Regulated upon activation, normal T cell expressed and secreted (RANTES) levels in the peripheral blood of patients with Alzheimer’s disease
Gabriela Vacínová , Daniela Vejražkova Robert Rusina, Iva Holmerová ,Hana Vaňková Eva Jarolímová Josef Včelák Běla Bendlová Markéta Vaňková
Abstract Alzheimer’s disease (AD) is the most common type of dementia, but it is very difficult to diagnose with certainty, so many AD studies have attempted to find early and relevant diagnostic markers. Regulated upon activation, normal T cell expressed and secreted(RANTES, also known as C-C chemokine ligand) is a chemokine involved in the migration of T cells and other lymphoid cells. Changes in RANTES levels and its expression in blood or in cerebrospinal fluid have been reported in some neurodegenerative diseases, such as Parkinson’s disease and multiple sclerosis, but also in metabolic diseases in which inflammation plays a role. The aim of this observational study was to assess RANTES levels in peripheral blood as clinical indicators of AD. Plasma levels of RANTES were investigated in 85 AD patients in a relatively early phase of AD (median 8.5 months after diagnosis;39 men and 46 women; average age 75.7 years), and in 78 control subjects (24 men and 54 women; average age 66 years). We found much higher plasma levels of RANTES in AD patients compared to controls. A negative correlation of RANTES levels with age,disease duration, Fazekas scale score, and the medial temporal lobe atrophy (MTA) score(Scheltens’s scale) was found in AD patients, i.e., the higher levels corresponded to earlier stages of the disease. Plasma RANTES levels were not correlated with cognitive scores. In AD patients, RANTES levels were positively correlated with the levels of pro-inflammatory cytokines interleukin-6 and tumor necrosis factor-α, which is consistent with the wellknown fact that AD is associated with inflammatory processes. RANTES levels were also positively correlated with insulin levels in AD patients, with insulin resistance (HOMA-R) and pancreatic beta cell function (HOMA-F). This study evaluated several clinical and metabolic factors that may affect plasma levels of RANTES, but these factors could not explain the increases in RANTES levels observed in AD patients. Plasma levels of RANTES appear to be an interesting peripheral marker for early stages of AD. The study was approved by the Ethics Committee of Institute of Endocrinology, Prague, Czech Republic on July 22, 2011.
Key Words: Alzheimer’s disease; biomarker; central nervous system; cognitive impairment;inflammation; RANTES
Introduction
Alzheimer’s disease (AD) is the most common type of dementia and is characterized by β-amyloid peptide and neurological tangle deposition in the brain. The frequency of AD recently increases every year. It is estimated that in 2050 there will be around 131 million people with AD worldwide(Alzheimer’s Disease International, 2015). It is thus necessary to find additional markers that can contribute to earlier diagnosis of AD.
Although inflammatory processes are not a major causative factor for AD, there is evidence that pro-inflammatory cytokines and chemokines contribute to the progression of this disease (Domingues et al., 2017). Neuroinflammation is initially a protective response in the brain, but an excess inflammatory response is detrimental and inhibits neuronal regeneration (Russo and McGavern, 2016). Long-term chronic neuroinflammation is generally important for the onset and progression of neurodegenerative diseases including AD(Kempuraj et al., 2016).
Regulated upon activation normal T cell expressed and secreted [RANTES, also known as CC chemokine ligand 5(CCL5)], a proinflammatory CC-chemokine, is a member of the chemokine family that regulates cell migration. It mediates the migration and navigation of classical lymphoid cells like T cells, monocytes, basophils, eosinophils, natural killer cells,dendritic and mast cells (Appay and Rowland-Jones, 2001).It also induces the migration of mononuclear phagocytes across the blood-brain barrier to sites of inflammation(Ubogu et al., 2006). RANTES has the ability to bind to multiple C-C chemokine receptors – CCR5, CCR3 and CCR1.T cell stimulation occurs via two ways. The first is via shortterm mobilization of calcium ions through G protein-coupled receptors that are situated on target cells. This leads to cell polarization and follow-up migration. The second is through the chronic increase of calcium ions, dependent on protein tyrosine kinase. The increase in Ca2+level leads to multiple responses including T cell proliferation or apoptosis, releasing interleukin-2 (IL-2), interleukin-5 (IL-5) or interferon gamma(IFN-γ). This response is typical for RANTES and unique among chemokines (Albert et al., 2017).
Although brain imaging techniques and neuropsychological testing have been successfully used to diagnose AD, and there are a number of biomarkers in cerebrospinal fluid that are used for the differential diagnosis of AD (Niemantsverdriet et al., 2018; Paterson et al., 2018), there remains a need to identify relevant biomarkers in the periphery. Detection of biomarkers in the periphery is less invasive and less expensive compared to detection of biomarkers in the cerebrospinal fluid or neuroimaging method.This study was therefore designed to evaluate plasma RANTES levels in AD patients as a marker for early stages of AD. Clinical and metabolic factors that can affect plasma RANTES levels were also investigated.
Participants and Methods
Participants
A total of 163 participants including 85 patients with AD (39 men and 46 women) and 78 healthy controls (24 men and 54 women) were included in this study. The 85 AD patients were recruited by the Department of Neurology, 3rdFaculty of Medicine, Charles University and Thomayer Hospital in Prague,the Czech Republic. Healthy controls were recruited into the study through targeted advertising in senior organizations as part of a research study by the Ministry of Health of the Czech Republic. The diagnosis of AD was established according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRA) clinical criteria (McKhann et al., 2011). The diagnosis of AD was supported by clinical evaluations, MRI findings (temporal and parietal atrophy) and cerebrospinal fluid biomarkers: amyloid beta (Aβ42), total tau and phosphorylated tau protein levels. Neuropsychological assessments were performed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in both AD and control groups, the Mini Mental State Exam (MMSE)and the Frontal Assessment Battery (FAB) in AD patients only,the Montreal Cognitive Assessment (MoCA) and the Geriatric Depression Scale (GDS) only in controls. On MRI scans, the medial temporal lobe atrophy (MTA) score (Scheltens’s scale;Schelten’s et al., 1992) was used to assess cortical atrophy,and the Fazekas scale (Fazekas et al., 1987) was used to assess the impact of ischemic white matter changes on the cognitive impairment in AD patients. Controls with normal cognitive performance and the absence of hippocampal atrophy and ischemic white matter lesions on MRI underwent the same test protocol as patients with AD, except for cerebrospinal fluid analysis. Treatment of AD patients was as follows: donepezil and rivastigmin in 44 patients (52%), memantine in 11 patients(13%), selective serotonin reuptake inhibitors (SSRI; citalopram,escitalopram, sertraline) in 22 patients (26%), and other antidepressants (trazodon, mirtazapine) in 7 patients (8%).
Body weight, height, and waist and hip circumferences were measured to calculate body mass index (BMI), body adiposity index (BAI), and to evaluate body fat distribution by means of the waist circumference and waist to hip ratio (WHR). Based on anamnestic data and medical treatment information, we recorded the presence of type 2 diabetes, hypertension,dyslipidaemia and metabolic syndrome (National Cholesterol Education Program, 2002). Details regarding the baseline characteristics of patients in the two groups are listed in Table 1.
The study protocol was approved by the Ethics Committee of Institute of Endocrinology, Prague, Czech Republic on July 22,2011. All participants gave their written informed consent to participate in the study.
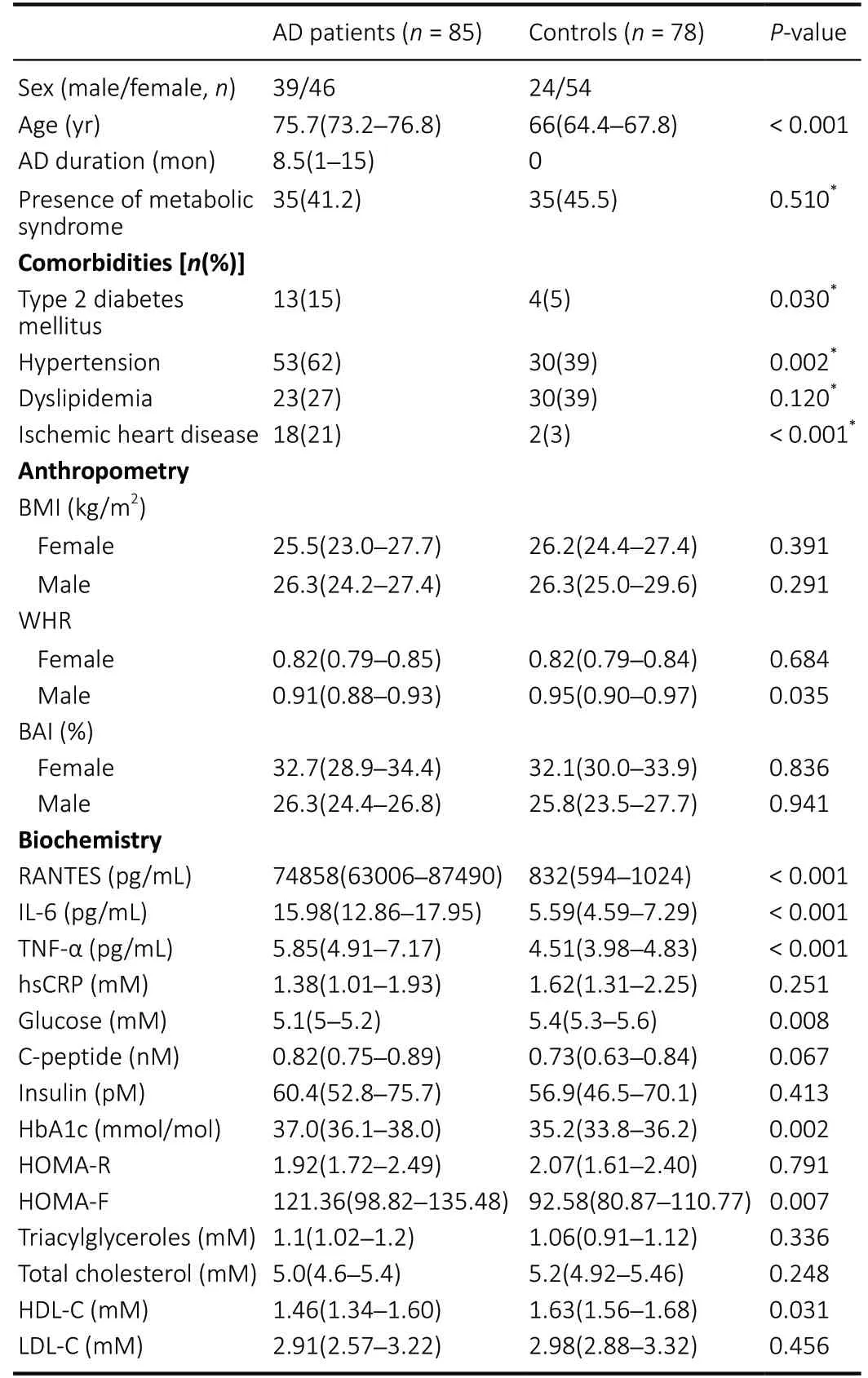
Table 1 |Clinical, anthropometric, and biochemical characteristics of patients with AD patients and controls
R ANTES analysis
To evaluate biochemical parameters, blood samples were drawn in the morning in a fasting state. Blood samples for the multiplex evaluation of fasting plasma biomarkers were treated with DPPIV Protease Inhibitor Coctail (Sigma-Aldrich,St., MO, USA), centrifuged, and stored at –80˚C until analyzed.Peripheral RANTES levels were measured using an HNDG3MAG-36K Human Neurodegenerative Disease Magnetic Bead Panel 3 kit (Millipore, Darmstadt, Germany).
Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α)levels were measured with a Bio-Plex ProTMHuman Th17 Cytokine Panel 15-Plex kit (LuminexTM, Hercules, CA, USA)on a Bio-Plex 200 (Bio-Rad, Hercules, CA, USA). Highsensitivity C-reactive protein (hsCRP) was measured by an immunoturbidimetric test (Cobas 6000, Roche Diagnostics,Manheim, Germany).
Biochemical characteristics
For lipid profile assessment, total cholesterol levels were quantified by an enzymatic colorimetric test, high density lipoprotein-cholesterol (HDL-C) and low density lipoproteincholesterol (LDL-C) levels by a homogeneous enzymatic colorimetric test, and triacylglycerol concentrations by an enzymatic colorimetric test (Cobas 6000, Roche Diagnostics,Mannheim, Germany).
To assess peripheral insulin sensitivity, the homeostasis model of insulin resistance HOMA-R was calculated, and to assess insulin secretion, the homeostasis model of beta cell function HOMA-F was calculated (Matthews et al. 1985). For these calculations, blood glucose levels were measured using an enzymatic reference method with hexokinase, insulin and C peptide by ECLIA (Cobas 6000, Roche Diagnostics, Mannheim,Germany). Glycated haemoglobin (HbA1c) was assayed by a turbidimetric inhibition method (Cobas 6000, Roche Diagnostics, Mannheim, Germany).
Metabolic syndrome
The presence of metabolic syndrome was diagnosed based on NCEP ATP III criteria (NCEP, 2002) for individual parameters of metabolic syndrome (fasting glucose > 5.56 mM,triacylglycerols > 1.69 mM, HDL-cholesterol < 1.036 mM (men),< 1.295 mM (women), waist circumference > 101.6 cm (men),> 88.9 cm (women) and systolic hypertension > 130 mmHg or diastolic hypertension > 85 mmHg) or based on the existing treatment for these parameters.
Statistical analysis
We chose methods respecting the skewed distribution and non-constant variance. A non-parametric Kruskal-Wallis oneway analysis of variance by ranks, Spearman’s rank correlation and the chi-square test or Fisher exact test (NCSS 2019, LLC,Kaysville, UT, USA) were used for comparing anthropometric,biochemical, and clinical parameters between the groups.P<0.05 was considered statistically significant. Data are shown as median with 95% confidence limit.
Results
Clinical, anthropometric, and biochemical characteristics
Clinical, anthropometric, and biochemical characteristics of the AD patients and controls are presented in Table 1. The median duration of AD in patients was 8.5 months (range 1–15 months). According to the MTA score, the proportion of patients was as follows — score 1: 21%, score 2: 43%, score 3: 19.5% and score 4: 16.5%; regarding the Fazekas scale –the distribution of AD cases was as follows: periventricular white matter (PWMH) — score 0: 4.5%, score 1: 55.2%, score 2: 26.9%, score 3: 13.4% and deep white matter (DWMH) —score 0: 3%, score 1: 53.7%, score 2: 25.4%, score 3: 17.9%.
Neuropsychological assessments were performed in AD patients using RBANS [median 62.5 (59–69; 95% LCL–95%UCL)], MMSE [median 22 (20–24; 95% LCL–95%UCL)] and FAB[median 13 (12–14; 95% LCL–95% UCL)]. Neuropsychological assessments were performed in controls using RBANS [median 107 (102–114; 95% LCL–95% UCL)], MoCA [median 29 (28–30;95% LCL–95% UCL)], and GDS [90% controls ≤ score 5].
RANTES results
Plasma levels of RANTES were much higher in AD patients compared to controls (P< 0.001). A gender difference was detected in controls, with men having higher RANTES values than women [median (95% LCL–95% UCL) in men 1680 (855–3132) vs. in women 655 (477–833);P= 0.005].
There were negative Spearman correlations between RANTES levels and age (rs= –0.301,P= 0.005) as well as the duration of disease (rs= –0.3469,P= 0.001) in AD patients. The MTA score and Fazekas scale of PWMH were also negatively correlated with RANTES levels (rs= –0.3078,P= 0.011;rs= –0.2569,P=0.036; Table 2).
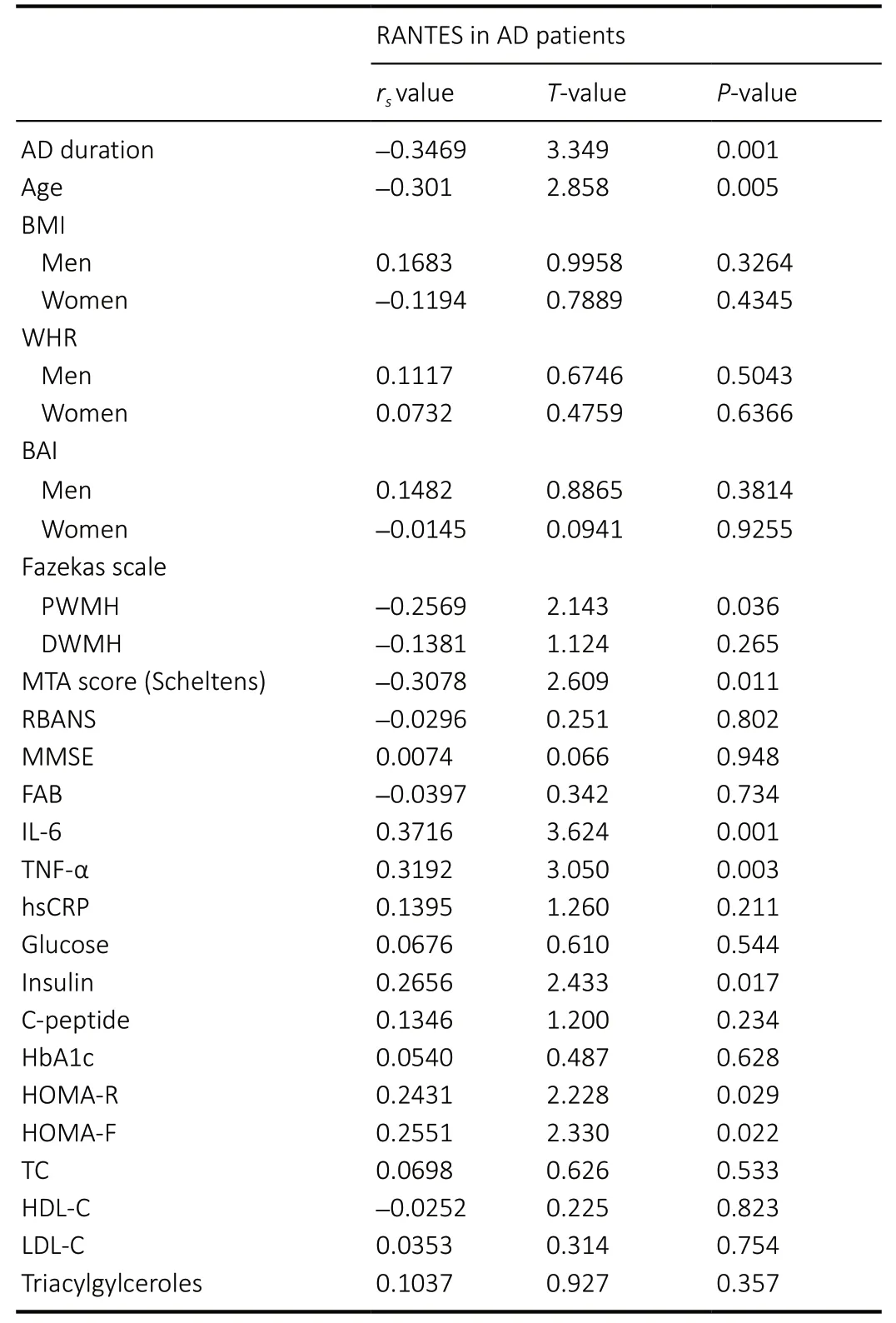
Table 2 |Spearman correlation of RANTES levels and basic characterization in AD patients
RANTES correlations
In AD patients, RANTES levels were positively correlated with the levels of IL-6 (rs=0.3716,P= 0.001), TNF-α (rs= 0.3192,P= 0.003), insulin levels (rs=0.2656,P= 0.017), higher insulin resistance (HOMA-R;P= 0.029) and higher pancreatic β cell function (HOMA-F;rs= 0.2551;P= 0.022). RANTES levels were negatively correlated with cognitive scores, lipid spectrum, or anthropometric characteristics in AD patients (Table 2). No correlations were observed between treatments for metabolic or cardiovascular diseases or the presence of metabolic syndrome and RANTES levels in either study group (Table 3).
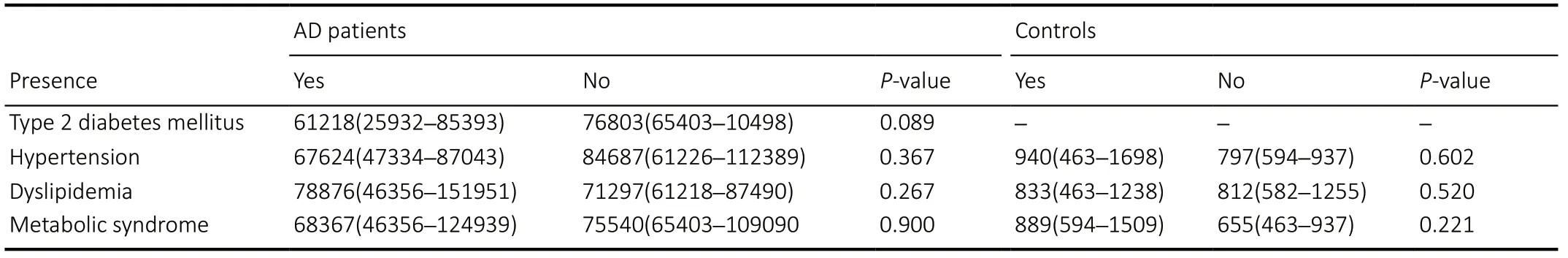
Table 3 |RANTES levels (pg/mL) and presence of metabolic and cardiovascular diseases
Discussion
Many researchers are attempting to find biomarkers in peripheral blood that would be reliable enough to help diagnose and predict AD. Our study showed that the chemokine RANTES could be an appropriate marker, especially for early stages of the disease. In our study, RANTES levels were up to 100 times higher in AD patients compared to control subjects. Higher levels of systemic RANTES have been reported in AD patients (Iarlori et al., 2005; Pellicanò et al.,2010; Marksteiner et al., 2011; Stuart and Baune, 2014), but we were unable to find such large differences in comparison with controls in any publication. This may be because our patients were in a relatively early phase of the disease (median 8.5 months after diagnosis).
Indeed, inflammation in the pathogenesis of neurodegeneration in AD is a dynamic process with two peaks – an early“protective” peak and a later “pro-inflammatory” peak(Cuello, 2017; Fan et al., 2017; Sarlus and Heneka, 2017). Early inflammation is likely to begin as soon as there is a threshold of accumulated beta-amyloid oligomeric peptides before the presence of amyloid plaques, whereas the later process starts when the first amyloid plaques are established (Cuello, 2017).This is also consistent with the results in microglia, which show acute activation in the early stages of the disease with the active phagocytosis of beta-amyloid and then a chronic stage at later stages of the disease with compromised betaamyloid plaque clearance (Sarlus et al. 2017). Our Spearman correlations confirmed this finding with RANTES levels negatively correlated with age and disease duration in our AD patients, i.e. the highest levels were measured in younger patients relatively soon after AD diagnosis. This finding is in accordance with the results of an ingenious study where RANTES levels in experimental human blood-brain barrier models were evaluated (Vérité et al. 2018) in which RANTES levels were higher in media from patients with mild AD compared to moderate AD patients. There have even been studies supporting the role of RANTES in neuroprotection(Bruno et al., 2000; Tripathy et al., 2010).
Besides RANTES, our AD patients had other significantly higher pro-inflammatory cytokines such as IL-6 and TNF-α compared to controls, but differences in their levels were not as marked as for RANTES. In addition, RANTES levels were positively correlated with these parameters in AD patients but not in controls. There was no difference in hsCRP between these groups and the hsCRP levels were low in both groups,confirming that there was no acute bacterial infection. Though some studies are in accordance with our results (e.g. Gong et al., 2016), it should be mentioned that a meta-analysis revealed no differences in peripheral levels of the proinflammatory markers IL-6, TNF-α and CRP between elderly AD patients and controls (Ng et al., 2018). But this large cohort of patients was heterogeneous with respect to the phase of the disease.
In our study, the earlier stage of the disease was also reflected in minor brain damage - RANTES levels negatively correlated with the Fazekas scale – PWMH and MTA score (Scheltens),but surprisingly not with cognitive decline (MMSE, RBANS,FAB). Julian et al. (2015) reported no correlation between RANTES levels and cognitive assessment.
We are aware that the chemokine RANTES is a non-specific indicator of inflammation, and that there are a number of confounding factors to be considered when interpreting the outcome of our study. These independent factors include,but not limited to, ageing per se (Bruunsgaard et al., 2001;Ward et al., 2015), obesity (Wu et al., 2007; Aguilar-Valles et al., 2015), type 2 diabetes mellitus (Nomura et al., 2000),dyslipidemia (Schmid et al., 2016), hypertension (Mikolajczyk et al., 2016), fatty liver (Marra and Tacke, 2014; Roh and Seki,2018), and metabolic syndrome (Matter and Handschin, 2007;Ueba et al., 2014). In addition to these metabolic diseases,which are more common at older ages, RANTES levels are also affected by other diseases such as cancer (Aldinucci and Colombatti, 2014).
Regarding obesity, our AD patients did not differ from controls in any measured anthropometric parameter except for a slightly lower WHR in AD men. We found that RANTES levels were not correlated with BMI, WHR, or the amount of adiposetissue expressed by BAI in either controls or AD patients.
The association of AD with insulin resistance and type 2 diabetes mellitus is well known (Dworacka et al., 2014;Baglietto-Vargas et al., 2016; Pugazhenthi et al., 2017). In this study, there were more diabetic patients in AD patients (13/85)than in controls (4/78), and this was associated with higher glycated haemoglobin HbA1c level. AD patients also displayed a significantly higher HOMA-F index, which is calculated from fasting glucose and insulin levels and reflects higher pancreatic beta-cell secretion. Interestingly, AD patients had lower fasting glucose compared to controls, but the peripheral insulin level and Homeostatic Model Assessment for Insulin Resistance were similar in both groups. However, a positive correlation of RANTES with insulin levels, HOMAR and HOMAF indices was found in AD patients but not in controls. A close relationship between RANTES levels and insulin signaling was reported in a recent study (Chou et al. 2016), showing that RANTES regulates insulin signaling in the hypothalamus via their CCR5 receptors, contributing to systemic insulin sensitivity and glucose metabolism.
The influence of some metabolic diseases and their treatments (type 2 diabetes mellitus, hypertension,dyslipidemia, and overt metabolic syndrome) on RANTES levels was also evaluated, but they did not influence RANTES levels in either AD patients or the control group.
High RANTES levels have also been reported in other neurodegenerative diseases such as Parkinson’s disease (Tang et al., 2014), multiple sclerosis (Pittaluga, 2017), posttraumatic stress disorder (Ogłodek et al., 2015), neuropathic pain(Bhangoo et al., 2007), and brain injury (Lumpkins et al.,2008). None of these conditions were diagnosed in our patients or controls, so they likely did not influence RANTES levels in our study.
This study has several limitations. Firstly, the controls were significantly younger than the AD patients. However, RANTES levels were negatively correlated with age in the AD group,and RANTES levels were not correlated with age in the control group. Secondly, RANTES levels were measured only in the peripheral blood and we did not compare RANTES levels in peripheral blood with those in cerebrospinal fluid,so we cannot evaluate the situation in the brain itself. It is known that the peripheral immune system and brain communicate and influence each other in both physiological and pathological conditions (Holmes and Butchart, 2011;Hernández-Romero et al., 2012; Hoogland et al., 2015).
In conclusion, our study demonstrated that patients with early stage of Alzheimer’s disease had many times higher plasma RANTES levels compared to controls, and this was not explained by any of the possible confounding clinical and metabolic factors studied. This supports the potential of RANTES as a biomarker of early neurodegeneration. However,it is important to note that the specificity and sensitivity of this marker should be evaluated in large cohorts of patients with different types of neurodegenerative and metabolic diseases.
Author contributions:Sample collection, biochemical and data analysis, and manuscript preparation: GV; Statistical analysis, manuscript proofreading: DV;selection of Alzheimer’s disease patients and tests: RR; selection of controls and tests: IH; tests of controls and data analysis: HV and EJ; biochemical analysis: JV; manuscript revision: BB; data collection, statistical analysis,manuscript revision: MV; approval of final version of the manuscript: all authors.
Conflicts of interest:There are no conflicts of interest associated with publication of this paper.
Financial support:This work was supported by the grant MH CZ NV 18-01-00399 from the Czech Ministry of Health.
Institutional review board statement:The study was approved by the Ethics Committee of Institute of Endocrinology, Prague, Czech Republic on July 22,2011, and performed in accordance with the Declaration of Helsinki.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the forms, the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:Statistical methods of this study were reviewed by biostatisticians of Institute of Endocrinology, Czech Republic.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Patterning inconsistencies restrict the true potential of dopaminergic neurons derived from human induced pluripotent stem cells
- Efficacy of epothilones in central nervous system trauma treatment: what has age got to do with it?
- Association between plasma immunoproteasome and 90-day prognosis after first-ever ischemic stroke
- Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer’s disease
- Co-nanoencapsulated meloxicam and curcumin improves cognitive impairment induced by amyloid-beta through modulation of cyclooxygenase-2 in mice
- Comparison of cerebral activation between motor execution and motor imagery of self-feeding activity

