Comparison of cerebral activation between motor execution and motor imagery of self-feeding activity
Moemi Matsuo , Naoki Iso, Kengo Fujiwara , Takefumi MoriuchiDaiki Matsuda , Wataru Mitsunaga , Akira Nakashima Toshio Higashi
Abstract Motor imagery is defined as an act wherein an individual contemplates a mental action of motor execution without apparent action. Mental practice executed by repetitive motor imagery can improve motor performance without simultaneous sensory input or overt output. We aimed to investigate cerebral hemodynamics during motor imagery and motor execution of a self-feeding activity using chopsticks. This study included 21 healthy right-handed volunteers. The self-feeding activity task comprised either motor imagery or motor execution of eating sliced cucumber pickles with chopsticks to examine eight regions of interest: pre-supplementary motor area, supplementary motor area, bilateral prefrontal cortex, premotor area, and sensorimotor cortex. The mean oxyhemoglobin levels were detected using near-infrared spectroscopy to reflect cerebral activation. The mean oxyhemoglobin levels during motor execution were significantly higher in the left sensorimotor cortex than in the supplementary motor area and the left premotor area.Moreover, significantly higher oxyhemoglobin levels were detected in the supplementary motor area and the left premotor area during motor imagery, compared to motor execution. Supplementary motor area and premotor area had important roles in the motor imagery of self-feeding activity. Moreover, the activation levels of the supplementary motor area and the premotor area during motor execution and motor imagery are likely affected by intentional cognitive processes. Levels of cerebral activation differed in some areas during motor execution and motor imagery of a self-feeding activity. This study was approved by the Ethical Review Committee of Nagasaki University (approval No. 18110801)on December 10, 2018.
Key Words: Activities of Daily Living; brain function; hemodynamics; imagery(psychotherapy); mental practice; motor cortex; near-infrared neuroimaging; neuroscience;rehabilitation; spectroscopy
Introduction
Motor imagery (MI) is an act wherein an individual contemplates mental action of motor execution without obvious action (Decety, 1996; Blefari et al., 2015). Mental practice (MP), executed by repetitive MI, aims to improve motor performances without any simultaneous sensory input or overt output (Decety and Ingvar, 1990). MP can complement physical exercise because it can be undertaken by patients who are in acute recovery, wherein they cannot conduct motor execution (ME) due to reduced voluntary movement post-injury. Moreover, MP can be executed safely anywhere and has been shown to improve upper limb function after stroke (Park et al., 2018). In addition, a combination of MP and physical practice can improve motor performance more than physical practice alone (Wriessnegger et al., 2014).
The effects of MP have been demonstrated by many studies that compared cerebral activity during ME and MI, and reported that near-identical brain areas were activated during ME and MI (Jeannerod, 1995; Decety, 1996; Wriessnegger et al., 2008; Wang et al., 2014). A meta-analysis of activationlikelihood estimation suggested that MI relies on a network involving motor-related regions, including the premotor area (PMA), supplementary motor area (SMA), parietal lobule, cerebellum, and basal ganglion, which supports the view that ME and MI are very similar processes (Hetu et al., 2013). However, most of these reports used simple movements as tasks; only a few reports, to date, have used complex movements that are essential in clinical scenarios in rehabilitation medicine.
A thorough understanding of cerebral hemodynamics during MI of complex movements is essential to use MP in relevant clinical scenarios. Cerebral hemodynamics during MI of the self-feeding activity task, one of the activities of daily living,remain unclear. We selected self-feeding as the MI task because it is frequently used for stroke patients undergoing rehabilitation, and it is a complex activity that engages various movements, including arm and finger movements, chewing and swallowing, and using cutlery (e.g., chopsticks). Therefore,MP with MI of self-feeding is likely beneficial for patients with dominant-hand hemiplegia.
In our previous research, we investigated differences in cerebral hemodynamics between self-feeding MI using chopsticks with the right and left hands. However, we did not compare the ME and MI of the task (Matsuo et al., 2019).Among the previous studies involving tool-use MI, a functional magnetic resonance imaging-based assessment indicated that near-identical cerebral regions were excited with either tool-use ME or MI, although differences in excitability were observed (Higuchi et al., 2007). However, a study of nearinfrared spectroscopy (NIRS) suggested that MI task with tool use can significantly increase oxyhemoglobin (oxyHb) levels in motor-related cerebral areas than MI task without tool use (Oikawa et al., 2017). Thus, our main objective was to investigate the cerebral hemodynamics associated with the MI and ME of a self-feeding activity with chopsticks.
Subjects and Methods
Subjects
This observational study recruited 21 right-handed volunteers,14 females and 7 females, aged 29.4 ± 10.2 years, from Nagasaki University via recruitment advertisement. The righthandedness of participants was confirmed by the Edinburgh Handedness Inventory (Oldfield, 1971). The safety regulations of this research work were explained to all participants. All participants assured that none of their identifying information would be disclosed. Therefore, all participants provided written informed consent for study participation. Additional informed consent was also obtained from all individual subjects whose identifiable information was included in this study. None of the study participants had a history of major physical disorders, including neurological illness, brain injury,or psychiatric illnesses. This study was approved by the Ethical Review Committee of Nagasaki University (approval No. 18110801) on December 10, 2018 and was performed in accordance with the principle of theDeclaration of Helsinki(World Medical Association, 2013) and its later amendments.
Experimental Protocol
Task
The participants were required to eat sliced cucumber pickles with the right hand using chopsticks, which involve actions including reaching to the mouth, chewing, and then swallowing. Participants were asked to execute serial movements of the task within 20 seconds. These movements were conducted under ME and MI conditions.
All participants practiced each movement before the experimental session until they could undertake the task sufficiently well during the experiment. The participants checked the actual slices of cucumber pickles on a dish with chopsticks, and repeated the task practice while monitoring
the time required to perform it using a stopwatch.
Experimental setup
The participants sat on a chair with a backrest in a silent room.They placed their forearms in an intermediate and relaxed position on the table and were instructed to carry out the actual self-feeding activity with their eyes open during the ME condition, and to imagine the task with their eyes closed without other movements during the MI condition. Moreover,they were instructed not to move their head, to maintain the same posture as much as possible, and not to speak during both conditions throughout the experiment. In addition, they were asked to maintain the same posture and relax without thinking for the remainder of the session.
After the MI task, the visual analog scale was used to investigate MI vividness, which has been previously described(Lotze et al., 2003; Lotze and Halsband, 2006; Malouin et al., 2008; Ikeda et al., 2012). All participants assessed the vividness of the MI task, which they marked on the visual analog scale: range from 0 to 100 mm (0%; none, 100%; very vivid).
NIRS measurements
Cerebral activation was investigated using the NIRS,which has been applied in clinical scenarios. The levels of oxyHb, deoxygenated hemoglobin, and total hemoglobin were measured with the 24-channel ETG-4000 (Hitachi Medical Co., Tokyo, Japan). The 24-channel ETG-4000 has 8 emitters and detectors, and comprises 4 × 4 probe sets.Every probe was positioned 3 cm away from each other and placed in accordance with the international 10–20 electroencephalography placement method (Okamoto et al., 2004). Then, Cz was marked as a reproducible marker in the probes, as described previously (Okamoto et al., 2004;Saimpont et al., 2015; Figure 1).
Eight regions of interests (ROIs) were examined: the presupplementary motor area (Pre-SMA), SMA, the bilateral prefrontal cortex (PFC), PMA, and sensorimotor cortex (SMC;Figure 1). The activation of the Pre-SMA, SMA, and left and right PFC (L-PFC/R-PFC) was detected by channels 2, 5, 6,and 9; channels 9, 12, 13, and 16; channels 1 and 4; and channels 3 and 7, respectively. The activation of the left and right PMA (L-PMA/R-PMA) was detected by channels 8, 11,and 15 and by channels 10, 14, 17, respectively, whereas the activation of the left and right SMC (L-SMC/R-SMC) was detected by channels 18 and 22, and by channels 21 and 24,respectively (Hatakenaka et al., 2007; Sagari et al., 2015; Iso et al., 2016; Matsuo et al., 2019). The levels of oxyHb and deoxygenated hemoglobin were calculated based on the levels of hemoglobin detected by the modified Beer–Lambert approach (Cope and Delpy, 1988; Obrig and Villringer, 2003).
After completion of the task practice session, NIRS measurements were taken with three cycles of 20-second task and 30-second rest in a blocked design (Figure 2) as described previously (Iso et al., 2016; Matsuo et al., 2019). The blocked design was used in each condition; thus, there were three cycles of the task each under MI and ME conditions. All participants were assessed under each condition, with the condition order (ME or MI) equilibrated. The participants were instructed to execute or imagine eating a pickle once for a 20-second duration. Under the MI condition, the participants were instructed to imagine they were eating the pickle. Thus,this study involved the participants imagining a first-person view with kinesthetic imagery, which included sounds. Thetiming of the task or rest was indicated by the words “start” or“stop”, spoken by the examiner.
Data analysis
Changes in the concentration of oxyHb were used to indicate brain activation during tasks, because oxyHb is the most sensitive parameter for detecting cerebral activity (Hoshi et al., 2001). We used the 5-second moving average method to exclude short-period motion artifacts from the analyzed data,and analyzed the data using the integral mode to calculate average values.
For data analysis, we considered the data measured at 5 seconds before the task as the pre-task baseline, and the data measured at 5 seconds after the task was completed as the post-task baseline; thereafter, we applied linear fitting to the data between these baselines. Moreover, we excluded the first 5 seconds of tasks from the analyses to ensure the data were unaffected by the time required for cerebral activation to occur. Thus, we included the last 5 to 20 seconds of the task duration for analyses (Figure 3). Next, we calculated data from three cycles with the signal-averaging technique. The 3-Hz high-pass filter of the wave analysis marked channels that included high noise levels to exclude noise such as hyperactivation caused by skin and blood dynamics (Takahashi et al., 2011).
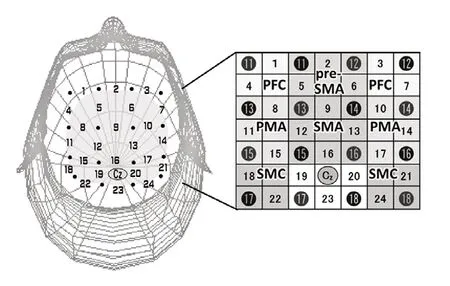
Figure 1 |NIRS probe sets.
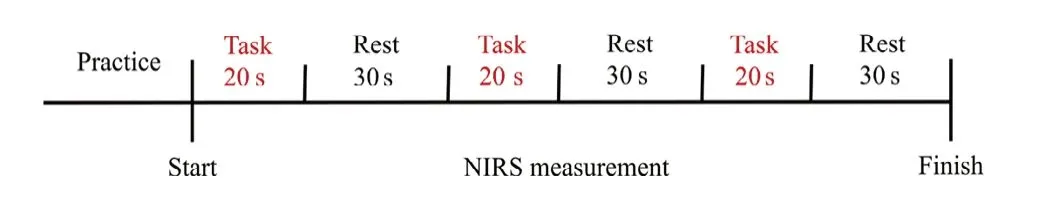
Figure 2 |Experimental protocol.

Figure 3 |Method of calculating OxyHb changes.
In addition, surface electromyography was applied to ensure that participants did not move under the MI condition. The surface electromyography electrodes were positioned over the right masseter and the first dorsal interosseous muscles of the right hand, and signals were amplified and filtered at 5 to 3 kHz of bandwidth with the Neuropack Sigma MEB-5504 digital signal processor (Nihon Kohden, Tokyo, Japan). An A/D converter transferred the signals to a PC for offline analysis(PowerLab16/30, AD Instruments, Sydney, Australia). During surface electromyography measurements, we ensured that the finger and face postures of all participants were immobile,without overt muscle movement. Therefore, all the data were used for analyses.
Statistical analysis
The mean oxyHb levels under the ME and MI conditions were calculated, and data were converted to the Z-score. We obtained mean 0 and up to 1 standard deviation (Tsunashima and Yanagisawa, 2009), also the higher score indicates higher oxyHb, through calculations undertaken in the ETG-4000. The two-way repeated measures analysis of variance (ANOVA) was used taking “ROIs” (Pre-SMA, SMA, L- and R-PFC, L- and R-PMA,as well as L- and R-SMC) and “conditions” (ME and MI) as factors to compare differences in the Z-scores. Furthermore,the Bonferroni test was used to compare the Z-scores. SPSS 22.0 (IBM, Tokyo, Japan) was used for data analysis. A level ofP< 0.05 was considered statistically significant.
Results
Handedness and the vividness of MI
The mean ± SD Edinburgh Handedness Inventory score of all participants was 88.6 ± 12.0; in addition, 76% of participants answered “always using the dominant hand” to the question relevant to self-feeding. Furthermore, none of the participants answered “always using the non-dominant hand”.All participants self-reported that they have used chopsticks with their dominant hand nearly every day for approximately 20 years or more, indicating that they had a proficient level of skill in using chopsticks. Moreover, the mean visual analog scale value (vividness of the MI task) of all participants was 73.1 ± 10.4 mm.
Comparison of cerebral hemodynamics between ME and MI
Figure 4 shows the grand average waveforms of oxyHb during tasks for all subjects. During tasks, the oxyHb levels increased over the baseline value in most ROIs. The significant main effect of “ROIs” (F(7,14)= 4.918,P= 0.000,η2= 0.193), and a significant “ROIs *conditions” interaction (F(7,14)= 2.221,P=0.036,η2= 0.114) were revealed by two-way ANOVA, whereas there was no main effect of “conditions” (F(1,20)= 0.002,P
= 0.968,η2= 0.000). Based on the main effect of ROI and interaction of ROI*conditions, we assessed Z-score differences
with the Bonferroni test, and found that the Z-score under the ME condition was significantly higher in the L-SMC than in the SMA (P= 0.033) and L-PMA (P= 0.039), whereas there were no apparent differences between ROIs during the MI.
Furthermore, significantly higher oxyHb levels were detected in the SMA (P= 0.017) and L-PMA (P= 0.012) during MI, in contrast to the levels that were observed during the ME;however, there were no apparent differences in the other ROIs (Figure 5).
Discussion
In this study, we investigated the cerebral hemodynamics during ME and MI of a self-feeding activity with chopsticks using NIRS. A previous study using a simple finger-tapping task suggested that increases in oxyHb levels in the SMA and PMA during MI were similar to those observed during ME(Iso et al., 2016), although another study that used the same task suggested that the PFC was more activated during MI than during ME, whereas the left SMA was more activated during ME than MI (Wu et al., 2018). Furthermore, an fMRI study suggested that stronger cerebral activations were observed in the PMA and the SMA during ME than during MI of swallowing, which is a simple task (Kober et al., 2019).As such, many previous MI studies using simple tasks have suggested that near-identical brain areas are activated during MI and ME, although the brain areas were more activated during ME than during MI. However, our results suggested that, although MI and ME of self-feeding activity as a tooluse complex task activated mostly overlapping cerebral areas, these activations were not identical, with MI inducing higher activation levels in some areas compared to ME. The Z-scores during ME were significantly higher in the L-SMC than in the SMA and the L-PMA. Moreover, cerebral activation differences were observed between ME and MI conditions,with significantly higher oxyHb levels detected under the MI condition than the ME condition in the SMA and the L-PMA;these results were not identical to those reported with simple MI tasks.
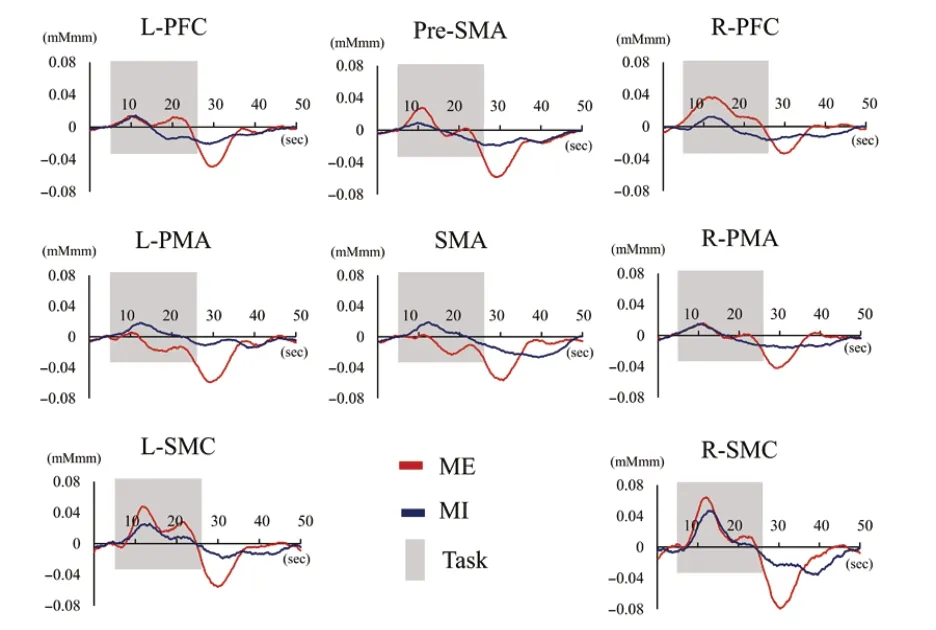
Figure 4 |OxyHb time course.
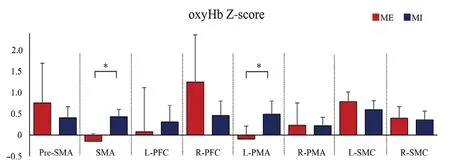
Figure 5 |Comparison of cerebral Hemodynamics between ME and MI.
The SMA and PMA are known to play roles in motor planning,motor preparation, and motor learning (Hoshi and Tanji, 2004;Nakayama et al., 2008; Wang et al., 2016). MI is equivalent to the motor planning and preparation for ME (Tong et al., 2017).A previous study suggested that SMA activation was mainly observed in MI, with partially overlapping activation during ME and MI (Stephan et al., 1995). Moreover, other studies suggested that SMA activation affected the inhibition of the SMC during MI (Di Rienzo et al., 2014; Gao et al., 2014). Some neurons of the PMA may be solely involved in the MI task(Raffin et al., 2012); the PMA serves as a hub that connects cognition and motor activities (Hanakawa, 2011). Both SMA and PMA activations during ME and MI are affected by the task experiences and the task-proficiency levels; moreover,they are dependent on the task specificity and are changeable by the degree of practice. Thus, low activity may indicate low nerve activation levels; however, low activity can also imply better task proficiency (Debarnot et al., 2014).
All participants were familiar to the self-feeding activity using chopsticks with the right hand, which was used in the present study because they had executed the task nearly every day for at least 20 years. Thus, the task was easy for them and participants could carry it out expertly and did not need to intentionally implement the cognitive process. Thus, SMA and PMA activation might have been reduced during ME, and the differences in the SMA and PMA activation between ME and MI might be related to a need for intentional cognitive processes. Overall, our results further expand the knowledge with regard to MI and may be useful in developing MP in rehabilitative fields.
Research limitations
The participants were all right-handed volunteers who were skilled in the use of chopsticks; therefore, it is unclear whether our results can be generalizable to individuals unskilled in the use of chopsticks, left-handed people, or patients with motor diseases or disabilities. Furthermore, the task was limited to a self-feeding activity; thus, it is unclear whether cerebral hemodynamics during other complex tasks are similar to those observed during the self-feeding activity. In addition,our study only included a small number of participants.Because of these limitations, future studies should include more subjects with various conditions for MP, as well as left-handed subjects, and investigate cerebral activation during various tasks, including other complex tasks to confirm the effectiveness of MP in rehabilitation.
Conclusion
Levels of cerebral activation differed in some areas during ME and MI of a self-feeding activity. SMA and PMA have important roles in the MI of self-feeding activity. Furthermore, levels of activation in the SMA and PMA during ME and MI were affected by the necessity of intentional cognitive processes.Our findings expand the knowledge base of MI, and provides further understanding of the use of MI in rehabilitation for activities of daily living. Therefore, this study might contribute to the application of MP in clinical situations.
Acknowledgments:We would like to thank a statistician Dr. Sumihisa Honda for supporting and giving us knowledge about statistical analysis.
Author contributions:MM contributed to the research concept,design, definition of intellectual content, literature search, experimental studies, data acquisition and analysis, statistical analysis, manuscript preparation, editing, and review. NI and KF contributed to the research concept, design, definition of intellectual content, literature search, and experimental studies. TM contributed to definition of intellectual content,statistical analysis, manuscript editing and review. DM contributed to experimental studies. WM and AN contributed to literature search. TH contributed to the research concept, design, definition of intellectual content, literature search, statistical analysis, manuscript editing and review. All authors approved the final version of this paper.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Financial support:None.
Institutional review board statement:This study was approved by the Ethical Review Committee of Nagasaki University (approval No.18110801) on December 10, 2018 and was performed in accordance with the principles of the Declaration of Helsinki (World Medical Association,2013) and its later amendments.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening theReporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Sumihisa Honda, Nagasaki University in Japan.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ryota Nishiyori, University of Southern California,USA; Jianjun Sun, Third Hospital of Peking University, China.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Patterning inconsistencies restrict the true potential of dopaminergic neurons derived from human induced pluripotent stem cells
- Efficacy of epothilones in central nervous system trauma treatment: what has age got to do with it?
- Association between plasma immunoproteasome and 90-day prognosis after first-ever ischemic stroke
- Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer’s disease
- Co-nanoencapsulated meloxicam and curcumin improves cognitive impairment induced by amyloid-beta through modulation of cyclooxygenase-2 in mice
- Regulated upon activation, normal T cell expressed and secreted (RANTES) levels in the peripheral blood of patients with Alzheimer’s disease

