Effects of Magnolia denudata Leaf Litter on Growth and Photosynthesis of Microcystis aeruginosa
FAN Zhongxiu(范中秀), HUANG Kaiwen(黄凯文), WANG Xiaoxiong(汪小雄)*, XIAO Yankun(肖炎坤), GAO Jingsi(高静思), JIANG Chengchun(姜成春), SZETO Yim Tong(司徒炎棠)
1 School of Materials and Environmental Engineering, Shenzhen Polytechnic, Shenzhen 518055, China
2 School of Civil Engineering, Guangzhou University, Guangzhou 510006, China
3 Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, HKSAR 999077, China
Abstract:Magnolia denudata(M. denudata) leaf litter extract(MDLLE) was tested on the response of Microcystis aeruginosa(M. aeruginosa). The inhibition of M. aeruginosa increased from 7.6% to 49.8% at 0.4 g·L-1 dosage while it increased from 15.7% to 77.4 % at 2.0 g·L-1 dosage during 15 days of incubation. The parameter variation of relative electron transport rate(rETR), maximal quantum yield(Fv/Fm ), and electron transport rates(rERTmax) indicated that photosynthesis was an important target impaired by MDLLE, including the photosynthetic center photosystem II(PSII) of M. aeruginosa which demonstrated from the rapid light curves(RLCs). The results of Chlorophyll-a(Chl-a) fluorescence, RLCs, and fluorescein diacetate(FDA) staining assays were consistent with those of growth inhibition tests. The results demonstrated that the application of MDLLE offered great potential on cyanobacterial blooms control especially at the initial stage of development. Furthermore, several kinds of volatile chemicals had been isolated in MDLLE, and the role on suppression of growth for M. aeruginosa should be further studied separately.
Key words: allelopathy; esterase activity; flow cytometry; Magnolia denudata(M.denudata)
Introduction
The frequency of cyanobacterial harmful algal blooms(CHABs) has recently increased in freshwater ecosystems and drawn great attention for the environmental, social, and economic impacts[1-2]. Different pollutants enter into water bodies, and global warming exacerbates the situation[3]. CHABs cause negative impacts, such as aesthetic changes to water bodies in color, taste, oxygen level, unpleasant odour problems, suppressing the survival of aquatic animals[4]as well as blocking water treatment systems because of overloading algae. Microcystins which are produced by CHABs from their metabolites[5]are hepatotoxic to animals or humans, and are lethal[6]. Thus, controlling the development of CHABs in fresh water bodies is crucial.
Allelopathy is a promising method for cyanobacterial blooms control through the production and release of allelochemicals to interact with cyanobacterial species[7-8]. Multiple macrophytes possessing strong allelopathic activity against various cyanobacteria have been reported[9]and proposed as a novel approach to prevent or reduce CHABs, which is believed to be environmental friendly and acceptable[10].Potamogetonmaackianus,Potamogetonpectinatus, andAcoruscalamusare believed able to inhibit the growth ofMicrocystisaeruginosa(M.aeruginosa)[11-12]. Recent findings suggest that the use of agricultural waste products is the effective and environmentally friendly method to control cyanobacterial growth[13-14]. However, a large amount of agricultural waste straw(8.0-10.0 g·L-1dry weight) is required for this purpose which might bring excess nutrients such as nitrogen and phosphorus into water bodies[13-14]. Therefore, it is necessary to explore the candidates of allelopathy with high efficacy and low nutrient content.
Numerous plants have been reported to possess of algistatic effects on many kinds of algae, especially using agricultural straws[15]. Recently, it has been reported that the growth of three harmful algal blooms species is suppressed significantly bySpartinaalternifloraextract, an invasive species[16]. However, there are limited studies on the application of allelochemicals and the mechanism of arbor species on anti-algal properties, particularly using leaf litter[17]. Low algal growth of certain streams, rivers, and lakes is observed, which is likely because of receiving of leaf litter from land habitats. Leaf litter, for example brown-rotted wood, and deciduous and coniferous leaves[18], exerted stronger allelopathic activity against cyanobacteria than macrophytes[19]. Our previous research has shown a kind of arbor specie’s leaf litter,Dracontomelonduperreanumposed significant effect on algae inhibition[20].Magnoliadenudata(M.denudata) mainly distributes throughout East Asia, Southeast Asia and Southeast North America. Flower ofM.denudatais commonly used as medicinal herbs in many Asian countries[21]. However, few literatures studied the potential application ofM.denudataleaves on environmental protection, and the physiological aspects of anti-algal had not been investigated.
At first she determined1 to follow the old custom of keeping the young king ignorant of the duties he would have to perform some day; then, quite suddenly, she resigned the reins2 of government into his hands; but, unluckily, it was too late to train him properly for the post
Cyanobacteria existed in aquatic environments since 3.5 billion years ago and had a universal feature of photosynthetic process with high nutrient utilization efficiency[22]. Inhibiting photosynthesis to impact on growth of cyanobacteria is a solution. The substances released from decomposing leaf litter may mediate physical, biological and chemical inhibition mechanisms against algae growth[20]. There are some promising techniques which could be applied in the mechanistic study on allelochemicals[13-14, 20]. Chlorophyll-a(Chl-a) fluorescence can indicate the photosynthetic efficiency and has been widely used in the detection and evaluation of stresses on plant photosystem under the illumination of actinic light[23]. In addition, phytoplankton analyzer pulse-amplitude-modulation(Phyto-PAM) and flow cytometry(FCM) allow rapidinvivodetermination of photosynthetic activity and facilitate understanding the mechanism of toxicity to algal cells.
The light response curves ofM.aeruginosadecreased with increasing MDLLE dosage initially. It showed a similar pattern of rETR in response to photosynthetically available radiation(PAR) generated by PAM according to the control and MDLLE treatment. The rETR curves ofM.aeruginosatreated with MDLLE compared to PAM fluorometry are shown in Fig. 3. The solid line represents model results. Comparisons were performed using the Ratkowsky method for non-linear models[25]. There was no obvious difference of the rETR in response to PAR between the treatment and control on 1 d (shown in Fig. 3(a)). Nevertheless, the values of rETR significantly decreased(P<0.01) at high extract concentrations(1.2, 1.6, and 2.0 g·L-1) on 4 d (shown in Fig. 3(b)). On 7 d and 15 d, the rETR values increased gradually corresponding to PAR and MDLLE concentrations (shown in Figs. 3(c) and 3(d)).
1 Materials and Methods
1.1 MDLLE preparation
M.denudataleaf litter was collected from Shenzhen City, Guangdong Province of China. Leaves were washed with tap water, cut into pieces with about 0.5 cm lengths, dried at 40 ℃ for 4 d in oven to constant weight and then crushed. To prepare the extract, 50 g sample was placed in 0.5 L deionized water, and then incubated at(26±1)℃ for 4 d in darkness. The solution was first filtered with glass fiber filters(Whatman GF/C, Britain), then the mixture was filtered using cellulose acetate membrane( pore size, 0.22 μm), and the stock solution of 0.1 g·mL-1was kept at 4 ℃ until use.
1.2 Culture conditions
M.aeruginosa(FACHB-915) was provided by the Freshwater Algae Culture Collection, Institute of Hydrobiology, Chinese Academy of Sciences(Wuhan, China). Cells were cultivated in BG-11 medium at 26 ℃ and under irradiance of 30 μmol photons·m-2·s-1(12/12 h light/dark cycle), and shaken two times a day. The MDLLE was added to microalgae cultures at the final concentrations of 0, 0.4, 0.8, 1.2, 1.6, and 2.0 g·L-1as described in Refs. [17,20]. After exposing to MDLLE, samples were taken and measured on 1, 4, 7, 10, and 15 d.
1.3 Measurement of Chl-a
Through measurement of Chl-afluorescence to probe photosynthesis is a widely adopted method. The mean fluorescence intensity(MFI) of Chl-aforM.aeruginosawas analyzed by FCM on 1, 5, and 15 d, respectively(shown in Fig.2). Results showed that decreasing in Chl-afluorescence of algal cells was caused by all levels of MDLLE concentration. The MFI of Chl-awas 46.8% compared to control at 2.0 g·L-1dose of extract on day 1. After 5 d and 15 d, the MFI was 62.3% and 68.2% of the control when exposed to 2.0 g·L-1of extract, respectively. It showed similar trend of declining with the exposure to MDLLE according to the results of growth and MFI forM.aeruginosa(shown in Figs. 1 and 2).
(1)
whereC0andCare Chl-acontent in the control and extract-treated cultures, respectively.
1.4 Determination of photosynthetic activity
Till now, the targets by which algicides inhibit growth of cyanobacteria are not fully understood[34]. One of the most prominent effects of algicides is that they may impair the photosynthetic system of phytoplankton[35-36]. In the study, photosynthetic activities of algae were evaluated by two key photosynthetic parameters and RLCs. Our results showed that MDLLE inhibitedFv/Fmto different degrees during the experiment at the first time of culture. In the photosynthetic system, the reduction inFv/Fmand RLCs(or rETRmax) can reflect both the inhibition of the energy transfer and capacity of the electron transport chain[31]. Generally, the parameters associated with photosynthetic activity decreased with the increasing dose. This showed the dose dependent manner for the extract on impairing the photosynthetic system. However, the impairment was found temporary and rather than irreversible at lower concentrations, which agreed with previous study using garlic and diallyl trisulfide to inhibit the growth and photosynthesis ofM.aeruginosa[34].
And the youngest daughter rejoiced in the good luck that had come to her, and they had a splendid wedding when the days of mourning for her mother and sister were ended
1.5 FCM analysis
The effects of MDLLE on Chl-afluorescence, cell integrity and esterase activity ofM.aeruginosawere investigated by FCM analysis. Accuri 6 FCM(Becton Dickinson, USA) equipped with FL3 detectors(>670 nm) was used to measure the fluorescence signal of Chl-a, while the changes on cell membrane integrity and esterase activity were analyzed by FL2(585/40 nm) and FL1(533/30 nm) detectors, respectively. Cells were stained with 65.2 mg·L-1propidium iodide(PI, Sigma, St Louis, MO, USA) and 10 mg·L-1of fluorescein diacetate(FDA, Sigma, St Louis, MO, USA) for 15 min. Twelve μL·min-1flow rate was applied and about 12 000 cells were counted by FCM for each sample. Data were collected and analyzed using BD-Accuri 6 software. Detailed procedures were described in Ref. [13].
1.6 Identification of anti-algal compounds in MDLLE
The constituents in MDLLE were analyzed by GC-MS(7890B-5977A, Agilent, Santa Clara, California, USA) equipped with DB-5 MS quartz capillary column after extracted using 10 mL hexane three times. For GC-MS condition, the injection temperature was 280 ℃ and initial oven temperature was 80 ℃. The programmed rate increased to 180 ℃ at 10 ℃·min-1and maintained for 10 min. Mass spectra were recorded over 50-550 amu range with electron impact ionization energy of 70 eV. One μL sample was injected at splitless mode. Helium acted as carrier gas at a rate of 1 mL·min-1. Preliminary identification of constituents was identified by peak matching against standards in the standard NIST library spectra. Relative amount of the constituents was calculated in each case from GC-MS peak areas.
He stood on tiptoe gazing and gazing, till the candle in the attic was put out; the student had blown it out and had gone to bed, but the Goblin remained standing6 outside listening to the music, which very softly and sweetly was now singing the student a lullaby
1.7 Statistical analysis
After the MDLLE incubation with theM.aeruginosa, slight inhibition the growth of algae was observed on 1 d. More significant inhibition was observed on 4 d. Nevertheless, the anti-algal effect seemed slightly less promising at higher MDLLE(1.6-2.0 g·L-1) dosage. It was possible that the anti-algal activity was partially offset by the promoting activity due to additional nutrients introduced to the system[13]. Previous studies also showed that many aquatic and terrestrial plants exerted stronger allelopathic activity against cyanobacteria harmful algae[26]. In addition, previous reports have shown that 3-month extraction of barley straw yields a significant algistatic effect on species ofScenedesmus[27]andM.aeruginosa[28]. In this study, only 4 d decomposition ofM.denudataleaf litter was sufficient to inhibit the growth ofM.aeruginosa. Thus, the allelopathic compounds released from leaf litter ofM.denudatamay be quite different from barley straw or rice straw. This finding aligned with our previous studies usingDracontomelonduperreanum(D.duperreanum) leaf litter extract[20]. The reported effective concentrations from 0.1 g·L-1to 10.0 g·L-1for barley straw or rice straw are required to control algal growth[13-14, 26]. However, an increase of the dosage introduces more nutrients into lakes or reservoirs, which is the potential risk of CHABs. In this experiment, the highest concentration of MDLLE was 2.0 g·L-1, which might limit the heterogenous nutrients to be introduced into water bodies.
2 Results and Discussion
2.1 Growth inhibition effects of M. aeruginosa exposure to MDLLE
Algal growth did not differ significantly between groups within the 1 d test period after treatment with 0- 2.0 g·L-1MDLLE(P>0.05), whereas the extract began to exert strong inhibition activity after 4 d exposure. The inhibition rate was 70.6% with 2.0 g·L-1treatment and it showed significant difference with the control on 4 d(P<0.01). On 10 d, the inhibition rate was 82.5% and the inhibition effect was more pronounce under the using of the highest concentration of extract. After 15 d of exposure, at all doses of extract, the inhibition effect weakened. The inhibition rates were 84.7% on 7 d, but only 77.4% on 15 d in the 2.0 g·L-1treatment(shown in Fig. 1).
Yes, it has been given to us, said the young wife, but thislife is nothing more than one long scene of trial and hardship to manythousands. How many have been cast into this world only to endurepoverty, shame, illness, and misfortune? If there were no future life,everything here would be too unequally divided, and God would not be the personification of justice.
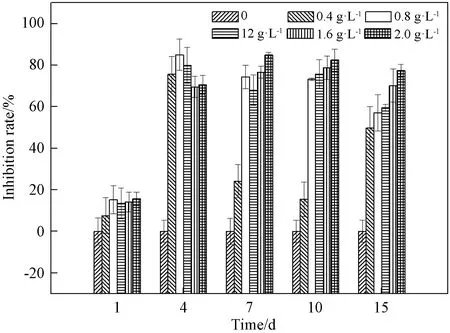
Fig. 1 Changes in inhibition activity of M. aeruginosa exposure to MDLLE after 1, 4, 7, 10, and 15 d
All assays were conducted in triplicate. Means and standard deviation were calculated and presented. The data were analyzed by SPSS 16.0 software(SPSS Inc., Chicago, IL, USA). Results were analyzed by one-way analysis of variance(Origin version 8.5, Microcal Software Inc., Northampton, MA, USA).P<0.05 was considered statistically significant. Comparisons of the fitted growth curves were performed based on the method for non-linear models[25].
2.2 In vivo Chl-a fluorescence measured by FCM
The Chl-acontent was determined with Phyto Win v2.13 by Phyto-PAM(Walz, Germany) on 1, 4, 7, 10, and 15 d. The inhibition ratioIrwas used for expressing the effects of MDLLE treatment, which was calculated by
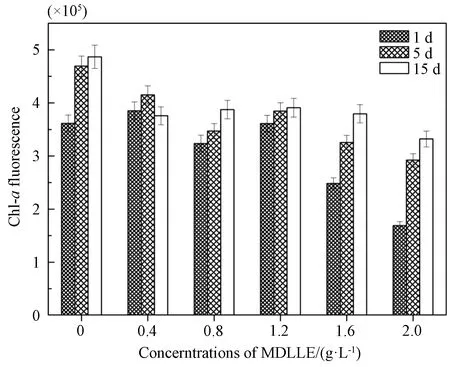
Fig. 2 Changes in Chl-a fluorescence of M. aeruginosa cells after 1, 5, and 15 d MDLLE exposure
The capacity of electron transport rate is crucial to the photosynthesis[31]. The electron transfer and charge separation have effect on quantum efficiency directly, which can affect ATP formation, carbon dioxide fixation and limit the growth of algae, respectively[32]. Allelochemicals luteolin can hinder the electron transport process and lead to the failure of photosynthesis forM.aeruginosa[22]. High artemisinin can decrease the electron transport rate and inhibit electron transport in the acceptor side. This results to the decrease of photosynthesis and inactivation of PSII reaction centers ofM.aeruginosa[33]. The photosynthetic parameters of rETR may reflect the state of algal cell at different stages. In this study, the rETR remained at high level when the dosages of MDLLE were 0.4 g·L-1and 0.8 g·L-1but decreased at higher concentration(1.2, 1.6, and 2.0 g·L-1) at early time(4 d), which agreed with the decreased mass ofM.aeruginosaafter MDLLE exposure. This demonstrated the dose dependent manner of extract on these two photosynthetic parameters.
2.3 Effects of MDLLE on rapid photosynthesis-irradiance response of M. aeruginosa
The aim of the study was to examine the potential allelopathic activity ofM.denudataleaf litter on the growth of typical bloom-formingM.aeruginosa. The photosynthetic activity, cellular esterase activity and the composition of volatile chemicals inM.denudataleaf litter extract(MDLLE) were determined.
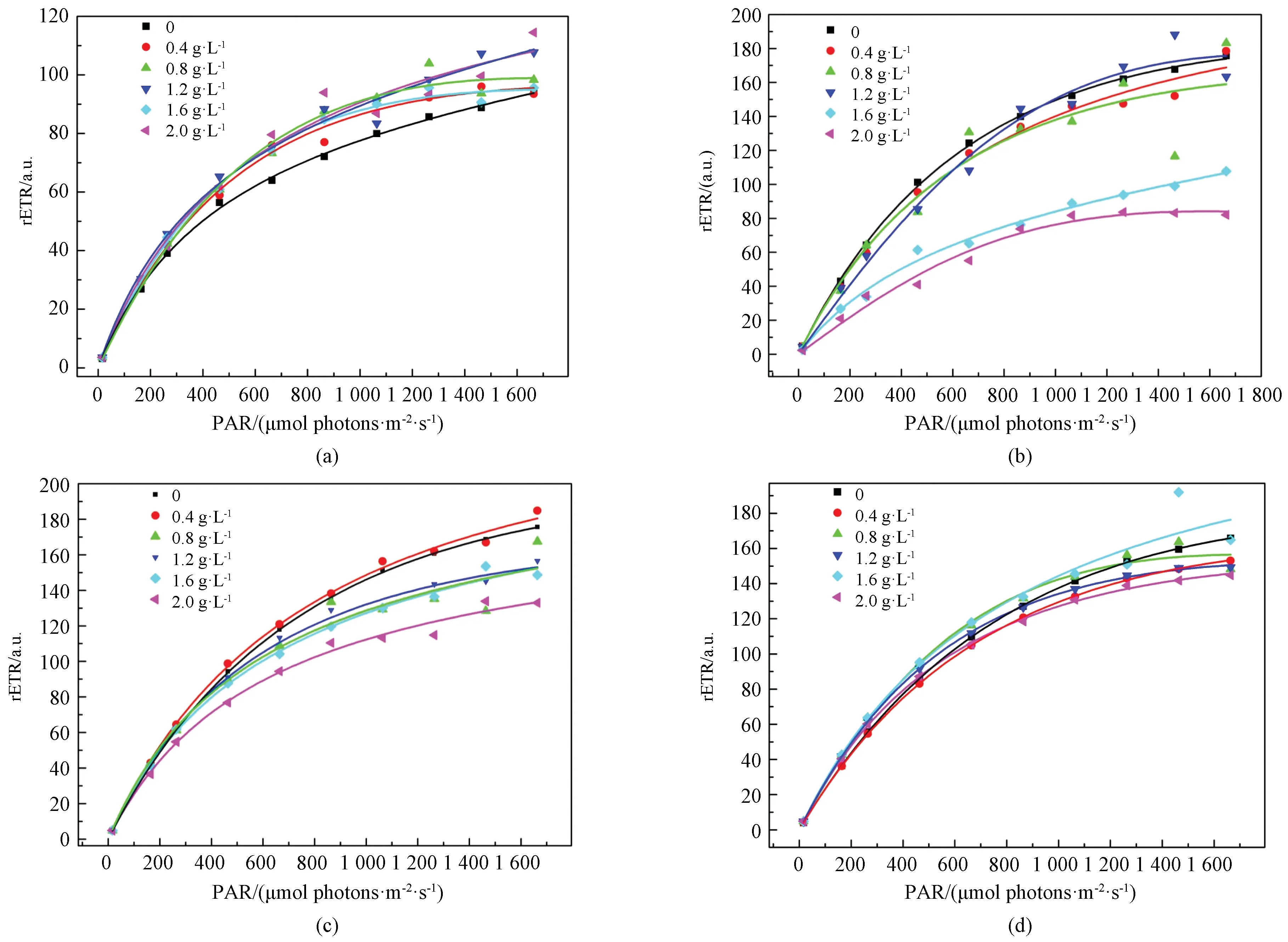
Fig. 3 Rapid photosynthesis-irradiance response curves of M. aeruginosa treated with different dosages of MDLLE: (a) 1 d; (b) 4 d; (c) 7 d; (d) 15 d
Chl-ais the major photosynthetic pigment in phytoplankton and provides information about the photosynthesis process, such as energy absorption, distribution, and utilization[29]. The degradation of Chl-awould damage the photosynthetic system and weaken photosynthesis, and therefore inhibit the growth of algae[30]. Biocides such as copper and chlorine induce declining of Chl-afluorescence within 24 h. Previous investigation showed that the effect of plant extract appeared to be algistatic rather than algicidal[13]. There are several dry plant products affecting the photosynthetic activity, such as agricultural waste straw[13-14]and leaf litter extract[20]. In addition, the extract of rice straw reduces Chl-afluorescence significantly on 7 d but not on 15 d forM.aeruginosa[14]. MDLLE could efficiently diminishinvivoChl-afluorescence at medium or high dosages in the current experiment. Nevertheless, the MFI level increased slightly over time and the inhibition effect weakened on initial stage( 1-10 d) at all doses of extract, which implied the need of continuous supply of MDLLE.
2.4 Effects of MDLLE on maximal quantum yield(Fv/Fm ) and electron transport rate (rETRmax) of M. aeruginosa
The effects of MDLLE onFv/Fmand rERTmax within the treatment time are shown in Fig. 4. As seen in Fig. 4(a), 1.6 g·L-1and 2.0 g·L-1treatment significantly reduced theFv/Fmfrom 0.78 to 0.61 and 0.53 during the first 4 d of cultivation, respectively. But theFv/Fmvalues increased gradually after 4 d (shown in Fig. 4(a)), and there was no significant difference between MDLLE treatments and the control. rETRmax ofM.aeruginosashowed a significant decrease with increasing MDLLE, which reduced from 170.2 to 127.2, and 95.6 mmol electrons·m-2·s-1at 1.6 g·L-1and 2.0 g·L-1within 4 d of exposure, respectively. On 15 d, the rERTmax was 155.4 mmol electrons·m-2·s-1when exposed to 2.0 g·L-1MDLLE, while the control was 197.3 mmol electrons·m-2·s-1(shown in Fig. 4(b)).
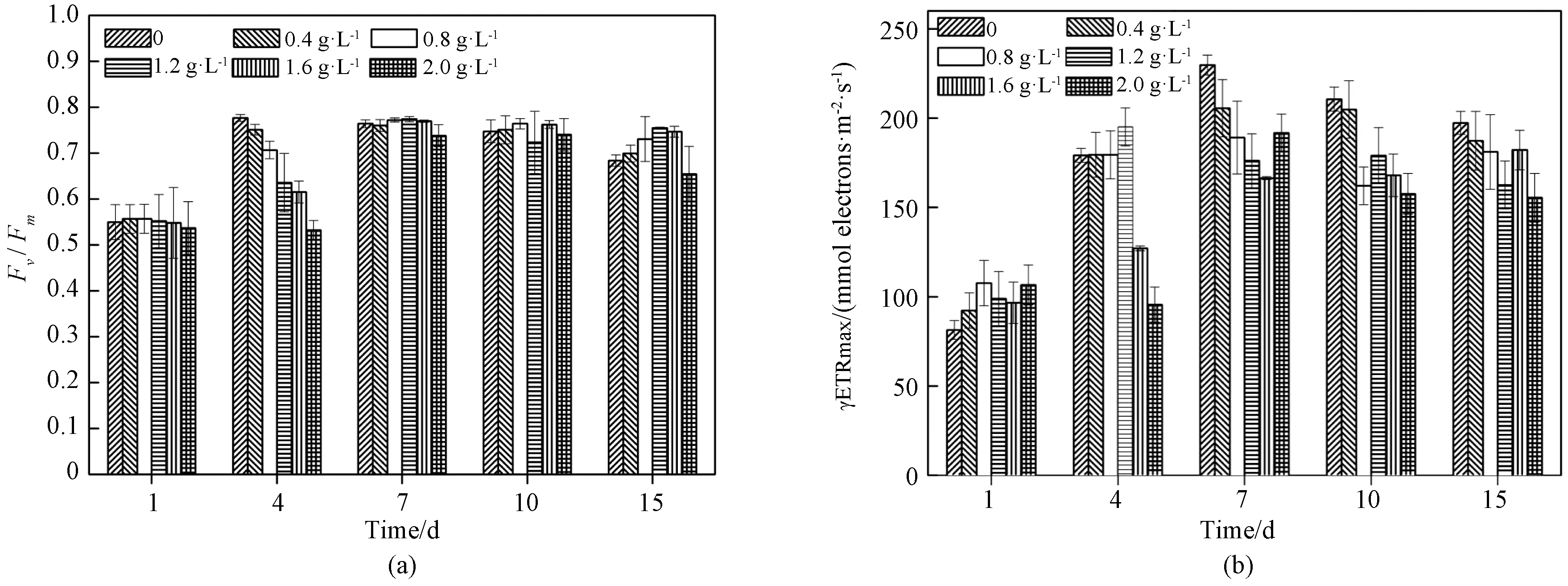
Fig. 4 M. aeruginosa treated with different dosages of MDLLE: (a)Fv/Fm; (b)rETRmax
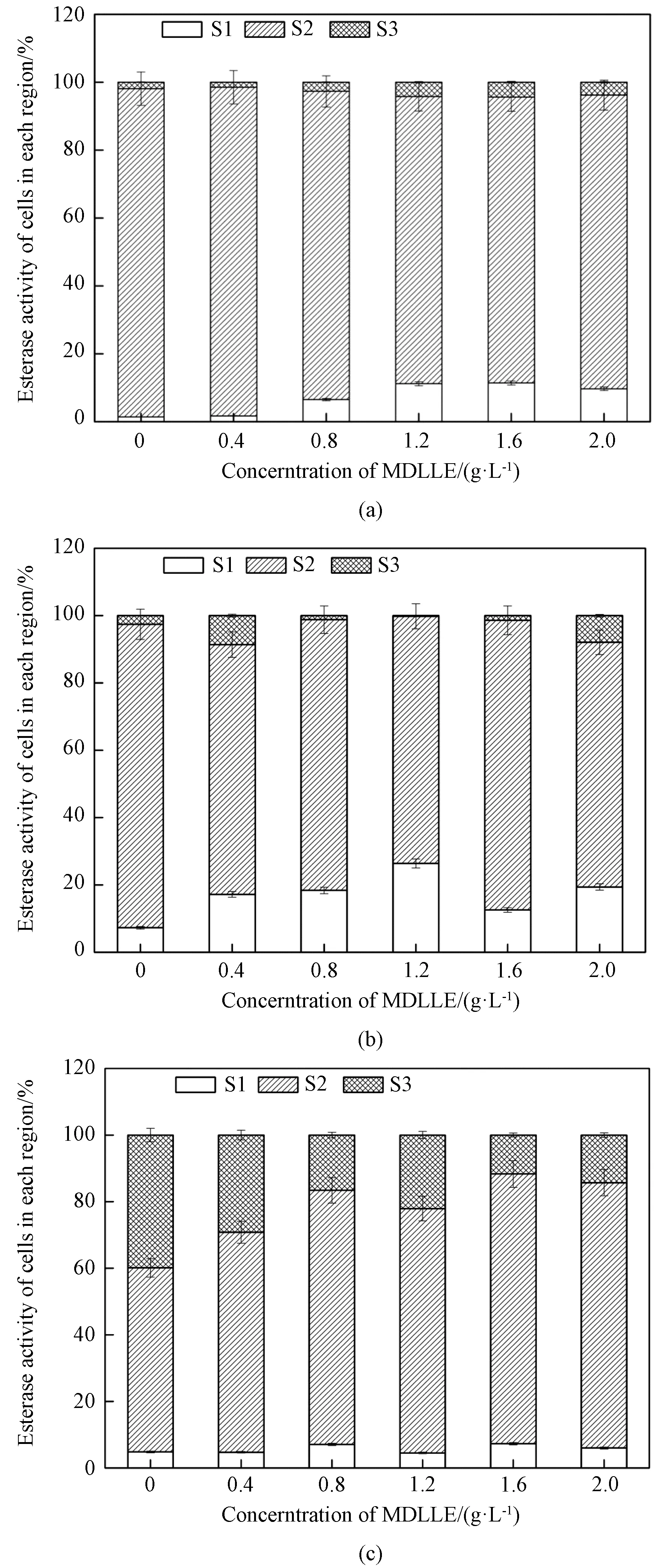
Fig. 5 Distribution of cells in different esterase activities of M. aeruginosa after MDLLE exposure: (a) 1 d; (b) 5 d; (c) 15 d (S1, S2 and S3 represent decrease in esterase activity, esterase activity in control cells, and increase in esterase activity, respectively)
The rapid light curves(RLCs) based on measurement of relative electron transport rates(rETRs) were employed to determine the photosynthetic activity ofM.aeruginosatreated with MDLLE. Values of rETR of samples exposed to 10 intensities of actinic light increasing from 16 μmol photons·m-2·s-1to 1 664 μmol·photons·m-2·s-1were obtained from the PAM. The samples were adapted for 2 min in darkness and the actinic light irradiance step was applied for 30 s. Then, the RLCs produced after the corresponding rETRs were calculated[19]. The RLCs were fitted using the model described previously[24].
2.5 Algistatic versus algicidal properties and esterase activity of M. aeruginosa exposure to MDLLE
Cellular membrane integrity can be determined by FCM, usually with fluorescent PI[36]. The cell membrane would be damaged if the algicidal property is in action, otherwise, namely algistatic action[37]. In this experiment, only a slight change of membrane integrity was seen even if at the highest MDLLE concentration. The lowest membrane integrity rate was 96.2% with different dosages of MDLLE after 1, 5, and 15 d. No statistical significant difference was seen compared to the control group(P>0.05, shown in Table 1). This indicated the cell membranes remained intact and the mechanism ofM.denudataleaf litter on algal growth control was algistatic in nature, which was consistent with previous reports[13-14, 20].
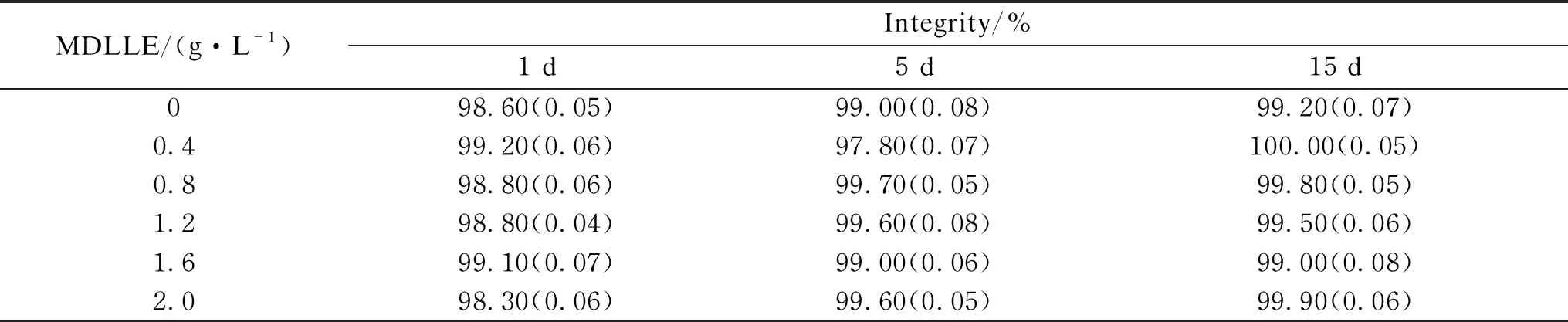
Table 1 Cell membrane integrity of M. aeruginosa treated with different dosages of MDLLE after 1, 5, and 15 d exposure
The cellular metabolic activity is evaluated by measuring the absorbance of FDA. AfterM.aeruginosaexposure to different concentrations of MDLLE and time points, substantial increases or decreases in esterase activity were observed(shown in Fig. 5). After 1 d exposure, approximate 1.4% - 14.0% of cells shifted to the lower esterase activity state(1.4% in the control) while 1.9% - 4.4% of cells shifted into the higher activity state(1.9% in the control) (shown in Fig.5(a)). On 5 d, 19.4% of cells shifted into the lower activity state at 2.0 g·L-1dosage(7.3% in the control), while the higher esterase activity state of cells increased to 0.2% - 7.9%(2.6% in the control) (shown in Fig. 5(b) ). After 15 d of exposure, the higher esterase activity state of cells increased when exposed to each concentration of MDLLE including the control, reaching approximately 39.9% in the control(14.3% at 2.0 g·L-1dosage), while the lower esterase activity state of cells remained to 4.5% - 7.5%(4.8% in the control) (shown in Fig. 5(c) ).
I have sprained18 my foot, and cannot well walk through the streets; thou shalt put on my wedding-clothes and take my place; a greater honour than that thou canst not have! Maid Maleen, however, refused it, and said, I wish for no honour which is not suitable for me
The esterase activity was evaluated by measuring the change of fluorescence, which was a sensitive, rapid and simple method to assess metabolic activity in microalgae. The esterase activity of phytoplankton is either enhanced or suppressed in many plants, such as barley straw andD.duperreanumdefoliation extract[13, 20]. The cellular esterase activities shifted to the inhibited state and continued to inhibit cellular metabolic activity exposure to barley straw extract[13], while the rice straw extract had a decreased inhibitory effect on esterase activity[14]. InFructusligustrilucidicontrolling the growth of algae, the cellular metabolic activities ofM.aeruginosashifted into the inhibited state after 1 d exposure[38]. In this study, MDLLE interfered with the cell esterase activity on 5 d, while the higher esterase activity of cells reached to 21.9%. TheM.aeruginosaesterase activity was slightly inhibited by MDLLE(P>0.05). The possible explanations were the low extract concentration or half-lives of allelochemicals in MDLLE were too short to inhibit the esterase and the activity of algal cell esterase was the target at early stage.
2.6 Potential allelochemicals identified from MDLLE
Many kinds of allelochemicals have been applied to solve the problems posed by harmful algal blooms, such as lipids, ketone, alkaloid, and diclofenac[39]. In order to deduce the major allelochemicals in MDLLE, volatile components and their relative amount were preliminary analyzed by GC-MS in this experiment. Some chemicals present in abundant quantities were found, namely sulfurous acid-octadecyl pentyl ester, 6-ethenyltetrahydro-2,2,6-trimethyl-2H-pyran-3-ol and(Z)-13-docosenamide, respectively. In addition, other lower amount components were also found in MDLLE(shown in Table 2).
He made it out of the concentration camp, and immigrated2 to America. In 1957, his friends had fixed3 him up on a blind date . He had no idea who the woman was. He picked her up, and during dinner began talking of Poland and the concentration camp. She said she was in Poland at that time. She said she used to talk to a boy and gave him apples daily. He asked if this boy was tall, skinny and if he had told her that she shouldn t come back because he was leaving. She said yes.
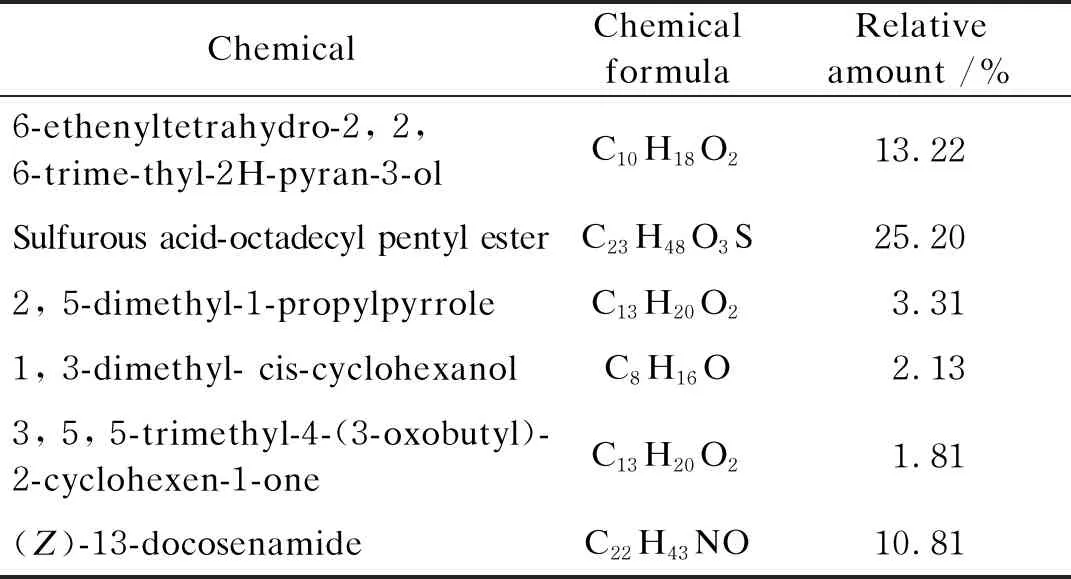
Table 2 Major alleochemicals in MDLLE by GC-MS analysis
By GC-MS technology, many kinds of allelochemicals have been successfully identified in plant extract. Examples are hexanoic, hexadecanoic and octadecanoic acid secreting from the root ofCannaindica, which play important role againstM.aeruginosa[40]. Cis-6-octadecenoic and cis-9-octadecenoic acids are able to inhibit the growth ofM.aeruginosasignificantly[41]. Other studies reported that there were mainly small molecular alcohols, ketones and esters in the extract ofM.grandifloraleaves which could inhibit the growth ofM.aeruginosa[42]. InD.duperreanumleaf litter extract, some fatty acids may act as allelochemicals to inhibit the growth ofM.aeruginosa[17]. Salcolin, a kind of flavonolignan is the key allelochemicals derived from barley straw and its anti-cyanobacterial activities have been confirmed[43]. In this experiment, sulfurous acid-octadecyl pentyl-ester found in MDLLE might act as allelochemicals to inhibit algae growth. Further experimental verification on the effect against the growth ofM.aeruginosafor(Z)-13-docosenamide, 6-ethenyltetrahydro-2, 2, 6-trimethyl-2H-pyran-3-ol and 1, 3-dimethyl-cis-cyclohexanol is required.
They both looked exactly alike, and the Tanuki only saw that one was bigger than the other, and would hold more fish, so he sprang into the large one, while the hare climbed into the one which was made of wood
3 Conclusions
Various parameters namely MFI, rETR,Fv/Fm, and rERTmax of photosynthetic center PS II indicated that photosynthesis was an important target for the allelopathic effect of MDLLE onM.aeruginosa. We suggested that MDLLE was firstly transported into the cytosol ofM.aeruginosaand disrupted enzyme activity. The PSII reaction center and photosynthesis forM.aeruginosawere affected and inhibited by MDLLE after 4 d exposure. The cyanobacterial cells then started to repair themselves, as indicated by the changes in their physiology(enzyme activity and photosynthesis). Therefore, it showed a strong inhibitory effect to the growth of algae, which could be an environmentally friendly mitigation technique to limit cyanobacteria to grow in the natural environment. However, further study should evaluate the potential risk of CHABs application of MDLLE, which may probably introduce more nutrients into water bodies.
 Journal of Donghua University(English Edition)2021年5期
Journal of Donghua University(English Edition)2021年5期
- Journal of Donghua University(English Edition)的其它文章
- Ecological Dyeing of Polyamide 6, 6(PA66) Fabrics with Monascus Pigments in Decamethylcyclopentasiloxane(D5) Solvent
- Effects of Microstructure on Quasi-Static Transverse Loading Behavior of 3D Circular Braided Composite Tubes
- Electrical-Mechanical Coupling Behaviors and Thermal-Resistance Effects of 3D Braided Composites
- Efficient Probabilistic Load Flow Calculation Considering Vine Copula-Based Dependence Structure of Renewable Energy Generation
- Turing Instability of Diffusive Predator-Prey System with Gompertz Growth
- Blockchain-Based Log Verification System for Cloud Forensics
