Does acid pickling of Mg-Ca alloy enhance biomineralization?
Sheeer A Rhim,VP Muhmmd Reeh,M A Joseph,T Hns,,∗
a Department of Mechanical Engineering,National Institute of Technology Calicut,Kerala 673601,India
b Nanomaterials Research Laboratory,School of Materials Science and Engineering,National Institute of Technology Calicut,Kerala 673601,India
Abstract The mechanical and physical properties of biodegradable magnesium(Mg)alloys make them suitable for temporary orthopaedic implants.The success of these alloys depends on their performance in the physiological environment.In the present work,surface modification of Mg-Ca binary alloy by acid pickling for better biomineralization and controlled biodegradation is explored.The corrosion rates of nitric and phosphoric acid treated samples were analysed by conducting electrochemical corrosion tests.In vitro degradation behaviour was studied using immersion test in simulated body fluid(SBF).The sample surfaces were characterized using scanning electron microscope(SEM),energy dispersive X-ray spectroscopy(EDS),Fourier transform infrared spectroscopy(FTIR)and X-ray photoelectron spectroscopy(XPS).It is seen that acid pickling leads to significant improvement in biomineralization and develop in situ calcium phosphate(CaP)coating on the sample surfaces.In addition,the treated samples recorded a reduced degradation rate in the SBF compared to untreated samples.Thus,acid pickling is suggested as an effective surface treatment method to tailor the biomineralization and degradation behaviour of the Mg-Ca alloy in the physiological environment.© 2021 Chongqing University.Publishing services provided by Elsevier B.V.on behalf of KeAi Communications Co.Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Inorganic acid pickling;Magnesium alloy;Surface pretreatment;Surface characterization;Biomineralization;Degradation rate.
1.Introduction
Biodegradation and biomineralization are the two important aspects to be considered for designing biodegradable orthopaedic implants.While biodegradation helps in removing the temporary implant from the site without surgery,the biomineralization helps in better tissue implant interactions.Magnesium based alloys are considered to be suitable for developing degradable orthopaedic implants[1–4].In the case of Mg,the biodegradation happens by the reaction with the physiological environment and biomineralization is expected to occur through nucleation and growth of biomimetic calcium phosphate(CaP).However,the rapid reactivity of magnesium in a physiological environment[5]makes the surface unstable and impede the nucleation and growth of CaP.Several techniques classified into metallurgical[6–9]and surface modifications[10–12]can be adopted to control the rapid reaction and promote biomineralization.Providing surface layers or coatings that have bioactive agents and architecture similar to that of extracellular matrix help in promoting biomineralization[13].However,the majority of such coatings have a porous morphology which can cause direct exposure of metal beneath the aggressive environment[14,15].Proper pretreatment of the surface before applying such a layer will not only help in protecting the metallic surface but also improves the coating adhesion on the substrate.One of the easiest and affordable techniques for surface pretreatment is acid treatment.Nwaogu et al.studied the effect of organic and inorganic acid pickling on the corrosion behaviour of AZ31 alloys and found that acid treatment is beneficial in three different ways to enhance the corrosion resistance of the metallic substrate:
(i)Acid pickling can form a metal oxide/hydroxide layer on the surface[16,17].
(ii)Acid etching removes the surface contaminations that can accelerate the degradation of Mg alloys[16–18].Common contaminants include elements such as iron(Fe),manganese(Mn),copper(Cu),aluminium(Al)and zinc(Zn).Such contaminations mostly occur during the production and/or processing of the alloys.These contaminants result in the development of strong micro-galvanic cells and accelerate the degradation rate[19–22].
(iii)The acid pickling improves the surface energy of the substrate and enhances the adhesion of the coating on the surface[17,18].
The findings of Supplit et al.also showed an improvement in the corrosion resistance of Mg alloy during acid treatment[23].Likewise,the studies reported so far on acid treatment focussed on corrosion resistance mainly aimed at light-weight industrial applications[24–26].As for the biomedical applications,Park et al.reported that the biological response of acid treated titanium implants indicated enhancement in biocompatibility and biomineralization[27].However,an exclusive study on the effect of inorganic acid treatment on the performance of Mg alloys in the physiological environment is not yet reported.This work aims to explore nitric acid and phosphoric acid pickling of Mg-Ca alloy as a surface treatment technique for biodegradable implant applications.Nitric acid is chosen based on the corrosion resistance imparted and phosphoric acid for its ability to form biocompatible phosphates[16].The samples were tested for their corrosion resistance in NaCl solution to obtain the optimum processes parameters.The best performing samples were tested for their biomineralization and biodegradation characteristics by conducting immersion studies in simulated body fluid(SBF).
2.Methodology
2.1.Sample preparation
Mg-Ca alloy with Ca<0.6wt.% and the remaining Mg(Nextgen Steels,Mumbai,India)was used as the bare sample.Nitric acid,phosphoric acid and other chemicals used for processing the samples were of laboratory grade.The cut samples were subjected to belt-grinding(60 grade)and then polished using silicon carbide papers up to 1000 grade to the required size of 20×20×2mm3.Enough samples were made to carry out the experiment in triplets.The samples were then annealed at temperature 340°C for 60 min followed by furnace cooling.The samples were again finely polished using 1000 grade papers and washed in acetone followed by drying in air.The samples were then treated in nitric acid and phosphoric acids of 1 molar concentration for different durations viz 30,60,90,120 and 180 s.The samples were then coded as BM,NA-30,NA-60,NA-90,NA-120,and NA-180;where‘BM’stands for bare Mg-Ca alloy and‘NA’for nitric acid treated Mg-Ca alloy.The number code corresponds to the acid treatment time in seconds(s).Similarly,the samples treated with phosphoric acid were coded as‘PA-30 to PA-180′.
Table 1Surface roughness parameters.
2.2.Surface characterisation
The acid treated surfaces were characterized using the field emission scanning electron microscope(SEM,Hitachi SU6600)at an accelerating voltage of 5kV and energy dispersive X-ray spectroscopy(EDS,Jeol 6390LA,OXFORD XMX N)at an accelerating voltage of 15–20kV.The surface chemical groups after acid treatment were characterized using Fourier transform infrared(FTIR,PerkinElmer Frontier)spectroscopy by attenuated total reflectance(ATR)method under atmospheric pressure and room temperature.The IR absorption spectra were plotted for the range between 4000 and 450 cm–1.The surface chemistry of the acid treated samples was further analysed using X-ray photoelectron spectroscopy(XPS,PHI 5000 VersaProbe-II,ULVAC)using Al-KαX-ray source.The survey spectra and narrow scan were recorded and the XPS data were processed using PHI’s Multipak software.To evaluate the suitability of acid treated surfaces for biodegradable applications,the wettability was assessed by contact angle measurements using a goniometer(Digidrop-MCAT,GBX).For contact angle measurement using static drop method,a volume of 3μl of distilled water was dropped on the sample surfaces and the measurements were calculated as the average of readings from 5 different locations on the sample.The surface roughness parameters as described in Table 1 were analysed using 3D profilometer(InfiniteFocus G5,Bruker Alicona).These parameters were obtained at a magnification of 10×for a lateral measurement range of 1.62mm.
2.3.Degradation behaviour
2.3.1.Degradation behaviour by electrochemical corrosion test
The electrochemical corrosion test by potentiodynamic polarization(PDP)method was conducted in a medium of biomimetic NaCl solution of 8.04g/l concentration[28]using an electrochemical workstation(CH 1608E).The measurement system consisted of a three-electrode cell immersed in 400ml of the prepared NaCl solution with sample surface exposed to the medium as the working electrode.The saturated calomel electrode as the reference electrode and platinum wire was made as the counter electrode.The PDP curves were plotted at a scanning rate of 1mV/s.The corrosion potential and corrosion current of the bare sample was compared with the various acid treated samples using the PDP curves to obtain the optimum treatment time for both the acid solutions.Hereafter,the samples were denoted without the number code that corresponds to their treatment time.
2.3.2.Immersion test
In vitrodegradation was studied by immersing bare and optimum acid treated samples of size 10×10×2mm3in simulated body fluid(SBF).The SBF having an ion concentration equivalent to the human blood plasma was prepared according to Kokubo and Takadama[29].The sample weights were noted before immersing in the SBF for specific durations of 1,3,7,14 and 28 days.Throughout the test,the medium temperature was kept constant at 37±1°C using a constant temperature bath.The samples were weighed post-immersion to assess the weight gain/loss.The change of weight was normalized with the area of the samples to avoid the effect of the surface area.The morphology and composition of the sample surfaces after the immersion were analysed using SEM and EDS.The degradation deposits were then removed by washing the samples using 180g/l concentrated boiling chromic acid.The weights of these samples were taken and the surface morphology was again investigated using SEM.The degradation rate(DR)was obtained in millimetres per year(mm/yr)as per the ASTM G31–72 standard using the following equation:

where‘ΔW’is the weight loss(g)after removing the deposits for an immersion time‘T’(h),‘A’is the total surface area of the sample exposed to degradation in cm2and the density‘D’of the sample was taken as 1.740g/cm3.
2.4.Statistical analysis
The results are reported as the average values for at least 3 samples.T-test was performed to evaluate the statistical significance of the results using a significance level ofp<0.05.
3.Results and discussions
3.1.Surface characterization
Surface morphology of the bare and treated samples is shown in Fig.1.The samples treated with nitric acid exhibited a metallic texture,while the samples treated with phosphoric acid displayed a greyish surface.The polishing marks observed in bare samples are removed as a result of the chemical etching during acid treatment.However,a closer look at the morphology of the samples treated for short durations indicates incomplete etching of these marks.The polishing marks disappeared after 90s and 60s,respectively for nitric acid and phosphoric acid treated samples.Furthermore,the reaction between the alloy surface and acids resulted in the formation of a conversion coating.These thin layer of deposition formed by the chemical conversion was exhibited for all treatment durations.The morphology of the coating deposits also depends on the type of acid and the treatment time.However,longer treatment time can cause undesirable levels of material removal.This is because during prolonged exposure to the aggressive acid medium,material experiences more chemical etching even to the subsurface layers.
The IR spectra of the conversion coatings formed on the treated surfaces are shown in Fig.2.The untreated sample cleaned using ethanol had no significant infrared absorption,that indicates the absence of any chemical groups on the surface[30].Fig.2(a)shows the FTIR spectra of nitric acid treated samples in comparison with the bare sample.The sample exhibited an absorption band at 652 cm–1indicates O2–attributed to the stretching of magnesium oxide(Mg–O)bond[31].The strong absorption band near 1419 cm–1can be ascribed to the asymmetric stretching of the adsorbed NO3–ions in magnesium nitrate[32].The broad band that corresponds to vibration of magnesium hydroxide(Mg–OH)groups were also identified near 3280 cm–1.The FTIR spectrum of phosphoric acid treated samples(Fig.2(b))exhibits absorbence band at 1036 and 1610 cm–1corresponds to the structure of magnesium phosphate[33].Additionally,as observed for NA samples,the phosphoric acid treated sample also exhibited peaks that correspond to Mg–O and Mg–OH.
The samples were further characterized using XPS to obtain a survey spectrum for the qualitative analysis as shown in Fig.3.The survey scan for NA samples(Fig.3(a))confirms the presence of photoelectrons of O 1s,N 1s,Ca 2p,C 1s,Mg 2s and Mg 2p.As for the PA samples,the photoelectrons present were O 1s,Ca 2p,C 1s,P 2s,P 2p,Mg 2s and Mg 2p;shown in Fig.3(b).These findings support the results obtained in IR spectroscopy.The high-resolution spectra for each element in the NA sample are shown in Fig.3(c–f).The distinct peaks exhibited by Mg 2s is related to the binding energy of magnesium hydroxide(49.25eV),magnesium carbonate(50.21eV)and magnesium nitrate(51.90eV)[29–31].The corresponding peaks were also displayed by the O 1s with binding energies 531.49eV,531.75eV and 532.06eV,respectively for the hydroxyl,carbonyl and nitryl bonding[34–36].Nevertheless,the intense peak for C 1s corresponding to MgCO3is adventitious due to the contamination from the carbon tape and atmosphere while handling the sample in XPS[37].The limited presence of nitrogen for NA samples is attributed to the high reactivity of MgNO3compounds with moisture to form hydroxides.The PA samples had a similar result except that there were peaks related to the binding energies of P 2s and P 2p instead of N 1s.This confirms the formation of phosphate deposits on the sample surface.Fig.3(g–j)shows the high-resolution spectra for P-A samples.The two peaks Mg 2s spectra is associated with the formation of oxide and phosphate on the surface.The Mg 2s peak at 50.23eV and O 1s peak at 531.45eV are related to the formation of Mg–O[31].The single peak of P 2p at 133.98eV together with Mg 2s peak at 50.81eV and O 1s peak at 532.27eV corresponds to phosphate deposits on magnesium that can be HPO42–or PO43–[33,39].
The findings from the surface characterization using IR and XPS of acid treated samples are in good agreement with the chemical reaction of Mg with NA and PA[40]:



Fig.1.Surface morphology of bare and treated samples.

Fig.2.IR spectrum of acid treated in comparison untreated sample:(a)NA and(b)PA.
3.2.Corrosion test by PDP
Fig.4 shows the PDP curves for the treated and bare samples obtained from the electrochemical corrosion test.The corresponding corrosion parameters are summarized in Table 2.An anodic shift in corrosion potential(Ecorr)was exhibited by the nitric acid treated samples with an increase in the treatment time up to 90s(Fig.4(a)).The measured value of Ecorrfor BM sample was˗1.717V that increased to a maximum of˗1.576V corresponding to NA-90.However,when the treatment time was increased beyond 90s,as in the case of NA-120 and NA-180 samples,lower Ecorrvalues of ˗1.718V and˗1.801V were recorded respectively indicating a reduction in the corrosion resistance.Corrosion current density(Icorr)also exhibited a similar trend and the minimum Icorrof 22.59μA/cm2was recorded by NA-90 samples.This improvement in corrosion resistance is due to the formation of a protective oxide layer on the surface[16].The reduction in the corrosion resistance of samples treated beyond 90s is due to the peeling of the protective layer as a result of lower Pilling Bedworth Ratio(PBR)of magnesium oxide[41,42].The phosphoric acid treatment also exhibited a similar trend as shown in Fig.4(b).However,the corrosion resistance decreased after 60s of treatment.The maximum Ecorrvalue of ˗1.634V was obtained for PA-60 sample that also displayed the lowest Icorrvalue of 24.66μA/cm2.The improvement in corrosion resistance of the phosphoric acid treated sample is attributed to the formation of magnesium phosphate deposits on the surface.From the above results,it is concluded that the optimum duration for acid treatment using 1M solutions of nitric acid and phosphoric acid are 90s and 60s,respectively.

Fig.3.Survey scan for(a)NA sample(b)PA sample;high-resolution spectrum for(c–f)elements of NA sample and(g–j)elements of PA sample.
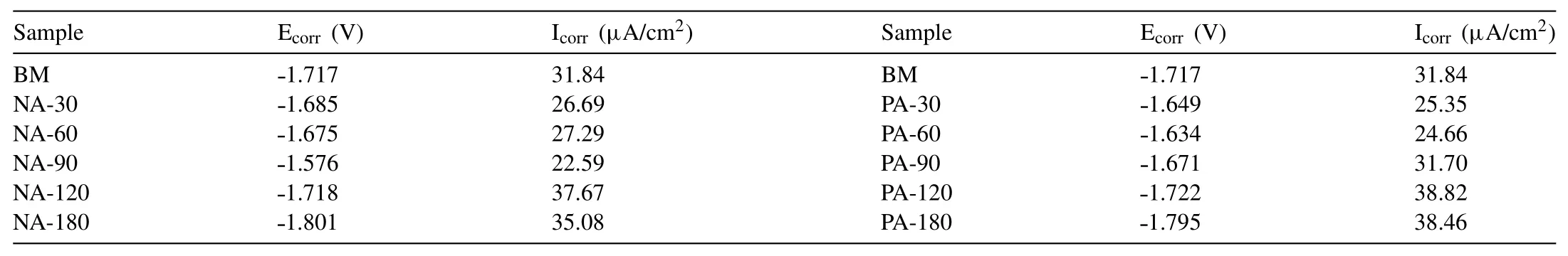
Table 2Electrochemical parameters of the alloy for different treatment conditions.
Fig 5.shows the surface morphology of the samples during the PDP test.BM sample has a significant amount of corrosion deposits in comparison with acid treated samples.The deposits are primarily MgCl2formed during the reaction between MgO/Mg(OH)2and NaCl solution[37,38].MgCl2dissolve easily in aqueous media and can cause pitting corrosion in the bare sample[43].However,the chemical conversion coatings on the acid treated samples are still observable on the SEM morphology even after electrochemical corrosion tests without the formation of any detrimental corrosion deposits.
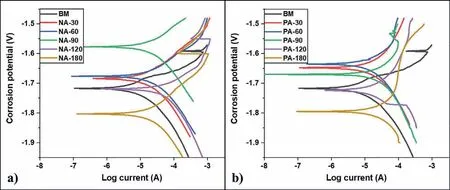
Fig.4.PDP curves of untreated and treated samples.(a)NA vs BM,and(b)PA vs BM.

Fig.5.Surface morphology of samples after PDP:(a)bare magnesium alloy,(b)nitric acid,and(c)phosphoric acid treated.
3.3.Contact angle measurement
The contact angle measurements for the optimum samples are represented in Fig.6.Though the bare sample showed hydrophilicity,the acid treatment further improved the wettability.The improvement in wettability can be attributed to the presence of conversion coatings.Surface with improved wettability can aid improved cell adhesion and proliferation in the physiological environments[44].This indicates that the chemical conversion coatings can help in improving biocompatibility.However,the PA treated samples displayed a very low contact angle(<10º),indicating they are highly hydrophilic.The high hydrophilicity is undesirable as it can inhibit the cell interactions that are detrimental during bioresorbable applications[45].

Fig.6.Wettability comparison.
3.4.Surface roughness
The surface roughness parameters also play an important role in cell adhesion and proliferation.However,an extremely rough surface can trigger the corrosion rate of Mg alloys[46].The roughness parameters of the bare and acid treated samples are compiled in Table 3.The results reveal that chemical etching caused by acid treatment has a significant effect on surface roughness.Among the treated samples the NA surface showed high Ravalues compared to phosphoric acid treated samples.This increase in surface roughness can be used to enhance cellular attachment and promote cellular activities as reported by Lamolle et al.[47].Thein vitroandin vivoresults of Elias et al.also point out that the rough surfaces facilitate protein entrapment and enhance osteogenic cell attachment thereby improving the interaction between the implant and host tissue[48].

Fig.7.SEM and EDS of samples before and after immersion test.

Table 3Values of roughness parameters.
3.5.Immersion test
3.5.1.In vitrobiomineralization
The elemental composition of the sample surfaces analysed using SEM and EDS before and after the immersion test is compared in Fig 7.Before the immersion test,all the samples exhibited strong peaks of Mg.Ca present as an alloying element in the system was not detected by the EDS due to its low weight fraction.Apart from Mg,the treated samples confirmed the presence of oxygen on the surface due to the formation of magnesium oxide and hydroxide as discussed in Section 3.1.In addition to oxygen,the PA samples recorded the presence of phosphorus too,confirming the phosphate formation during acid treatment.SEM images after immersion show the formation of deposits on the sample surfaces and the EDS confirmed that these deposits contained Mg,Ca,P,Cl and more O.Though the relative quantities of all these elements varied from sample to sample,it confirms the biomineralization on the sample surfaces during immersion.The NA and PA samples exhibited more intense peaks of Ca and P compared to the BM sample.This enhancement in biomineralization was achieved due to the presence of the surface layers formed during the acid treatment.These layers offered partial protection to the surface beneath and functioned as a stable scaffold for nucleation and growth of the CaP layer.Thus the surface of the treated samples was made more conducive for biomineralization compared to the BM samples.However,as the immersion duration increases the Mg(OH)2gets converted to MgCl2due to the high concentration of Cl¯ions in the SBF.The MgCl2is soluble in aqueous media that leads to rapid pitting corrosion[43].The BM sample shows a comparatively higher amount of Cl¯ions that can accelerate the degradation rate.After 28 days of immersion,the NA and PA recorded more amount of Ca and P confirming the enhanced biomineralization compared to the BM sample.
During the immersion test,enhancement in biomineralization was quantified based on the weight gain/loss as represented in Fig.8.Throughout the immersion test,the BM samples exhibited weight loss.Though the treated samples(NA and PA)exhibited weight loss during the initial stages of immersion,it reduced with immersion time and exhibited significant(p<0.05)biomineralization than BM samples.Additionally,after 28 days of immersion acid treated samples exhibited more significant(p≤0.001)weight gain due to the enhanced biomineralization.As discussed before the enhanced biomineralization can be attributed to the formation of protective conversion coatings.On the other hand,the BM had no protective coating that could promote the biomineralization.Among the treated samples,the NA samples exhibited more weight gain which substantiates the outcomes from the SEM morphology observed in Fig.7.

Fig.8.Acid pickled samples(NA and PA)showing significant biomineralization during immersion in SBF.(∗)and(∗∗)indicates p<0.05 and p≤0.001,respectively.
3.5.2.In vitrodegradation
The surface morphology of the samples immersed in SBF after the removal of degradation and biomineralization products are shown in Fig.9.The surface morphology shows that cracks and pits were formed on the samples due to degradation reactions.While the effect of degradation was rapid on BM samples,the acid treated samples were relatively stable during the initial duration of immersion.The formation of surface cracks on BM samples exposed subsurface layers to aggressive Cl¯ions and triggered pitting corrosion.After 14 days,visible cracks were seen on these surfaces which led to severe pitting corrosion as well as layer by layer depletion of the material from the surface.Conversely,acid treated samples had protective conversion coatings that acted as a barrier against Cl¯attack.Even though these conversion coatings were significant during the initial days of immersion,they appear to become unstable and fragile against Cl¯attack after long days of immersion.

Fig.10.Acid pickled samples(NA and PA)showing significant degradation rate during initial days of immersion in SBF.(∗)indicates p<0.05.

Fig.9.SEM image of samples after in vitro degradation.
Fig.10 represents the degradation rate of bare and acid treated samples during immersion in SBF.The BM sample exhibited the highest degradation rate throughout the experiment.This confirms the previous findings as observed from SEM morphology.The acid treated samples exhibited comparatively lower degradation rate significantly(p<0.05)during the initial days of immersion due to the CaP layer formation as discussed in the previous section.However,there was no significant difference(p>0.05)in the degradation rate between NA and PA samples.During the initial stages of immersion,the degradation rate was quite higher due to the formation of cracks.The rate decreased in the subsequent days of immersion can be attributed to the formation of CaP deposits due to the bioactivity of magnesium in the physiological environment[45,46].

Fig.11.Schematic representation of the degradation mechanism.
4.Discussion
Biodegradation and biomineralization are the two important phenomena that occur when Mg alloys are subjected to a physiological environment.The high reactivity of Mg results in the accelerated degradation rate in such a severe environment and it is detrimental for thein vivoapplications.The major reactions involved in the degradation process are as follows[43]:

It can be seen that the degradation process results in the evolution of H2gas.The rapid release of H2gas from the surface will make it non-conducive for nucleation and growth of CaP layer[49,50].In addition to this,there will be mechanical disturbances during bone regeneration[51]and the subcutaneous hydrogen gas formation can block the blood flow that leads to necrosis[52].Furthermore,the degradation reaction also forms MgCl2soluble in aqueous media and cause rapid pitting corrosion.Thus it can be noticed that the unprotected alloy surface becomes unstable as soon as it is exposed to the physiological environment.Previous studies have reported that when Mg surface that is made conducive for nucleation and growth of CaP can exhibit enhanced biomineralization[53–55].The CaP layer formed can also act as a protective layer for the surface beneath and control the degradation rate.Thus we can see that the biomineralization and biodegradation phenomena become competitive in nature and one can hinder the other in case of Mg alloys.Unlike the bare samples,an acid treated sample has a protective layer formed on the surface before immersion in SBF.This protective layer helps in reducing the degradation reactions and makes the surface more conducive for biomineralization.Such a surface will be not only stable but can promote the formation of biomimetic CaP and can help in reducing the degradation rate further.
A schematic representation of the degradation mechanism in this work is represented in Fig.11.Immediately after immersion,the BM samples react with water molecules of the SBF to form Mg(OH)2layers on the surface.Additionally,the bioactivity of Mg causes the formation of biominerals(CaP layer)over the substrate.However,the Cl¯ions present in the medium attacks these deposits to form pits on the substrate surface.In the case of acid treated samples,the chemical conversion coatings acts as a barrier between the Cl¯ions and substrate surface.The coating hinders the degradation rate significantly during the initial stages of immersion.This improvement in degradation resistance is due to the combined action of conversion coating and formation of CaP coating.Nevertheless,over a period of time,such layer will develop cracks and allow the medium to penetrate and leads to biodegradation.Thus it is noted that though the acid treatment can help in promoting biomineralization and reduce degradation during the initial days of immersion,the protection will not be substantial at longer durations.Hence it is suggested that acid treatment can be used as a surface pretreatment process on Mg alloys that are subjected to surface deposition coatings for controlling biodegradation.
5.Conclusions
Mg-Ca alloy was treated with nitric acid and phosphoric acid solutions.The treated samples exhibited improved biomineralization due to the formation of conversion coatings that acted as a scaffold for nucleation and growth of the CaP layer.Among the acid treatments,the nitric acid treatment provided better stability for the surface as it is evident from the higher weight gain on these samples during the immersion test.Furthermore,during the initial stages ofin vitrodegradation,the conversion coatings reduced the degradation rate.The enhancement in biomineralization and degradation resistance due to acid pickling can be further combined with an appropriate deposition coating to obtain a synergistic effect on the degradation behaviour of magnesium alloys for biodegradable implant applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge the financial grant No.5/4–5/3ORTHO/2019-NCD-I Dt.16–09–2019 from Indian Council of Medical Research(ICMR)and the grant from DST-FIST-No.SR/FST/ETI-388/2015,Govt.of India used for setting up 3D Profilometer facility at NIT Calicut.The expert assistance of Dr.Saju Pillai,Scientist-in-charge of XPS facility at NIIST-CSIR is also acknowledged.
 Journal of Magnesium and Alloys2021年3期
Journal of Magnesium and Alloys2021年3期
- Journal of Magnesium and Alloys的其它文章
- Modifying microstructures and tensile properties of Mg-Sm based alloy via extrusion ratio
- The effects of Ca and Mn on the microstructure,texture and mechanical properties of Mg-4 Zn alloy
- H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution—A large-scale and quick strategy to get hydrogen
- The slip activity during the transition from elastic to plastic tensile deformation of the Mg-Al-Mn sheet
- Rotational and translational domains of beta precipitate in aged binary Mg−Ce alloys
- In situ growth process of Mg–Fe layered double hydroxide conversion film on MgCa alloy
