的特点有针对性地选择,而且修饰改性方式也会影响NCM的性能。Zr包覆在NCM表面,部分Zr在材料表面形成均匀膜,部分Zr分布在晶格内部,内部与表面的双重作用稳定晶格结构,极大提高放电容量,100次循环后的容量保持率为92%,高于未包覆时的75%[51];ZrO2原位修饰的NCM在100次循环后达到82.5%的容量保持率,高于改性前的52.4%[52];钨酸铵修饰NCM811,在材料表面形成钨酸锂,消耗产生的气体,900次循环后容量仅下降20%,提高了循环性能[53];表面修饰AlPO4能有效改善NCM622与电解液的界面行为,抑制副反应,提高热稳定性与安全性能,表面改性机理见图7[54];膨胀石墨中嵌入NCM622前驱体,焙烧后在石墨层间形成NCM622,将石墨层撑开,形成石墨烯/NCM622/石墨烯的多层结构,限制NCM622的颗粒生长,减少电极材料与电解液的接触,提高了导电性、高温性能和循环稳定性[55]。
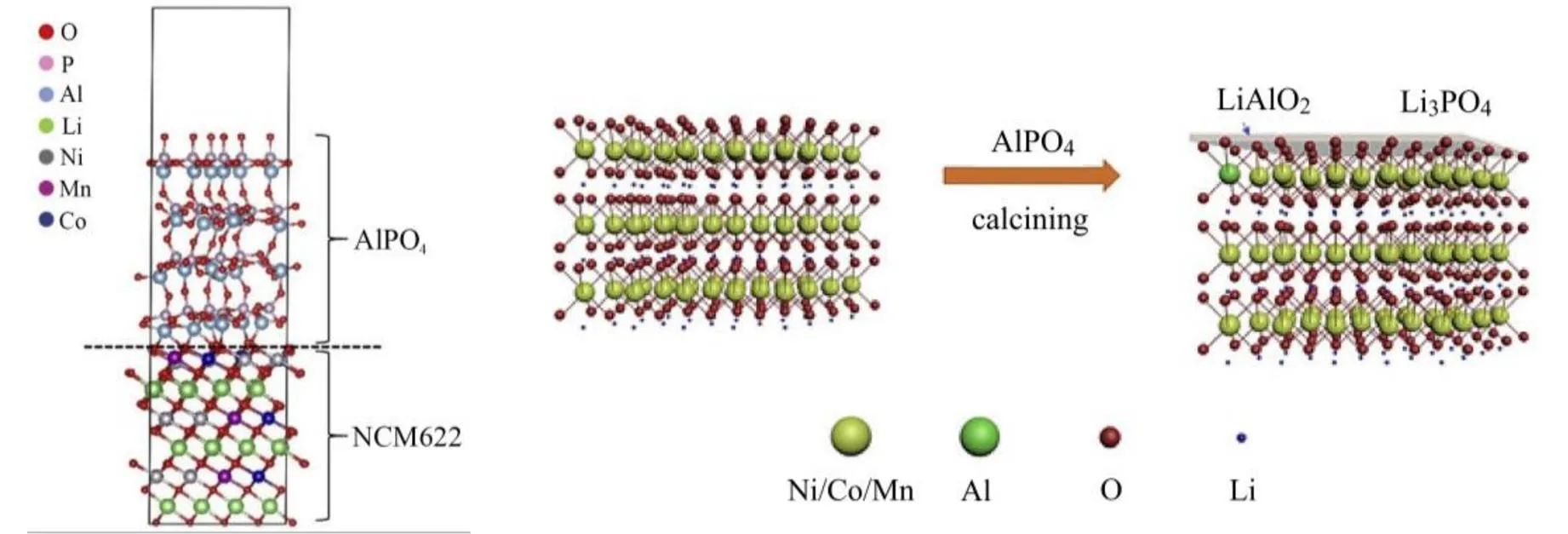
图7 NCM622与AlPO4间的界面松弛模型和AlPO4对NCM622的可能促进机制[54]
针对NCM循环过程中放热严重及热失控的问题,本课题组开展了利用负热膨胀(negative thermal expansion, NTE)材料对NCM改性的研究,取得了一些原创性成果[54-61]。NTE材料原位吸收电极循环过程中的热而体积缩小,为电极活性材料的体积膨胀提供空间,调和内部阴(负热膨胀材料)阳(能源材料),减少电极整体体积变化,降低热失控风险、热引起的结构破坏、内应力、形变、副反应、燃烧、爆炸等问题,改善电极与电解液的界面行为,提高材料的热稳定性,通过调控热、形变和界面,提高倍率特性、高温性能、循环稳定性和安全性,工作原理如图8。与常见材料的“热胀冷缩”特性相反,负热膨胀材料具有“热缩冷胀”特性,在受热时体积收缩,在一定温度范围内平均线膨胀系数为负值[64],有望与其他材料复合制备低膨胀或零膨胀材料,应用前景广阔。众多NTE材料中,ZrW2O8在0.3 ~ 1 050 K的热膨胀系数为负值[65],覆盖多数电极材料的工作温度范围[66],明显提升电极材料的性能[54-61]。ZrW2O8含量极大影响电极性能,过少不足以吸收电极释放的热并减少形变,过多则因自身的非电化学活性而阻碍Li+和电子的转移,有可能引起负的形变,5%的ZrW2O8能有效提高NCM622[56,58,62]和NCM811[61]在大电流和高温下的容量及其保持率,降低极化和电荷转移阻抗及形变,改善循环性能,提高热稳定性与安全。
图8 NTE材料对能源材料改性机理图[57]
修饰可以阻止电解液与材料的直接接触,改善电极与电解液间的界面行为,减少副反应,利用NTE材料还可以同时调控电极循环过程中的热和形变,减少热引起的危害和内应力诱发的裂纹,保持完整结构,提高材料热稳定性和循环稳定性,但是包覆层的成分、厚度、分布及修饰方式等需要重点关注。
2.4 复合改性
目前单一策略不能有效解决NCM的所有问题,需要组合多种方法或材料进行复合改性,但是与NCM不一定形成复合物,目前很多报道没有严格区分修饰和复合[44,67-71]。耦合掺杂与特殊结构方面,用Mg2+掺杂核壳结构CS-NCM622,减少阳离子混排,循环100次后的容量保持率为86%[67];将表面包覆ZrO2薄膜的NCM材料进行700℃热处理,部分Zr4+从包覆涂层中渗入NCM内部,离子掺杂和包覆的协同效应使NCM在150次循环后容量保持率高达98.5%[68];用Co氧化包覆与Ti掺杂对NCM进行改性,材料表面产生了由Co和Li组成的尖晶石结构涂层,有效保护了表面,提高了界面稳定性,Ti抑制了阳离子混排,增加层间间距,强化锂离子扩散,抑制不可逆相变的发生,显著提高电化学性能[69];结构重构与化学演化可以降低容量衰减和阻抗[70];Mg2+体相掺杂和聚吡咯包覆可以提高倍率性能和循环寿命[71]。NCM复合改性可以利用多种方法或材料的优点,多方面提高材料性能,是一种有前景的改性措施,但是组成分布与精准调控难。
3 不同改性方法的比较
综上可知,改性方法各有千秋,既有无需外加元素的素化内部改性,也有添加不同元素的外部改性。内部改性通过调控结构或形貌实现,外部改性从单一元素、单一维度发展到多元素、多维度的复合方式,其优缺点如表1所示。

表1 NCM材料改性方法比较
4 结 语
从混排、相变、微裂纹和热等方面剖析了NCM存在的问题,从组成、结构、形貌、表面、界面等方面综述了最新改性进展,归纳分析了不同改性方法的优缺点,指出了今后的发展方向。不同改性方法各有优缺点,能不同程度提高NCM材料的性能,有些方法可同时促进多方面的性能:特殊结构或形貌无需其他元素,推动能源材料素化,主要通过结构或形貌调控改变元素或粒径分布;掺杂侧重外加不同阳或阴离子,聚焦用其他元素替换相关元素,主要改变晶格结构;修饰侧重表面涂层、包覆、接枝等,主要改善电极表面和电极与电解液之间的界面行为;复合兼有不同方法的优势,弥补单一方法的不足,发展前景广阔。
虽然不同改性方法能不同程度地提高NCM性能,但是在使用过程中仍存在一定的容量衰减和安全问题。素化措施无法满足NCM的应用要求,需要结合其他的措施提高综合性能。今后需要进一步针对NCM内应力、热失控和容量衰减的问题,从结构、形变、内应力、产热、表面、界面、副产物、导电性、衰减机理和修复等方面出发,对热和形变进行有效调控,降低热和形变的不利影响,也可以开发新型低膨胀或零膨胀能源材料消除热和形变的影响。负热膨胀材料能有效调控NCM的结构、热、形变和界面行为等,在大电流、高温及长循环过程等放热较多的条件下优势更加明显。利用负热膨胀、相变、热缩等材料,调控结构、热、形变和界面行为,降低热危害和内应力,将会是镍钴锰酸锂今后的一个重要改性方向。
[1] WANG X X, DING Y L, DENG Y P, Chen, et al. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: promises and challenges[J]. Advanced Energy Materials 2020, 10: 1903864. DOI: 10.1002/aenm. 201903864.
[2] CHOI J U, VORONINA N, SUN Y K, et al. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion batteries: yesterday, today, and tomorrow[J]. Advanced Energy Materials 2020, 10, 2002027. DOI: 10.1002/aenm.202002027.
[3] SUN H H, RYU H H, KIM U H, et al. Beyond doping and coating: prospective strategies for stable high-capacitylayered Ni-rich cathodes[J]. ACS energy letters, 2020, 5(4):1136-1146. DOI: 10.1021/ACSENERGYLETT.0C00191.
[4] LEE W, MUHAMMAD S, SERGEY C, et al. Advances in the cathode materials for lithium rechargeable batteries[J], Angewandte Chemie International Edition, 2020, 59: 2578-2605. DOI: 10.1002/anie.201902359.
[5] XIAO Y G, LIU T C, LIU J J, et al. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2cathode materials, Nano Energy, 2018, 49: 77-85. DOI: 10.1016/j.nanoen.2018.04.020.
[6] NEGI R S, CELIK E, PAN R J, et al. Insights into the positive effect of post-annealing on the electrochemical performance of Al2O3-coated Ni-rich NCM cathodes for lithium-ion batteries[J]. ACS Applied Energy Materials, 2021, 4(4): 3369-3380. DOI: 10.1021/acsaem.0c03135.
[7] ZHAO W G, ZOU L F, JIA H P, et al. Optimized Al doping improves both interphase stability and bulk structural integrity of Ni-Rich NMC cathode materials[J]. ACS applied energy materials, 2020, 3(4): 3369-3377. DOI: 10.1021/acsaem.9b02372.
[8] LI R, ZHANG P, HUANG J, et al. Enhanced high voltage performance of LiNi0.5Mn0.3Co0.2O2cathodethe synergistic effect of LiPO2F2and FEC in fluorinated electrolyte for lithium-ion batteries[J]. RSC advances, 2021, 11(14): 7886-7895. DOI: 10.1039/D0RA10280F.
[9] YUE P, WANG Z X, PENG W J, et al. Preparation and electrochemical properties of submicron LiNi0.6Co0.2Mn0.2O2as cathode material for lithium ion batteries[J]. Scripta materialia, 2011, 65(12): 1077-1080. DOI: 10.1016/j. scriptamat.2011.09.020.
[10] KOSOVA N V, DEVYATKINA E T, KAICHEV V V. Optimization of Ni2+/Ni3+ratio in layered Li(Ni,Mn,Co)O2cathodes for better electrochemistry[J]. Journal of power sources, 2007, 174(2): 965-969. DOI: 10.1016/j.jpowsour. 2007.06.051.
[11] SUN H H, CHOI W, LEE J K, et al. Control of electrochemical properties of nickel-rich layered cathode materials for lithium ion batteries by variation of the manganese to cobalt ratio[J]. Journal of power sources, 2015, 275: 877-883. DOI: 10.1016/j.jpowsour.2014.11.075.
[12] XIAO Z, LIU P, SONG L, et al. The correlation between structure and thermal properties of nickel-rich ternary cathode materials: a review[J]. Ionics, 2021, 27: 3207-3217. DOI: 10.1007/s11581-021-04103-z.
[13] PAN C C, ZHU Y R, YANG Y C, et al. Influences of transition metal on structural and electrochemical properties of Li[NiCoMn]O2(0.6≤≤0.8) cathode materials for lithium-ion batteries[J]. Transactions of nonferrous metals society of China, 2016, 26(5): 1396-1402. DOI: 10.1016/S1003-6326(16)64244-9.
[14] LI T, YUAN X Z, ZHANG L, et al. Degradation mechanisms and mitigation strategies of nickel-rich NMC- based lithium-ion batteries[J]. Electrochemical Energy Reviews, 2020, 3: 43-80. DOI: 10.1007/s41918-019-00053-3.
[15] KWON S N, SONG J H, MUMM D R. Effects of cathode fabrication conditions and cycling on the electrochemical performance of LiNiO2synthesized by combustion and calcination[J]. Ceramics international, 2011, 37(5): 1543-1548. DOI: 10.1016/j.ceramint.2011.01.028.
[16] CHEN H, DAWSON J A, HARDING J H. Effects of cationic substitution on structural defects in layered cathode materials LiNiO2[J]. Journal of materials chemistry A, 2014, 2(21): 7988-7996. DOI: 10.1039/c4ta00637b.
[17] SHI J L, ZHANG J N, HE M, et al. Mitigating voltage decay of Li-rich cathode material via increasing Ni content for lithium-ion batteries[J]. ACS applied materials & interfaces, 2016, 8(31): 20138-20146. DOI: 10.1021/acsami.6b06733.
[18] ZHENG J M, KAN W H, MANTHIRAM A. Role of Mn content on the electrochemical properties of nickel-rich layered LiNi(0.8-x)Co(0.1)Mn(0.1+x)O (0.0 ≤≤ 0.08) cathodes for lithium-ion batteries[J]. ACS applied materials & interfaces, 2015, 7(12): 6926-6934. DOI: 10.1021/acsami.5b00788.
[19] BAK S M, NAM K W, CHANG W N Y N, et al. Correlating structural changes and gas evolution during the thermal decomposition of charged LiNi0.8Co0.15Al0.05O2cathode materials[J]. Chemistry materials, 2013, 25(3): 337-351. DOI: 10.1021/CM303096E.
[20] MAKIMURA Y, ZHENG S J, IKUHARA Y, et al. Microstructural observation of LiNi0.8Co0.15Al0.05O2after charge and discharge by scanning transmission electron microscopy[J]. Journal of the electrochemical society, 2012, 159(7): A1070-A1073. DOI: 10.1149/2.073207jes.
[21] SUN H H, MANTHIRAM A. Impact of microcrack generation and surface degradation on a nickel-rich layeredLi[Ni0.9Co0.05Mn0.05]O2cathode for lithium-ion batteries[J]. Chemistry of materials, 2017, 29(19): 8486-8493. DOI: 10.1021/ACS.CHEMMATER.7B03268.
[22] YIN S, DENG W, CHEN J, et al. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries[J], Nano Energy, 2021, 83: 105854, DOI: 10.1016/j.nanoen.2021.105854.
[23] LI J, ZHOU Z, LUO Z, et al. Microcrack generation and modification of Ni-rich cathodes for Li-ion batteries: A review[J]. Sustainable Materials and Technologies, 2021, 29: e00305. DOI: 10.1016/j.susmat.2021.e00305.
[24] BI Y J, TAO J H, WU Y Q, et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode[J]. Science, 2020, 370(6522): 1313-1317. DOI: 10.1126/science.abc3167.
[25] RYU H H, PARK K J, YOON C S, et al.Capacity fading of Ni-Rich Li[NiCoMn1-x-y]O2(0.6<≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation?[J]. Chemistry of Material, 2018, 30: 1155-1163. DOI: org/10.1021/acs.chemmater.7b05269.
[26] YOU L Z, TANG J T, WU Q, et al. LiFePO4-coated LiNi0.6Co0.2Mn0.2O2for lithium-ion batteries with enhanced cycling performance at elevated temperatures and high voltages[J]. RSC advances, 2020, 10(62): 37916-37922. DOI: 10.1039/D0RA07764J.
[27] YOON C S, JUN D W, MYUNG S T, et al. Structural stability of LiNiO2cycled above 4.2V[J]. ACS energy letters, 2017, 2(5): 1150-1155. DOI: 10.1021/acsenergylett. 7B00304.
[28] WOO S U, YOON C S, AMINE K, et al. Significant improvement of electrochemical performance of AlF3-coated Li[Ni0.8Co0.1Mn0.1]O2cathode materials[J]. Journal of the Electrochemical Society, 2007, 154(11): A1005. DOI: 10.1149/1.2776160.
[29] LIU H S, ZHANG Z R, GONG Z L, et al. Origin of deterioration for LiNiO2cathode material during storage in air[J]. Electrochemical and solid-state letters, 2004, 7(7): A190. DOI: 10.1149/1.1738471.
[30] MAHESH K C, SURESH G S, BHATTACHARYYA A J, et al. Synthesis and electrochemical characterization of LiNi0.8Co0.2O2as cathode material for aqueous rechargeablelithium batteries[J]. Journal of the Electrochemical Society, 2012, 159(5): A571-A578. DOI: 10.1149/2.075205jes.
[31] JUNG C H, KIM D H, EUM D, et al. New insight into microstructure engineering of Ni-rich layered oxide cathode for high performance lithium ion batteries[J]. Advanced Functional Materials, 2021, 31: 2010095. DOI: 10.1002/adfm.202010095.
[32] ZAHNOW J, BERNGES T, WAGNER A, et al. Impedance analysis of NCM cathode materials: electronic and ionic partial conductivities and the influence of microstructure[J]. ACS Applied Energy Materials, 2021, 4(2): 1335-1345. DOI: 10.1021/acsaem.0c02606.
[33] WU K, LI Q, DANG R B, et al. A novel synthesis strategy to improve cycle stability of LiNi0.8Mn0.1Co0.1O2at high cut-off voltages through core-shell structuring[J]. Nano Research, 2019, 12(10): 2460-2467. DOI: 10.1007/ s12274-019-2469-6.
[34] SUN Y K, CHEN Z, NOH H J, et al. Nanostructured high-energy cathode materials for advanced lithium batteries[J]. Nature Materials, 2012, 11: 942-947. DOI: 10.1038/nmat3435.
[35] LIANG M, SUN Y M, SONG D W, et al. Superior electrochemical performance of quasi-concentration- gradient LiNi0.8Co0.15Al0.05O2cathode material synthesized with multi-shell precursor and new aluminum source[J]. Electrochimica Acta, 2019, 300: 426-436. DOI: 10.1016/ j.electacta.2019.01.125.
[36] WEIGEL T, SCHIPPER F, ERICKSON E M, et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2cathode materials doped by various cations[J]. ACS Energy Lett. 2019, 4, 2, 508-516. DOI: 10.1021/acsenergylett. 8b02302.
[37] DUAN J G, HU G R, CAO Y B, et al. Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2via Al gradient doping[J]. Journal of power sources, 2016, 326: 322-330. DOI: 10.1016/j. jpowsour.2016.07.008.
[38] ZHANG Y D, LI H, LIU J X, et al. LiNi0.90Co0.07Mg0.03O2cathode materials with Mg-concentration gradient for rechargeable lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(36): 20958-20964. DOI: 10.1039/ C9TA02803J.
[39] LIU X L, WANG S, WANG L, et al. Stabilizing the high-voltage cycle performance of LiNi0.8Co0.1Mn0.1O2cathode material by Mg doping[J]. Journal of Power Sources,2019, 438: 227017. DOI: 10.1016/j.jpowsour.2019.227017.
[40] KIM U H, JUN D W, PARK K J, et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries[J]. Energy & Environmental Science, 2018, 11(5): 1271-1279. DOI: 10.1039/C8EE00227D.
[41] HE T, LU Y, SU Y F, et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of Nickel-rich cathode materials for lithium-ion batteries[J]. ChemSusChem, 2018, 11(10): 1639-1648. DOI: 10.1002/cssc.201702451.
[42] PARK K J, JUNG H G, KUO L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2through microstructure modification by boron doping for Li-Ion batteries[J]. Advanced Energy Materials, 2018, 8(25): 1801202. DOI: 10.1002/aenm.201801202.
[43] LI C L, KAN W H, XIE H L, et al. Inducing favorable cation antisite by doping halogen in Ni-rich layered cathode with ultrahigh stability[J]. Advanced science, 2019, 6(4): 1801406. DOI: 10.1002/advs.201801406.
[44] LIU K, ZHANG Q Q, DAI S, et al. Synergistic effect of F-doping and LiF coating on improving the high-voltage cycling stability and rate capacity of LiNi0.5Co0.2Mn0.3O2cathode materials for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(40): 34153-34162. DOI: 10.1021/acsami.8b10016.
[45] CHU M, HUANG Z, ZHANG T, et al. Enhancing the electrochemical performance and structural stability of Ni-Rich layered cathode materials via dual-site doping. ACS Appl. Mater. Interfaces 2021, 13, 17, 19950-19958. DOI: 10.1021/acsami.1c00755.
[46] WOO S W, MYUNG S T, BANG H, et al. Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2positive electrode materials by multiple metal (Al, Mg) substitution[J]. Electrochimica acta, 2009, 54(15): 3851- 3856. DOI: 10.1016/j.electacta.2009.01.048.
[47] ELMOFID W, IVANOV S, KONKIN A, et al. A high performance layered transition metal oxide cathode material obtained by simultaneous aluminum and iron cationic substitution[J]. Journal of power sources, 2014, 268: 414-422. DOI: 10.1016/j.jpowsour.2014.06.048.
[48] CHEN Q C, YAN G J, LUO L M, et al. Enhanced cycling stability of Mg-F co-modified LiNi0.6Co0.2Mn0.2-yMgO2-zFfor lithium-ion batteries[J]. Transactions of nonferrous metals society of China, 2018, 28(7): 1397-1403. DOI: 10.1016/S1003-6326(18)64778-8.
[49] SU Y, CHEN G, CHEN L, et al. Advances and prospects of surface modification on Nickel-Rich materials for lithium-ion batteries[J]. Chinese Journal of Chemistry, 2020, 38: 1817-1831. DOI: 10.1002/cjoc.202000385
[50] WEBER D, TRIPKOVIĆĐ, KRETSCHMER K, et al. Surface modification strategies for improving the cycling performance of Ni-rich cathode materials[J]. European Journal of Inorganic Chemistry, 2020, 3117-3130. DOI: 10.1002/ejic.202000408.
[51] LI X, ZHANG K J, WANG M S, et al. Dual functions of zirconium modification on improving the electrochemicalperformance of Ni-rich LiNi0.8Co0.1Mn0.1O2[J]. Sustainable energy & fuels, 2018, 2(2): 413-421. DOI: 10.1039/ C7SE00513J.
[52] YAO L, LIANG F Q, JIN J, et al. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2cathode via in-situ ZrO2coating for high energy density lithium ion batteries[J]. Chemical engineering journal, 2020, 389: 124403. DOI: 10.1016/j.cej.2020.124403.
[53] BECKER D, BÖRNER M, NÖLLE R, et al. Surface modification of Ni-rich LiNi0.8Co0.1Mn0.1O2cathode material by tungsten oxide coating for improved electrochemical performance in lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(20): 18404-18414. DOI: 10.1021/acsami.9b02889.
[54] TANG W J, PENG Z F, SHI Y L, et al. Enhanced cyclability and safety performance of LiNi0.6Co0.2Mn0.2O2at elevated temperature by AlPO4modification[J]. Journal of Alloys and Compounds, 2019, 810: 151834. DOI: 10.1016/j.jallcom.2019.151834.
[55] PENG Z F, TANG W J, PENG Y J, et al. Enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2by expanded graphite[J]. Energy technology, 2019, 7(11): 1900614. DOI: 10.1002/ente.201900614.
[56] 彭振锋. 膨胀石墨和钨酸锆对LiNi0.6Co0.2Mn0.2O2的改性研究[D]. 四川大学硕士研究生论文, 2020.
[57] 魏云. 三种无机盐对LiNi0.8Co0.15Al0.05O2电池电化学性能与安全的影响研究[D]. 四川大学硕士研究生论文, 2020.
[58] 徐升. 三种负热膨胀材料对单晶LiNi0.6Co0.2Mn0.2O2电池的电化学性能影响研究[D]. 四川大学硕士研究生论文, 2021.
[59] 敬娜娜. 四种负热膨胀材料对Si基锂离子电池电化学性能和安全的影响研究[D]. 四川大学硕士研究生论文, 2021.
[60] JING N N, XU S, WANG Z Q, et al. Enhanced electrochemical performance and safety of silicon by a negative thermal expansion material of ZrW2O8[J]. ACS Applied Materials & Interfaces, 2021, 13(26): 30468- 30478. DOI: 10.1021/ACSAMI.1C01088.
[61] HAO H M, XU S, JING N N, et al. Negative thermal expansion material: promising for improving electrochemical performance and safety of lithium-ion batteries[J]. The Journal of Physical Chemistry Letters, 2021, 12(26): 6134-6142. DOI: 10.1021/ACS.JPCLETT.1C01332.
[62] XU S, JING N N, HAO H M, et al. Enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2by a negative-thermal-expansion material at elevated temperature[J]. Energy Technology, 2021, 9(8): 2100183. DOI: 10.1002/ente.202100183.
[63] WANG M Y, WEI Y, XU S, et al. Simultaneously adjusting deformation and heat using a negative thermal expansion material to enhance electrochemical performanceand safety of lithium-ion batteries[J]. Chemical EngineeringJournal, 2021, 425: 131434. DOI: 10.1016/j.cej.2021.131434.
[64] CHEN J, HU L, DENG J X, et al. Negative thermal expansion in functional materials: controllable thermal expansion by chemical modifications[J]. Chemical Society Reviews, 2015, 44(11): 3522-3567. DOI: 10.1039/ C4CS00461B.
[65] MARY T A, EVANS J S O, VOGT T, et al. Negative thermal expansion from 0.3 to 1050 kelvin in ZrW2O8[J]. Science, 1996, 272(5258): 90-92. DOI: 10.1126/science. 272.5258.90.
[66] MA S, JIANG M D, TAO P, et al. Temperature effect and thermal impact in lithium-ion batteries: a review[J]. Progress in Natural Science: Materials International, 2018, 28(6): 653-666. DOI: 10.1016/j.pnsc.2018.11.002.
[67] ZHANG N S, AI L, MAO L P, et al. Understanding the role of Mg-doped on core-shell structured layered oxide LiNi0.6Co0.2Mn0.2O2[J]. Electrochimica acta, 2019, 319: 822-831. DOI: 10.1016/j.electacta.2019.07.048.
[68] BAO W D, QIAN G N, ZHAO L Q, et al. Simultaneous enhancement of interfacial stability and kinetics of single-crystal LiNi0.6Mn0.2Co0.2O2through optimized surface coating and doping[J]. Nano Letters, 2020, 20(12): 8832-8840. DOI: 10.1021/acs.nanolett.0c03778.
[69] ZHANG X Y, QIU Y G, CHENG F Y, et al. Realization of a high-voltage and high-rate nickel-rich NCM cathode material for LiBs by Co and Ti dual modification[J]. ACS Applied Materials & Interfaces, 2021, 13(15): 17707-17716. DOI: 10.1021/acsami.1c03195.
[70] LIN F, MARKUS I M, NORDLUND D, et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries[J]. Nature communications, 2014, 5(1): 3529. DOI: 10.1038/ ncomms4529.
[71] YU H, ZHU H, YANG Z, et al. Bulk Mg-doping and surface polypyrrole-coating enable high-rate and long-lifefor Ni-rich layered cathodes, Chemical Engineering Journal, 2021, 412: 128625. DOI: 10.1016/j.cej.2021.128625.
Advances in the Improvement of Lithium Nickel Manganese Cobalt Oxide Electrode Materials
WANG Gui-xin, LI Chen-yue, HUANG Xin-yi, YANG Liu-yi, WEI Shuang, HAO Hu-ming, JING Na-na
(School of Chemical Engineering, Sichuan University, Chengdu 610065, China)
LiNiCoMn1−−O2(NCM) is a ternary cathode material with high practical capacity. However, its wide application is affected by capacity fade and safety problems caused by factors such as mixing elements, phase transition, poor thermal stability, microcracks, etc. Considering current problems of the ternary materials, recent progress in improving methods were reviewed, including special structure and morphology, doping, substituting, coating, modifying, and combining. Effects of different methods on the electrochemical performance, cyclability and safety of NCM were discussed as well as their advantages and disadvantages. Combining multidisciplinary knowledge of materials, electrochemistry, heat, stress, and the improvements of energy materials using negative thermal expansion (NTE) materials in our laboratory, some novel strategies were developed by simultaneously adjusting deformation and interface behaviors viausing the generated heat during cycles, which paves the way for solving the safety problems like thermal runaway and stress of batteries.
lithium nickel manganese cobalt oxide; improvement; adjusting heat and deformation; electrochemical performance; safety
2095-560X(2021)05-0359-09
TK02;TM911.11;TQ131.11;O646.21
A
10.3969/j.issn.2095-560X.2021.05.001
王贵欣(1978-),男,博士,研究员,主要从事新能源过程与材料、装备失效与防腐防污、工业三废资源化利用的研究。
收稿日期:2021-07-10
2021-09-11
国家自然科学基金项目(21978179)
王贵欣,E-mail:guixin66@scu.edu.cn

