The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches
Magdalini Tsintou , Kyriakos Dalamagkas , Tara L. Moore, Yogesh Rathi Marek Kubicki Douglas L. Rosene, Nikos Makris ,
Abstract Neural tissue engineering, nanotechnology and neuroregeneration are diverse biomedical disciplines that have been working together in recent decades to solve the complex problems linked to central nervous system (CNS) repair. It is known that the CNS demonstrates a very limited regenerative capacity because of a microenvironment that impedes effective regenerative processes, making development of CNS therapeutics challenging. Given the high prevalence of CNS conditions such as stroke that damage the brain and place a severe burden on afflicted individuals and on society, it is of utmost significance to explore the optimum methodologies for finding treatments that could be applied to humans for restoration of function to pre-injury levels. Extracellular vesicles(EVs), also known as exosomes, when derived from mesenchymal stem cells, are one of the most promising approaches that have been attempted thus far, as EVs deliver factors that stimulate recovery by acting at the nanoscale level on intercellular communication while avoiding the risks linked to stem cell transplantation. At the same time, advances in tissue engineering and regenerative medicine have offered the potential of using hydrogels as bio-scaffolds in order to provide the stroma required for neural repair to occur, as well as the release of biomolecules facilitating or inducing the reparative processes. This review introduces a novel experimental hypothesis regarding the benefits that could be offered if EVs were to be combined with biocompatible injectable hydrogels. The rationale behind this hypothesis is presented, analyzing how a hydrogel might prolong the retention of EVs and maximize the localized benefit to the brain. This sustained delivery of EVs would be coupled with essential guidance cues and structural support from the hydrogel until neural tissue remodeling and regeneration occur. Finally, the importance of including nonhuman primate models in the clinical translation pipeline, as well as the added benefit of multi-modal neuroimaging analysis to establish non-invasive, in vivo, quantifiable imagingbased biomarkers for CNS repair are discussed, aiming for more effective and safe clinical translation of such regenerative therapies to humans.
Key Words: cortical injury; exosomes; extracellular vesicles; hydrogels; neural tissue engineering; neural tissue repair; neuroregeneration; non-human primates; stroke
Introduction
Cortical injury, due to stroke or other insults, is a leading cause of long-term disability, including deficits in speech, motor function, cognition and affect. To gain a basic understanding of the clinical significance and pressing need to address this problem one has to consider the epidemiology of stroke. On average, someone in the United States has a stroke every 40 seconds, and there are about 401 deaths from stroke each day, based on 2017 data (Virani et al., 2020). At a molecular and cellular level, cell death and oxidative damage during the acute phase of cortical injury have been known to stimulate a prolonged pro-inflammatory state cascade (Warner et al.,2004; Crack and Taylor, 2005; Margaill et al., 2005; Wang et al., 2007; Lakhan et al., 2009). This, in turn, aggravates the initial damage and leads to continued death and decay in the brain, limiting recovery and function.
There is an undeniable critical need for therapeutic interventions to enhance recovery from cortical injury with the cascade of post-injury neurodegenerative events a critical target. To this end, several regenerative treatment strategies have been attempted, but each is linked to certain limitations.Various types of stem cells have been used for stroke therapies with promising results in preclinical animal models.However, only limited types of stem cells have reached clinical trials due to concerns that might be linked to their use (e.g.,ethical or tumorigenesis-related) and inadequate data for clinical translation. Currently, based on the registered clinical trials, the preferred major stem cell lines in use to treat stroke are mesenchymal stem cells (MSCs), hematopoietic stem cells and bone marrow mononuclear cells (Tsintou et al., 2020).
The need to a) minimize any adverse effects due to systemic administration of therapeutics, and b) directly target the brain lesions without the need for bioactive agents to cross the blood-brain barrier, initially led to the injection of drugs and biologics in aqueous solution (viz., phosphate buffered saline). It is worth noting that the results, which fell short of expectations, were due to dispersion of agents from the target site that motivated the implementation of biomaterial delivery systems to retain the agents at the injection site and to provide controlled release. Biomaterial delivery vehicles, some of which can also serve as sustained delivery system for therapeutics into the brain, have led to exploration of the ideal carriers.Thus, scaffolds to accommodate host cell migration/infiltration have been engineered and seeded with promising stem cells and molecules; proteins for example can be incorporated directly into the gel or first encapsulated in, or bound to, nanoor micro-particles including liposomes which in turn are added to the delivery gel. Only a small number of nanocarriers have yet been approved by the FDA (Bobo et al., 2016). In recent years, the efforts to replicate biological carriers are giving place to efforts to enhance carriers already existing in the human body, such as extracellular vesicles (EVs), including nanometersize exosomes; for the purpose of this review these terms will be used interchangeably. EVs are endogenous and natural nano-sized carriers that can be harvested from a variety of cultured cells, conferring different therapeutic potential.Bioengineering EVs though has also become possible, altering the EVs’ therapeutic potential if needed.
Due to their nano-size, EVs demonstrate unique characteristics in terms of delivery of biomolecules. EVs have been shown to retain a sensitive cargo and move unabated from one location of the body to another. The currently used routes of administration for EVs in most research protocols, as well as their biogenesis and effect in the central nervous system (CNS)are illustrated in Figure 1. Overall, they seem to hold promise as an ideal delivery system. Their advantages include decreased rate of elimination from tissues (approximately 6 hours for most tissues) (van der Meel et al., 2014), low clearance levels and degradation, and preservation of their cargo’s therapeutic activity (Smith et al., 2015). Nevertheless, there are reports suggesting that unconjugated EVs have challenges related to rapidin vivoclearance by the innate immune system (Imai et al., 2015). Conjugation of certain ligands to the EVs surface has been attempted to alter cellular interactions with the EVs and potentially the EVs’ biodistribution (Smyth et al., 2014).However, retaining unconjugated EVs in the damaged tissues for an extended period of time is certainly problematic (Subra et al., 2007; Imai et al., 2015).
Despite these issues, IV EV treatments have demonstrated very promising results in animal models with small,circumscribed experimental lesions, including non-human primates (NHPs) (Moore et al., 2019). In the study by Moore et al. (2019), EVs were isolated from MSCs culture media and, after being purified and reconstitutedex vivo, they were systemically injected for treating the NHPs with cortical injury. Still, medium or large size lesions that are frequently present in the human brain have not been experimentally addressed to date. Extended retention of EVs within the brain parenchyma could be crucial in such cases. Therefore,systemic treatment with EVs might not be sufficient for the kind of lesions frequently found in the human population,posing a problem for effective clinical translation. To this end,the use of local biomaterial delivery vehicles seems to be a logical approach. A promising type of scaffold suitable for CNS applications would be an injectable, biodegradable hydrogel matrix that mimics brain tissue in certain mechanical behavior.Thus, the combined use of EVs with hydrogels as scaffolds for delivery of EVs into the brain could represent a testable experimental model to assess translational implementation in human brains affected by injury such as stroke.
In this review, we introduce a hypothesis for an experimental model of a combinatorial therapeutic approach with intracerebral EV/hydrogel matrices and analyze the rationale behind the enhancement of an already promising therapeutic strategy using intravenous EVs following stroke. Theoretically,were we to combine promising therapies using EVs with a targeted sustained delivery system using a scaffold such as a hydrogel applied directly to a brain lesion, this might enhance the current positive results of EV treatment to promote an even higher level of functional recovery. This proposed combinatorial therapeutic intervention if optimized in a NHP model, despite differences in neuroanatomy and brain size, may be the key for altering the EV bioavailability and biodistribution enough to potentially allow treatment of large human brain lesions. Finally, this review highlights the importance of adapting non-invasive, quantifiable,in vivoneuroimaging methodologies to assess, longitudinally,biomarkers of brain tissue repair processes, bridging preclinical and clinical studies.
For the purposes of this review we conducted an electronic search on PubMed and Google Scholar using search terms such as “stroke AND extracellular vesicles,” “stroke AND stem cells AND extracellular vesicles,” “stroke AND extracellular vesicles AND monkeys,” “mesenchymal stem cells AND extracellular vesicles AND mechanisms of action,” “extracellular vesicles AND route of delivery,” “extracellular vesicles AND labeling AND imaging,” “stem cells AND extracellular vesicles AND central nervous system AND limitations,” “scaffolds AND tissue engineering history,” “extracellular vesicles AND scaffolds AND stroke,” “hydrogels AND stoke AND extracellular vesicles,” “form AND function AND philosophy.” All searches were repeated by substituting the word “exosomes” for“extracellular vesicles.” Articles were reviewed for each search after being sorted by “best match.” Subsequently, the results of the same search were sorted by “most recent.” The results were further screened by title and abstract to ensure relevance to the reviewed topics. Up to 100 articles were reviewed for each search outcome with no filtering based on publication dates to avoid missing important historic neuroanatomical data. Certain significant citations within the papers examined were also reviewed after independent searches. Clinical trials were also explored in Clinicaltrials.gov using the term “stroke”as the “Condition or disease” and the term “exosomes” as“Other terms” with the search repeated without including the condition “stroke” to capture the whole landscape of clinical trials using stem cells-derived exosomes.
Extracellular Vesicles and Recovery of Function after Brain Injury
EVs are endosome-derived small extracellular vesicles released from cells to the extracellular space after an intermediate endocytic compartment, the multivesicular body, is fused with the plasma membrane (Edgar, 2016). They were first observed by electron microscopy in the 1980s. However, until the early 2000s, they were considered to be cellular garbage carriers full of expired proteins without any other significant role. In the early 2000s this viewpoint changed when EVs were discovered to contain and transfer functional RNA to target cells (Valadi et al., 2007). They are now recognized as important and universal nano-sized agents (30–100 nm) of intercellular communication. EVs shuttle signaling molecules,proteins, lipids, messenger ribonucleic acid (mRNA), microribonucleic acid (miRNA), small interfering ribonucleic acid(siRNA), long non-coding ribonucleic acid (IncRNA), and extrachromosomal deoxyribonucleic acid (DNA) within the body(Kalra et al., 2012).
In the history of regenerative neurology there was originally a misconception that transplanted stem cells induced their therapeutic outcomes by differentiating into the target tissue.More recent studies suggest that transplanted stem cells are most likely acting in a paracrine manner, by secreting EVs(Ratajczak et al., 2012; Shen et al., 2013; Liang et al., 2014;Song et al., 2014) that promote tissue repair and neural regeneration. It is now believed that EV products induce epigenetic changes in the recipient’s cells, positively regulating their fates by promoting proliferation or inhibiting apoptosis(Zhou et al., 2013; Tan et al., 2014; Nakamura et al., 2015;Zhang et al., 2015; Nong et al., 2016; Qi et al., 2016; Zhang et al., 2016; Lee et al., 2018). In the acute phase of stroke,prolongation of the pro-inflammatory state cascade is directly linked to continuation of neuronal death and increased functional deficits. EVs derived from mesenchymal stem cells(MSCs) have shown promise for reducing inflammation and enhancing functional recovery in rodent models of stroke(Zhang and Chopp, 2016; Bang and Kim, 2019). There are currently three Phase I/II clinical trials in ClinicalTrials.gov assessing the safety and efficacy MSCs-derived EVs in patients with CNS conditions (e.g., ischemic stroke, depression, anxiety and dementias); two use intravenous administration for the EV treatments (ClinicalTrials.gov Identifiers: NCT03384433;first trial on ischemic stroke completed in December 2019, but with the results remaining unpublished; and NCT04202770,still recruiting by invitation) and the last one uses intranasal administration (ClinicalTrials.gov Identifier: NCT04388982, not yet recruiting).
Experimental Animal Models – Rodents and Monkeys
A recent research study in monkeys, published by Moore et al.(2019) indicate that intravenous (IV) delivery of MSCs-derived EVs after cortical injury promotes functional recovery that can be observed within 3–5 weeks of injury, likely through reduction of inflammation. This is the first study to our knowledge, in which IV administration of MSCs-derived EVs in monkeys after cortical injury produced significant functional recovery of fine motor function of the hand, with grasp patterns returning to pre-injury levels within the first weeks following injury.All monkeys treated with EVs in that study returned to preoperative grasp patterns and latency to retrieve a food reward within the first three to five weeks of recovery.
The activation status of microglia, the innate macrophages of the brain, is critical to cell fate after injury. Based on Go et al. (2019) analysis of our aged rhesus monkey brain tissue harvested 14–16 weeks post-injury, the IV EV treatment limited acute damage after cortical injury by directly acting on microglia to promote a rapid proinflammatory to anti-inflammatory cascade, and transition to a restorative microenvironment. These findings thus demonstrate that EV treatment after injury is associated with greater densities of ramified, homeostatic microglia,along with reduced pro-inflammatory microglial markers.These observations are consistent with a phenotypic switch of inflammatory hypertrophic microglia towards antiinflammatory, homeostatic functions, which correlated with enhanced functional recovery. Moreover, these data suggest that EVs reduce neuroinflammation and shift microglia toward restorative functions. Furthermore, these findings demonstrate the therapeutic potential of MSC-derived EVs for reducing neuroinflammation after cortical injury in the brains of aged monkeys, which holds promise for clinical translation.
In a follow-up study of the same NHP model of cortical injury(Medalla et al., 2020), in an attempt to further explore the EV treatment-induced changes to the brain tissue, Medalla and colleagues demonstrated a reduction of injury-related physiological and morphologic changes in perilesional layer 3 pyramidal neurons. This study indicated that the observed EV-mediated enhancement of recovery is “associated with amelioration of injury-related hyperexcitability and restoration of excitatory-inhibitory balance in perilesional ventral premotor cortex.” Therefore, this study highlights another mechanism by which the EV treatment supports the recovery of function after cortical injury.
These results in NHPs are supported by several rodent preclinical studies (Zhang and Chopp, 2016; Otero-Ortega et al., 2017; Otero-Ortega et al., 2018; Zhang et al., 2018;Bang and Kim, 2019). Otero-Ortega et al. (2017) performed extensive analysis that included biodistribution, proteomics analysis, functional evaluation, lesion size measurement,fiber tract integrity, axonal sprouting and white matter repair markers in order to study EVs effects in rodent models. In their subcortical preclinical stroke model, a single administration of EVs was shown to improve functional recovery, fiber tract integrity, axonal sprouting, and white matter repair markers.Molecular repair factors implicated in axonal sprouting, tract connectivity, remyelination and oligodendrogenesis were found to be partially responsible for the white matter integrity restoration after administration of EVs. The findings from rodent preclinical models were associated with improved functional recovery like that observed in our NHP model.
Nevertheless, despite promising preclinical outcomes,therapeutics based on MSC-derived EVs face challenges due to their short half-life and rapid clearance by the innate immune systemin vivo(Imai et al., 2015). The biodistribution of EVs in the body can reduce the efficacy of the therapy, because the EVs are not targeted only toward brain tissue but also found in peripheral organs soon after administration (Otero-Ortega et al., 2017, 2018). Another factor affecting the outcome is that damaged neural tissue, especially in larger lesions in humans,requires time to heal through a complex multiphase process.However, prior studies indicate that retaining unconjugated EVs at the brain lesion site for an extended period may be difficult (Imai et al., 2015). The use of nanotechnology andtissue engineering, as discussed below, could offer pioneering options to address these obstacles (Zhang et al., 2018).Surface functionalization of the EVs through conjugation of certain ligands to their surface is one of the attempts made to alter the cellular interactions with EVs and potentially the EVs’ biodistribution (Smyth et al., 2014). Nevertheless, a hydrogel could offer additional benefits. Hydrogel matrices have been considered highly biocompatible with brain tissue and can impede rapid clearance while accomplishing targeted,sustained release of EVs. Using a hydrogel system, the delivery of EVs can be tuned based on the period of time needed to attain a beneficial functional outcome with CNS tissue repair.
Peripheral versus Central Delivery of Extracellular Vesicles
One could argue that if peripheral delivery of EVs or other novel therapeutics produces significant restorative results, central delivery may not be needed. Nevertheless,intravenously administered, labelled MSC-derived EVs were found in brain tissue and in peripheral organs such as the lung, liver, and spleen at 24 hours after administration (Otero-Ortega et al., 2017, 2018). The EVs are rapidly cleared by the innate immune systemin vivo(Imai et al., 2015). Hence while peripheral systemic administration of EVs might be adequate for recovery after selective and circumscribed experimental brain lesions, brain lesions in humans are often large and irregular, perhaps explaining the longer time course of even spontaneous recovery (Tsintou et al., 2020). In these conditions it is plausible that more extended treatment and intracerebral retention of the EVs may be necessary to accomplish the optimal therapeutic outcomes. Hence intracerebral administration could potentially maximize the effect of the EVs in the brain by accomplishing a targeted,sustained local delivery in the brain lesion area and would,at the same time, minimize potential adverse effects of a non-targeted peripheral administration approach. Structural support in order to maintain the integrity of the brain tissue is also essential for restoration of function. Such support can be accomplished with the use of various scaffolds, but these must be injected intracerebrally to gelin situwhile conforming to the brain shape for structural and nutritional support stemming from the released EVs. Overall, the combination of intracerebral administration of a hydrogel that would embed EVs has considerable potential, when considering the clinical translatability of EVs therapy following stroke and other brain lesions.
Why Form Matters? – The Concept of Scaffolds and Biomaterials for Scaffolds Development
The concept of form and why it matters in biomedical sciences
To evaluate the significance of the introduction of scaffolds in the medical field, one should appreciate the true meaning of the concept of form (The Editors of Encyclopedia Britannica, 2011) and how it relates to restoration of function.Conventionally, the word “form” refers to the shape and material of an object. Aristotle in Metaphysics dealt with the concept of “form” in his theory of matter and form (Aristotle,Translated by Ross, 2006; Russell, 1989). “Aristotle would say that it is the form, when imposed on the matter, which makes the latter what it is” (Russell, 1989). Take, for instance,the raw material, the statue is made of, such as marble; this would be the matter. The form, instead, is the shape, the figure of the statue; “the shape that makes it the statue it is” (Robinson, 1995). Thus “it is form that is creative, matter being of course required too, but merely as raw material”(Russell, 1989). Moreover, it is clear that both, the form (or shape) and the matter (or material) of which a given object is made of are critical for this object’s function. The word “form”has been used in a number of ways throughout the history of philosophy and aesthetics and understanding its importance is crucial for medicine, and especially reconstructive neurosurgery.
Why is there such a large concern for maintaining form and structural integrity in medicine and disciplines such as reconstructive neurosurgery? To our knowledge, there is no straightforward answer to this complex issue. However,it seems that due to the limited potential of the CNS to be repaired and regain function after an injury or any other damage, it would not be ethical to attempt a surgery that potentially could put a patient at risk of not regaining function.Considering that endogenous tissue regeneration in the brain is essential for the plastic changes and reparative processes that start after CNS damage, in conjunction to the lack of cellular infiltration into the brain lesion cavity in the absence of a matrix substrate (e.g., bio-scaffold) that facilitates endogenous stem cell migration (Modo, 2019), one can detect a potential link of structure to the recovery of function.Given that we currently lack an objective and scientifically measurable answer to that question, our understanding is empirically based on clinical observations, a usual source of knowledge in the medical arena and clinical practice.
The relationship between form and function in biology and medicine has been historically a leading subject of discussion(Michael Ruse, 2016). Thought on morphogenesis has attempted to integrate several dimensions that influence the becoming of the form of an organism, including embryological and developmental as well as the interplay of genetic and epigenetic influences (Clark and Medawar, 1945; Richman et al., 1975; Caviness Jr. et al., 1995; Van Essen, 1997; Wedeen et al., 2012). Despite the inability to objectively assess the need to maintain form and structural integrity, researchers and surgeons tend to respect the shape of damaged tissues when attempting to restore function. To this end, the scaffolds widely known in the fields of mechanical and architectural engineering have inspired biomedical sciences to develop biological scaffolds, or bio-scaffolds. An eloquent parallelism between architectural engineering and tissue engineering is illustrated in Figure 2A, while Figure 2B highlights the conformation and precise spatial arrangement of injectable hydrogels to the brain lesion’s shape.
The concept of scaffolds in biomedical sciences and the genesis of tissue engineering
Scaffolds in biomedical sciences are structured, appropriately configured materials that have been engineered to support damaged tissues permanently or temporarily (until they degrade if they are biodegradable) until functional tissue replacement occurs. The work of several researchers has influenced the construction of bio-scaffolds. Judah Folkman was a distinguished medical scientist at the forefront of inquiries regarding cellular growth and histogenesis during the 1970s. Part of his work (Folkman and Moscona, 1978)revealed that a substrate could affect cellular shape and subsequently affect growth and cellular proliferation. He observed that dissociated cells were able to create structures if presented with cues from their native environment (Folkman and Haudenschild, 1980a, b). Such observations are still being used in tissue engineering when certain mechanical or other cues are introduced in the scaffolds to guide cellular growth and differentiation. Even though it was not until the 1990s that the term “tissue engineering” was used as it is applied today, principally to describe the formation of tissuein vitro,the seeds of tissue engineering extend back to the late 1970s when William T. Green, an orthopedic surgeon, used chondrocytes to grow cartilage (Green, 1977), using spicules of bone as a scaffold. Even though his attempts did not meet his expectations for the regeneration of cartilage, he laid the groundwork for the development of tissue engineering experiments. From earlier experimental work that influenced the genesis of tissue engineering (Russell, 1985; Atala and Mooney, 1997), it was discovered that isolated cells implanted into tissues as cell suspension would neither integrate with the tissue nor initiate regeneration. This was because they lacked a template guiding those processes. In other early work in the 1980s, the collaboration of Drs. Burke and Yannas at the Massachusetts General Hospital and Massachusetts Institute of Technology (M.I.T.) led to the development of a collagenglycosaminoglycan sponge-like dermal regeneration template to facilitate a regenerative response in skin wounds; this was the first clinically-successful achievement of biomaterialsbased regenerative medicine (i.e., regeneration of tissuein vivo). Subsequently, others demonstrated the generation of the first tissue-engineered skin substitute based on a collagen matrix seeded with fibroblasts (Vacanti, 2006). It was then in the 1990s that tissue engineering was seen as an emerging technology (Langer and Vacanti, 1993; Atala and Mooney, 1997; Schultheiss et al., 2000; Vacanti, 2006).That was when Joseph Vacanti approached Robert Langer to engineer scaffolds that could be manipulated for cell delivery as opposed to seeding cells on a naturally occurring matrix,which could act unpredictably. Tissue engineering entered public consciousness even later, after several centers had been founded around the world, in 1997 when the photo of the now infamous mouse with the human ear (Kruszelnicki,2006; Vacanti, 2006), fondly referred to as auriculosaurus from Vacanti’s laboratory, was circulated. Even though this photo was misleadingly used in anti-genetics campaigns that followed, it was a breakthrough for the field that had just been born, namely tissue engineering.
The evolution of bio-scaffolds and the importance of their composition depending on the application
Ever since the genesis of tissue engineering and regenerative medicine, biomaterial scaffolds have evolved with respect to their chemical make-up and structure/architecture. They are now successfully used for several applications, causing desirable cellular interactions. Many different materials with different classifications (i.e., natural or synthetic,biodegradable or permanent) have been investigated and used in tissue engineering in an attempt to maintain structural integrity of the damaged tissue and enhance endogenous or exogenous neuroregeneration following stroke (Wang,2017; Lim et al., 2019). In general, natural biomaterials offer the advantage of better biocompatibility and bioactivity, and some also have the advantage of possessing cell adhesion ligands. By contrast, synthetic biomaterials demonstrate less of a challenge in preparation. Exploring the appropriate composition of a scaffold to mimic the neural tissue, after considering the proposed application of the scaffold, can facilitate regulation of cell behavior and regeneration of injured nervous tissues.
Scaffolds can be used either alone or by incorporating cells,micro- or nano-carriers and other biomolecules such as growth factors or EVs depending on the intended application.The field has evolved to the level of engineering scaffolds at the nanoscale level (e.g., nanogels) (Tsintou et al., 2017).Overall, bio-scaffolds can be tuned in order to provide sustained release of the embedded cells or other incorporated agents. In this way they can continue to support the native tissue as long as needed for the repair to occur, providing structure and nutritional support for tissue remodeling and growth. For scaffolds to influence cellular behavior and growth that will become part of the native damaged tissue, they need to mimic the extracellular matrix of the native tissue and demonstrate similar viscoelastic properties.
In the CNS where the microenvironment is hostile for regeneration and for functional synapses to form after an injury, scaffolds can modulate the microenvironment in a favorable way. The axonal regeneration can be guided by mechanical and chemical cues so that elongation of tracts is not random, but part of a functional bridge creation for recovery of the damaged networks. Endogenous or exogenous stem cells can be attached, migrate and differentiate within the scaffold so that the tissue is remodeled using the sustained release of the nutrients from the scaffold, allowing the gap to be repaired by the time the scaffold degrades(Lim et al., 2019). Optimally, the degradation rate would need to be tuned for larger animal models and eventually for humans, yet the much larger size of the lesions would require longer recovery periods that cannot be tested in smaller animal models. Another factor that has become important as the field evolves and moves closer to clinical trials is the ability of biocompatible scaffolds for CNS applications to retain therapeutics, avoiding potential unwanted generalized effects or adverse reactions and maximizing the efficiency of the treatment with highly targeted localized release that can be tuned as needed. This was previously a major obstacle for neural repair, given the fact that therapeutic agents were often rapidly cleared or became unstable in the CNS microenvironment without proper structural support and protection (Liu et al., 2017; Zhang et al., 2018). Hence,the combinatorial therapeutic approaches with appropriate scaffolds become a rationale pathway for addressing the complexity of CNS regeneration and repair eventually in humans.
Emphasis on Hydrogels for Scaffolding and Slow Release of Extracellular Vesicles
Scaffolds for neural tissue engineering applications can take many forms, but one of the most promising for CNS repair is a hydrogel (Wang et al., 2018). A hydrogel is a threedimensional (3D) network of hydrophilic polymers. It is highly absorbent, being capable of containing over 90% water, while maintaining its structure due to chemical or physical crosslinking of the polymer chains. Hydrogels for biological use were first reported by Wichterle and Lím in 1960 (Wichterle and Lím, 1960). In tissue repair, hydrogels can offer not only structural support, but also a system of sustained delivery of bioactive substances (e.g., drugs, growth factors, EVs, cells,etc.) for targeted nutritional support of the lesion site. Not all hydrogels are made the same way, however, and not every hydrogel is a good choice for CNS repair. One should consider all the properties of a hydrogel, including its porosity, physical structure, and crosslinks, among others.
Several studies have explored the properties of the ideal hydrogel for CNS regeneration (Assunção-Silva et al., 2015;Tsintou et al., 2018; Tuladhar et al., 2018). Such a hydrogel should be (Tsintou et al., 2020) biodegradable for CNS repair applications, since the goal is to allow it to act as a true scaffold, offering structural and nutritional support for the neural tissue to grow and replace the hydrogel as it degrades without the need for a second surgery. Moreover, the degradation rate should be tuned along with other properties of the hydrogel (such as the porosity for the appropriate retention and sustained release of embedded biomolecules)so that the time is adequate for tissue remodeling and regeneration to occur. It is also important for the hydrogel to mimic the CNS microenvironment in terms of viscoelasticity,facilitating the migration and differentiation of endogenous stem cells within the hydrogel. Mechanical and topographical cues within the hydrogel can be used to guide axonal growth in the correct direction for the establishment of functional synapses, improving the regenerative outcomes. It should be noted that even though the use of such cues have been extensively studied for providing guidance of spinal cord axonal sprouting , the establishment of similar approaches for brain applications has been challenging (Nih et al., 2016),considering our limited understanding of factors that guide changes of intracortical connectivity patterns after brain damage (Nudo, 2013). Finally,in situgelation is essential when using a hydrogel for clinical applications in the CNS, since this allows for minimally invasive approaches, and therefore, faster recovery after the intervention, and also enables the hydrogel to conform to the space into which it is introduced, including a brain lesion where it offers support even in irregularly shaped tissue defects (Lim et al., 2019). Figure 2B is an illustrative demonstration of how a hydrogel behaves, fully supporting even irregular deep lesions.
Based on these features, it is reasonable to ask if a combinatorial approach using a hydrogel as the delivery substrate may help maximize the effect and impact of a therapeutic intervention with EVs. Figure 3Ademonstrates this combinatorial approach for intracerebral administration,which has not yet been tested. Figure 3B is an illustrative demonstration of our experimental hypothesis in a NHP model of cortical injury. A biodegradable injectable Chitosan-based hydrogel matrix has already been tested for the sustained delivery of EVs in a murine model of hindlimb ischemia (Zhang et al., 2018) among other applications (Ruel-Gariépy et al.,2004; Song et al., 2010). Based on the preclinical data (Zhang et al., 2018), the use of this or a similar hydrogel could be extended to a NHP model to offer structural support. Such an approach could, most importantly, offer a tunable targeted sustained delivery system for EVs. It could also facilitate endogenous stem cell adhesion, migration, and proliferation which might also be of value. In this way, EVs could theoretically avoid clearance in the brain and remain at the lesion site for sustained periods, thereby optimizing functional outcomes in the NHP model.

Figure 1 |The currently used routes of administration for EVs treatments for central nervous system repair, depending on whether genetically engineered cells producing labeled EVs or exogenous EVs previously labeled are used.
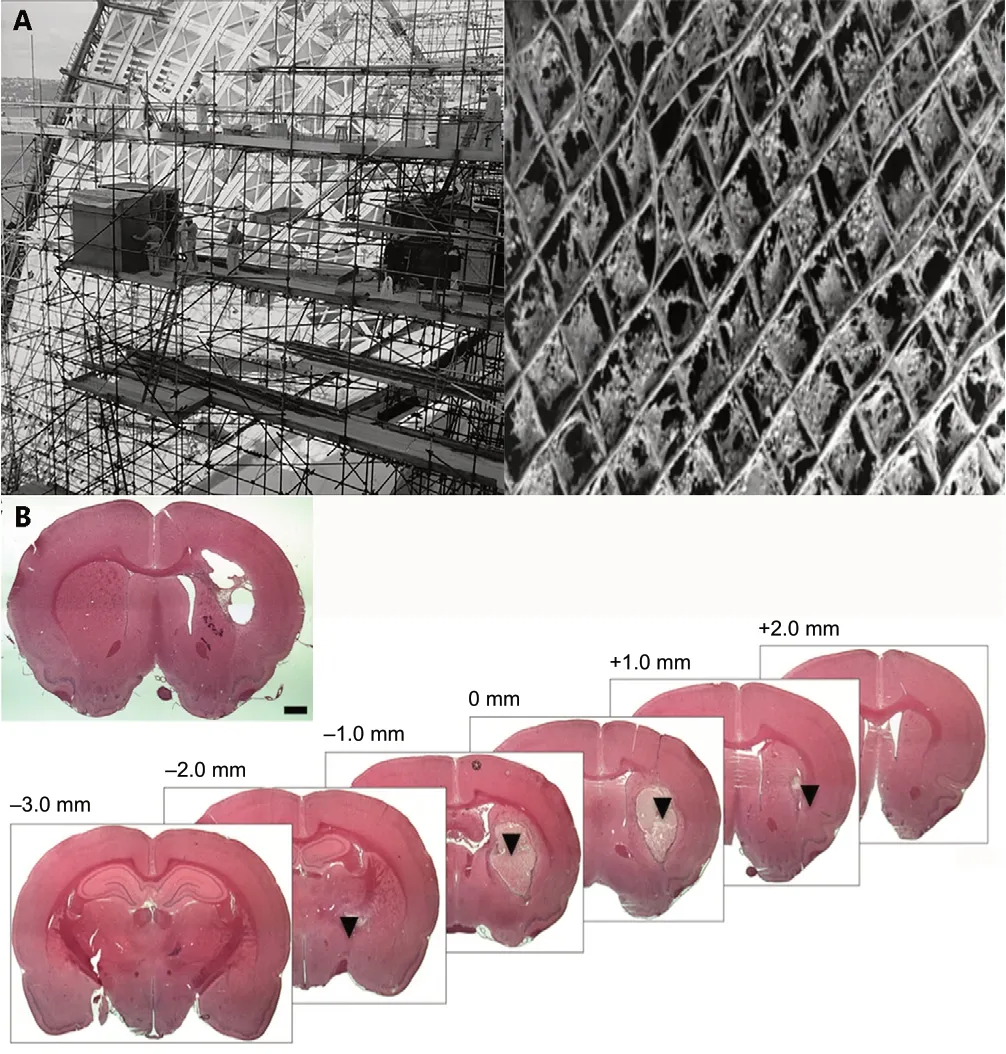
Figure 2 |Understanding the concept of scaffolding.
The Relevance of Current Neuroimaging for the Clinical Assessment of Brain Tissue Repair
With the advent ofin situneural tissue engineering in the brain, the significance of neuroimaging needs to be highlighted, especially when aiming for clinical translation of novel therapeutics. The use of neuroimaging methodologies as a non-invasive,in vivomeans of targeting the injected hydrogels and the released biomolecules (e.g., EVs)while objectively monitoring the remodeling of the brain tissue, is essential for clinical translation as postmortem histopathological approaches are unable to provide answers in living human subjects (Bible et al., 2012; Liu et al., 2020).Magnetic resonance imaging (MRI) is ideally suited for both pre-clinical and clinical applications as the most versatile tomography imaging method available. It is harmless, nondestructive, non-radioactive and can provide high resolution anatomical images of CNS lesions, guiding transplantation using 3D stereotactic coordinates (Nitzsche et al., 2009; Liu et al., 2016).
Using MRI forin vivodetection of hydrogels can be problematic, because hydrogels have high water content and appear similar to soft tissues in the MRI. Therefore,to distinguish hydrogels from the adjacent brain tissue,certain MRI contrast agents should be used. It is indeed the physicochemical nature of the MRI contrast agents that determines the modification strategy for the hydrogel so that it becomes MRI-visible (Lei et al., 2016). Thus, pre- and posttreatment MRI images enable us to visualize the biomaterial that fills a cavity produced by a lesion. Furthermore, the formation of de novo neural tissue or remodeling of a cellular/imaging has an inherent limitation of signal attenuation for deep imaging applications, and nuclear imaging has inferior spatial resolution and, more importantly, is linked to safety concerns because of exposure to ionizing radiation.MRI delivers no radiation and provides excellent soft tissue contrast and spatial resolution for deep tissues.
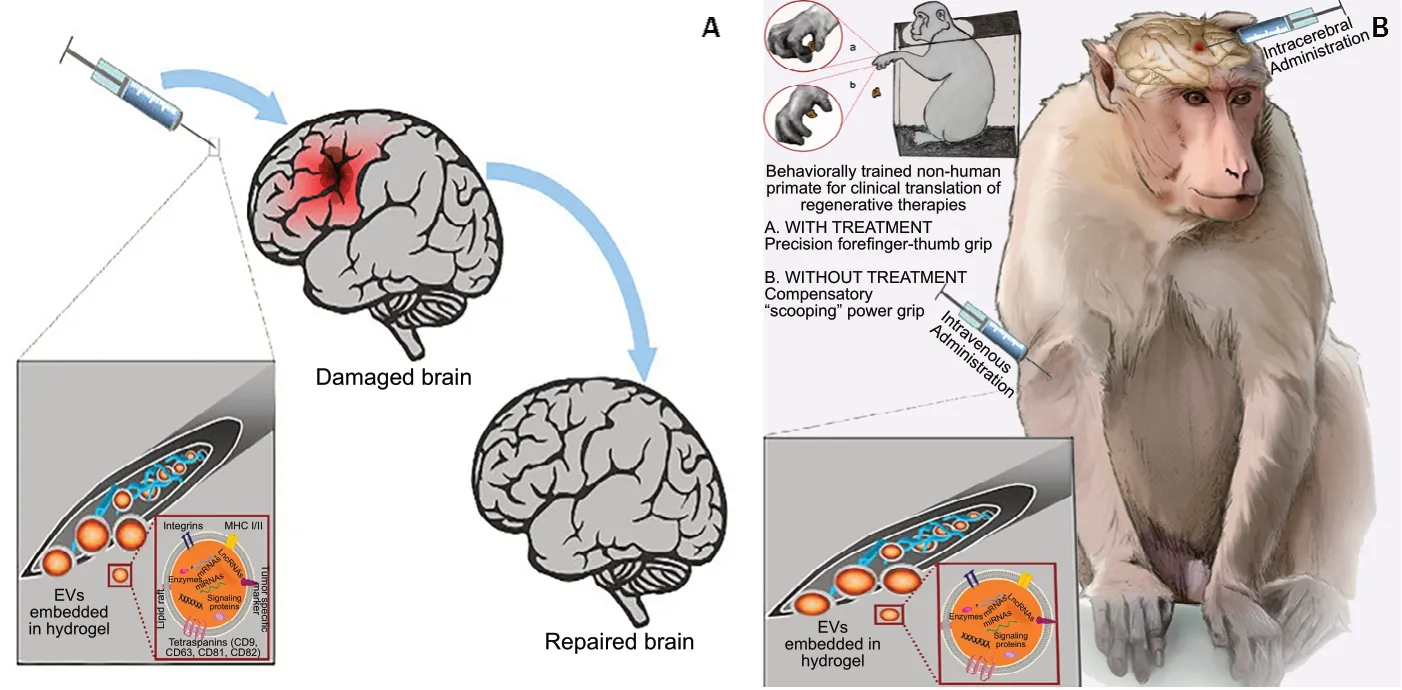
Figure 3 |Experimental hypothesis about a combinatorial hydrogel/EVs therapeutic approach.
Indeed, MRI imaging of EVs has been reported, but most studies have relied on MRI contrast agents (e.g.,superparamagnetic iron oxide nanoparticles (USPIO)) (Hu et al., 2015; Busato et al., 2016; Dabrowska et al., 2018). EVs can either be labeled directly with electroporation using USPIO, or the parent cells from which the EVs will be collected can be labeled with USPIOs. Electroporation might affect the function of the EVs because of the temporary breakage of the membrane of the EVs during the labeling process,whereas labeling of the parent cells with USPIOs leads to a dilution effect of the MRI signals as the cells proliferate. Thus,in an attempt to resolve those issues, several genetic MRI reporting systems, such as transferrin receptor (Wang et al.,2010), ferritin (Cohen et al., 2007; Iordanova and Ahrens,2012), magA (Sengupta et al., 2014), β-galactosidase (Gulaka et al., 2013), and tyrosinase (Qin et al., 2013), have become candidates for tracking the EVsin vivo. This is important because genetic modification of the EVs-producing cell would persistently lead to production of labeled EVs despite the cellular proliferation. Previous studies have demonstrated that labeling EVs with a reporter protein that emits chemiluminescence by using transfection of the EVs-producing extracellular matrix bio-scaffold could be detected by diffusion MRI, which is highly sensitive to the 3D movement of water molecules within tissues and the lesion cavity. Tissue regeneration within the lesion would increasingly restrict the movement of water molecules,making diffusion MRI an important imaging tool for information on the regenerative processes taking place.Based on the directional movement of water molecules along fiber tracts with diffusion tensor imaging, it is possible to generate a 3D fiber map exploring brain networks depending on the condition in question (Mori et al., 2001). Noninvasive, quantitative biomarkers have emerged from diffusion tensor imaging analysis, such as fractional anisotropy,which is considered a potential predictor of motor recovery if measured at an early phase following stroke (Dalamagkas et al., 2019; Moura et al., 2019).
In addition,in vivotracking of the EVs or other released biomolecules at the lesion site is crucial, and this has become possible due to advancements in neuroimaging analysis. Numerous imaging techniques for monitoring EVsin vivohave been tested, including optical imaging, nuclear imaging, and MRI (Choi and Lee, 2016; Di Rocco et al.,2016; Hood, 2016; Gangadaran et al.,2017; Piffoux et al., 2017; Chuo et al.,2018; Gangadaran et al., 2018; Shen et al., 2018), with each having advantages and disadvantages. It seems that the optimum methodology is MRI, especially in regard to clinical translation. Optical cells with a plasmid could trace exogenously administered EVsin vivo(Takahashi et al., 2013). This labeling approach was recently applied in the protocol of Zhang and colleagues(2018), which combined the use of a hydrogel and EVs for the treatment of a murine model with hindlimb ischemia with very promising results (Zhang et al., 2018). Another recent study (Liu et al., 2020) hypothesized that labeling EVs with the MRI reporter protein that generates change in contrast using a similar genetic modification approach can detect exogenously administered EVsin vivoby MRI.
Finally, resting-state functional magnetic resonance imaging(fMRI) is a valuable research tool for stroke (Ovadia-Caro Smadar et al., 2014). It has been considered an attractive technique for mapping neuroplasticity in a lesioned brain(Carter et al., 2010; Wang et al., 2010; Lu et al., 2011).Recently, different hand outcomes in stroke patients that directly influence the level of independence and quality of life of the patients, showed distinct functional reorganization patterns in large-scale brain networks (Hong et al., 2019).This highlights the clinically meaningful correlations that can emerge from resting-state fMRI analysis. Functional connectivity (FC) represents the synchrony of intrinsic blood oxygen level-dependent (BOLD) signal fluctuations among different brain regions. High FC indicates an effective connection of neuronal activity between and among different brain regions. In patients with ischemic motor stroke,apart from the reorganization of sensorimotor networks demonstrated by resting-state fMRI, the FC of the motor network has been found to be impaired within hours after stroke onset (Golestani et al., 2013), changing with motor deficit improvements during longitudinal observations (Wang et al., 2010; Fan et al., 2015; Zheng et al., 2016). In addition,variation in the clinical status of the patients post-stroke (e.g.,good or poor hand outcomes) has been recently linked to different connectivity patterns. Therefore, the FC of the motor network provides a potential imaging biomarker of recovery from cortical injury.
Overall, neuroimaging analysis is an essential addition for any protocols exploring the regenerative potential of novel therapeutics. Although the establishment of optimal imaging methodologies remains a matter of intensive investigation,using appropriate multi-modal imaging would allow the reparative processes to be trackedin vivoin a non-invasive and quantifiable way, so that the more subjective functional clinical scales could be informed by more objective indicators of repair.
Conclusions and Future Directions
It is evident that regenerative medicine, nanomedicine, and tissue engineering have many promising avenues to offer for accomplishing effective CNS repair. Nevertheless, many obstacles must be overcome to translate promising preclinical results in smaller animal models to clinical studies in humans. Harnessing the nano-size-related benefits that EVs have to offer without the risks associated with certain types of stem cells, novel therapeutics have been explored using EVs as a vehicle for inducing neural repair and for limiting the inflammatory cascade that typically leads to further neural damage after brain injuries. Such therapeutics has recently demonstrated promising reparative results even in NHP models of cortical injury. Nevertheless, systemic administration of EVs leads to their rapid clearance in the human body and makes retention in neural tissue challenging. The fact that EVs have demonstrated significant effects despite this suggests that even greater therapeutic effect might be expected if delivery and retention could be prolonged. This is especially important when prolonged retention may be required for repair of larger brain lesions that result from stroke in humans. The use of a biocompatible hydrogel as a sustained release system for EVs in the brain could provide critical structural support for the repair of larger lesions. Moreover, it could provide the necessary temporary guidance for neural repair with functional synapse formation,overcoming the challenges currently linked to EVs use. The EV/hydrogel combinatorial therapeutic approach has already been attempted in a murine model of hindlimb ischemia with very promising results. Thus, we hypothesize that such a combinatorial approach can be extended to our NHP model of cortical injury, further improving functional outcomes when compared to systemic administration of EVs alone. The assessment of the outcomes using robust behavioral studies informed by non-invasive and quantifiable imaging-based biomarkers would also enable the safe and effective clinical translation of such a novel experimental approach.
Acknowledgments:We would like to thank Drs. Myron Spector, Eng H. Lo and Christopher J Love for sharing expertise on hydrogels for stroke repair and the tissue engineering aspects of our hypothesis. We would like to thank Dr.Edward Yeterian (from Department of Psychology, Colby College, USA), and the anonymous reviewers for providing useful comments on the manuscript.
Author contributions:Review conception and design: MT, KD, NM; datacollation, analysis and interpretation: MT, KD; paper writing: MT; specific portions writing and editing: KD; editing portions related to animal models and regenerative and EV-related treatments: TLM; editing portions related to neuroimage analysis and reviewing reasoning of experimental hypothesis: YR and MK; reviewing the portions related to animal models and regenerative and EV-related treatments, as well as the reasoning of experimental hypothesis: DLR; paper review and review guiding: NM. All authors approved the final version of this paper.
Conflicts of interest:None.
Financial support:This work was supported by the National Center for Complementary and Integrative Health (NCCIH), No. R21AT008865 (to NM);the National Institute of Aging (NIA)/National Institute of Mental Health(NIMH), No. R01AG042512 (to NM). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Copyright license agreement:The Copyright License Agreement has beensigned by all authors before publication.
Data sharing statement:Datasets analyzed during the current study areavailable from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articlesare distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ana-Maria Buga, University of Medicine and Pharmacyof Craiova, Romania.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Patterning inconsistencies restrict the true potential of dopaminergic neurons derived from human induced pluripotent stem cells
- Efficacy of epothilones in central nervous system trauma treatment: what has age got to do with it?
- Association between plasma immunoproteasome and 90-day prognosis after first-ever ischemic stroke
- Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer’s disease
- Co-nanoencapsulated meloxicam and curcumin improves cognitive impairment induced by amyloid-beta through modulation of cyclooxygenase-2 in mice
- Regulated upon activation, normal T cell expressed and secreted (RANTES) levels in the peripheral blood of patients with Alzheimer’s disease

