In situ growth process of Mg–Fe layered double hydroxide conversion film on MgCa alloy
Jun Chen,Ju Feng,Lei Yan,Huan Li,Changqi Xiong,Sude Ma
Key Laboratory of Fluid and Power Machinery of Ministry of Education,School of Materials Science and Engineering,Xihua University,Chengdu 610039,China
Abstract In this study,an environment-friendly layered double hydroxide(LDH)film has been deposited on MgCa alloy by a two-step technique.To improve the chemical conversion technique and control the film properties,batch studies have been carried out to address various process parameters such as pH value,treatment temperature and immersion time.The chemical composition was determined by X-ray diffractometry and energy dispersive X-ray spectroscopy.The morphology was characterized by scanning electron microscopy.The corrosion resistance of the samples with various films was compared by polarization curves and immersion test.It is found that the transformation duration of Mg–Fe LDH is long.Too high pH value and temperature has harmful effect on the purity of the film composition.The corrosion resistance of the films formed at low value of pH or high temperature or short treatment time is deteriorative.The optimum process is as follows:the sample is first immersed in the solution containing Fe3+/HCO3−/CO32−with a pH value of 5 at a temperature of 55°C for 1h to form a precursor film,and then this precursor film is immersed into the solution containing Fe3+/HCO3−/CO32−with a pH of 11 at 55°C for 24h to obtain the Mg–Fe LDH conversion film.© 2020 Chongqing University.Publishing services provided by Elsevier B.V.on behalf of KeAi Communications Co.Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/)Peer review under responsibility of Chongqing University
Keywords:Magnesium alloy;Layered double hydroxide;Film;Corrosion;Parameter optimization.
1.Introduction
In recent years,magnesium(Mg)alloys,especially the alloys containing calcium(Ca),have attracted great attention as biodegradable materials[1-13].Several studies have reported that MgCa alloy had bio-safety with higher mechanical properties[7-14].However,since Ca exhibits a negative standard electrode potential,the fast corrosion of MgCa alloys in the human body environment imposes a challenging issue for clinical applications[15].In order to slow down the corrosion rate,many coating techniques have been developed.Chemical conversion coatings,with the advantages of simplicity to be operated and low operating and capital cost(low-temperature process,low energy consumption,short treatment time and high efficiency),can be widely used.The chemical reaction with the metal surface is expected to produce a strongly adherent and corrosion inhibiting coating[16-19].However,an ideal coating for biodegradable Mg alloys must exhibit corrosion resistance in the early stages of healing,and the corrosion product of the coating also should not be potentially toxic[20].Layered double hydroxides(LDHs)have the advantages of low cost,good corrosion resistance,good biocompatibility and low cytotoxicity,which are considered as a promising approach[21-27].For example,LDHs have been used as drug carriers and DNA delivery vector[25-27].
LDHs are typically composed of positively-charged mixed metal M2+-M3+hydroxide layers and galleries filled with the charge compensating anions(An–)and water molecules[28,29].Various approaches have been developed to synthesize LDHs films,including coprecipitation[30,31],hydrothermal transformation[32],and so on.Among these,in-situ growth of LDHs films on Mg alloys can be achieved by simple means[21-23,33-36].The authors’earlier work also directly formed Mg–Al LDH layer on Mg alloys by a two-step in-situ formation process[35,36].However,Al is believed to be a neurotoxic element,which may prevent bone calcification and inhibit bone formation leading to osteomalacia when implanted material contains too much of it[37].In contrast,iron(Fe)shows no toxic effect in a physiological environment.The work from Lin et al.presents a novel approach for directly growing Mg–Fe–CO3LDH on pure Mg[21].According to their results of corrosion test in revised simulated body fluid(R-SBF),the LDH coated sample had a much higher corrosion resistance than the substrate.The results of in vitro human mesenchymal stem cell adhesion tests showed that the Mg–Fe–CO3LDH coated sample had a better cell spreading and cell–cell interaction behavior than pure Mg.Therefore,insitu synthesis of Mg–Fe LDH film on Mg alloys is promising.
However,in comparison with Mg–Al LDH,little is known about the transformation of Mg–Fe LDH.Only the effect of post-treatment time on the transformation of Mg–Fe LDH had been studied[21].From our earlier research,we know that the formation conditions(pH of the treatment bath,treatment time,temperature,etc.)are critical for the LDH film quality and transformation[36].Accordingly,this work aims at preparing an environmental-friendly Mg–Fe LDH film on MgCa alloy.In addition,the influence of the formation process conditions in the composition,morphology and corrosion resistance of the film is investigated.It is expected to find the optimized process to synthesis a protecting Mg–Fe LDH film through this paper.
2.Experimental
2.1.Fabrication of the films
The material used in this study was extrusion MgCa alloy(0.97wt% Ca,0.007wt% Si and bal.Mg).The size of the samples was 30×25×3mm.The samples were ground to 2000 grit SiC paper,ultrasonically cleaned in ethyl alcohol,and then dried in cold air.The films were prepared by a two-step method.The processes were called as step 1 and step 2,respectively.To prepare a solution containing Fe3+/HCO3−/CO32−for the formation of Mg–Fe–CO3LDH,10g Fe powder was added to 1000mL of distilled water,and then a continuous bubbling of CO2gas to the solution was carried out for about 3h at room temperature(RT)to corrode the Fe powder.Afterward,the solution saturated with Fe compounds was filtered through filter paper to remove the Fe powder and rust.The solution of step 1 was prepared by bubbling CO2gas through 200ml of the above filtered solution for about 10min,and the pH value was achieved to about 5.The solutions of step 2 were prepared by dropwise addition of 2mol/L NaOH solution to the solution of step 1 to increase the pH to different values,and then heated to different temperatures.According to the experiment phenomenon during the fumbling process parameters and our previous experience[36],the parameters of step 1 did not affect the composition and qualities of the film much.Hence,only the influence of the process parameters of step 2 was investigated in this paper,and step 1 was carried out as follows:MgCa substrates were first immersed in the solution of step 1 with a continuous bubbling of CO2gas at a temperature of 55°C for 1h to form a precursor film.The aim of the continuous bubbling of CO2gas in step 1 is to keep the solution acidic.Step 2 was done as follows:the samples with precursor film were further immersed into different solutions for various times to obtain the final conversion films.The individual effect of pH value,temperature of the coating bath and the treatment time has been evaluated while keeping other parameters constant.The basic parameters were settled as follows:pH value of 11,temperature of 70°C,and 18h treatment time.Then,three levels have been chosen.
2.2.Characterization
The X-ray diffractometry(XRD)analysis was carried out using a Bruker D2 diffractometer with Cu target(λ=0.154nm)at a scanning rate of 0.01 s−1in the 2θrange from 10° to 60°.The morphology of the films was observed using a Quanta250 FEG environmental scanning electronic microscope(ESEM)equipped with an energy dispersive X-ray spectroscopy(EDS).Electrochemical tests were carried out using a ParStat 4000 potentiostat.A classical three-electrode system was applied.The samples,a saturated calomel electrode(SCE)and a platinum plate were used as working electrode,reference electrode and auxiliary electrode,respectively.The polarization curves were obtained on an exposed area of 1 cm2at a constant voltage scan rate of 0.5mV/s after an initial delay of 300s.The polarization curves were fitted using the CorrView software in the mode of Tafel(traditional)by intersecting the cathodic Tafel line and the level line at the OCP value.The immersion tests were performed according to the GB 10124-88 of China.The corrosion tests were conducted in Hank’s solution at 37±1°C.The composition of the SBF(per 1L,dissolved in distilled water)was as follows:8.0g NaCl,0.4g KCl,0.14g CaCl2,0.35g NaHCO3,1.0g C6H12O6,0.1g MgCl2•6H2O,0.06g MgSO4•7H2O,0.06g KH2PO4,0.06g Na2HPO4•12H2O.
3.Results and discussion
3.1.Process parameters optimization
3.1.1.Chemical composition of the films formed under different processes
The chemical composition of the films formed under different processes was analyzed by XRD and EDS.The XRD spectra of the films formed by different processes are shown in Fig.1.It is observed that all films are consisted of Mg6Fe2(OH)16CO3•4H2O,Mg(OH)2and CaCO3,but the relative content of each component is different.The typical diffraction pattern related to LDH can be observed with peaks at 11.4,22.6 and 58.8°.These peaks can be associated with(003),(006)and(110)reflections of LDH.The peaks at 18.7,38.0 and 51.1° in the patterns can be attributed to the presence of hydroxides.Fig.1a displays the XRD pattern of the films formed in the 70°C solutions with various pH values for 18h.It is observed that,when the pH value of the solution is 10,the curve displays different feature compared with the other two curves.The composition of the film formed at pH value of 11.5 is similar to that of pH 11.The intensity of peaks for the film material is weak,which may be because that the film formed in the solution at pH 10 is thinner than the other two.Furthermore,except the main phase Mg6Fe2(OH)16CO3•4H2O,there is a small amount of basic magnesium carbonate in the film when the pH value is low,but there are large quantity of Mg(OH)2and Fe(OH)3in the other two films.As we know,pH>10.5 is suitable for precipitating a large number of Mg hydroxides,and higher pH is beneficial for the film deposition[34].Hence,with the increase of pH value,the film becomes thicker,and the composition changes.It can be implied that,pH 10 is alkaline enough to form Mg–Fe LDH.This phenomenon is conflict with the Williams’research that the LDHs formation condition occurs over pH 10.5[28].However,it is in accordance with the research from Lin et al.,who had formed highlyoriented Mg–Fe–CO3LDH coating on pure Mg by immersing in pH 9.5 aqueous HCO3/CO32−[21].Certainly,the pH value should not be too low.Because when pH is too low,there is not enough OH−for the precursor film transforming into the LDH compound.Moreover,the transformation may also relate to the stability and solubility product constants of different hydroxides,since the values of these constants considerably are different.Hence,each LDH requires its own pH value of synthesis condition.
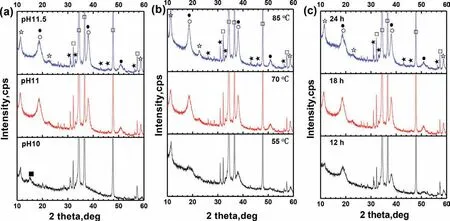
Fig.1.XRD spectrums of the films formed by different processes:(a)in the 70°C solutions with various pH values for 18h,(b)in the solution with pH value of 11 for 18h at various temperatures and(c)in the 70°C solution with pH value of 11 for various times(□-Mg,☆-Mg6Fe2(OH)16CO3•4H2O,°-Fe(OH)3,●-Mg(OH)2,★-CaCO3,■-Mg(CO3)4(OH)2•4H2O).
Fig.1b displays the XRD pattern of the films formed in the solution with pH value of 11 for 18h at various temperatures.The diffract intensity of the peaks for the film materials is increased with increasing of temperature,indicating the increase of film thickness.The highest Bragg reflections are observed for the film formed at the highest temperature of the solution.The peak intensity of the hydroxides impurity phases in the films increases significantly when the temperature is raised from 55 to 70°C.It suggests that the growth kinetics of hydroxides is significantly accelerated at temperature higher than 70°C.

Table 1The content of various elements in different films(C was not counted).
Fig.1c shows the XRD pattern of the films formed in the 70°C solution with pH value of 11 for various times.The curve of the film treated for 12h is absolutely different from the other two curves.It can be implied that most of the hydroxides have not been transformed into LDH yet.The relative content of LDH in the films is greatly increased from 12 to 18h,but gradually increased after then.It can be seen that the formation process of the Mg–Fe LDH conversion film is evidently influenced by the treatment time.The duration for the hydroxides transforming into LDH is long.
The content of various elements in different films is listed in Table 1.All films contain O,Mg,Fe,Ca and C(C was not counted).The content of Mg is very high in the film formed at pH 10,indicating that the signal from the matrix is strong,and the film is relatively thinner.It is in accordance with the XRD result.In addition,the content of Ca in this film is much smaller than that in other films.It may be because that only a small amount of CaCO3is generated when the pH value is low.The proportions of the elements in the films formed at pH 11 and 11.5 are similar.It is revealed from Table 1 that the content of Fe in the film formed at 55°C and the film formed at pH 10 is relatively smaller.It is in accordance with the XRD analysis,since the composition of the two films is basically Mg–Fe LDH,and the iron hydroxide impurity is much less than the other films.While the content of O and Fe in the film formed at 85°C is high,and the signal from the matrix Mg is weak,implying that the detected information is mainly attributed to the film.It is because that the film thickness is increased,and too much Fe(OH)3impurity deposits on the film surface when the temperature is raised to 85°C.The result is coincided with the XRD pattern in Fig.1b.Notably,the content of Fe is especially high in the film treated for 12h,but the content of Mg–Fe LDH is very small based on the XRD analysis.It can be implied that Fe3+content in the precursor film is excessive,and the atomic ratio of Fe and Mg elements is decreased during the long-time LDH transformation.Similar phenomenon was observed in the works from Chen et al.[35]and Lin et al.[38],who suggested that the Al-rich compounds should first leach out of Al3+during the Mg–Al LDH transformation.In addition,Lin et al.found that Fe3+was reduced to Fe in the Mg–Fe LDH film[21].As illustrated by Dr.Saji[18],there must be accomplished by a series of dissolution,oxidation,reduction and precipitation reactions.Although this two-step in situ growth mechanism of Mg–Fe LDH film is beyond the scope of this work,a further analysis will be proposed in future research.

Fig.2.SEM morphologies of the films formed in the 70°C solutions with various pH values for 18 h:(a–b)pH 10;(c–d)pH 11 and(e–f)pH 11.5.
3.1.2.Morphology of the films formed under different processes
Morphology of the films formed in the 70°C solutions with various pH values for 18h is shown in Fig.2.From the low-resolution image of the films,it can be seen that the pH value of the solution affects the quantities of the microcracks and sediments.When the pH is low,plenty of microcracks exist on the film surface.With increasing of pH value,the size of flaw and number of cracks decrease significantly.While the pH is up to 11,the ionization of Mg stops,thus the film is much more compact.However,the precipitates on the film surface increase greatly,and the surface of the two samples is not“clean”.It is because that in the strongly basic medium up to the pH value of 11,there are more OH−ions in the solution,resulting in the increase of precipitates.At higher resolution,films with a typical HT structure are observed,that curved hexagonal platelets lying perpendicular to the surface of substrate.However,with the increase of pH value,the composition of the films is not pure.Hence the flaky particles are not as uniform as the film formed at pH 10.
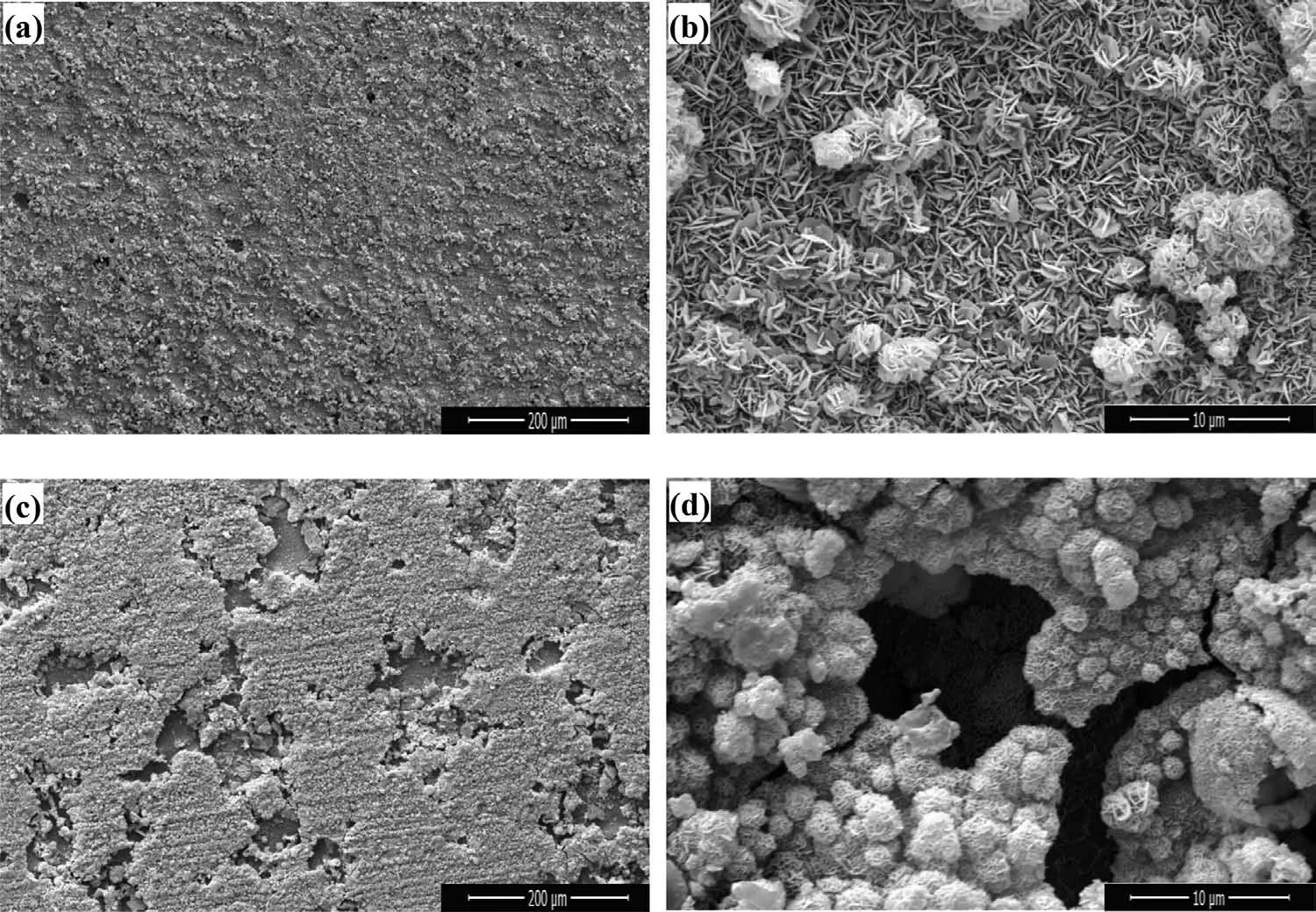
Fig.3.SEM morphologies of the films formed in the solution with pH value of 11 for 18h at various temperatures:(a–b)55°C and(c–d)85°C.
Fig.3 and Fig.2c–d show the morphology of the films formed in the solutions with pH value of 11 for 18h at various temperatures.As seen in these images,the temperature greatly affects the amount of precipitates on the film surface.When the temperature is as high as 85°C,a lot of small particles deposit loosely on the surface,and the film has obvious stratification phenomenon,that the upper layer does not completely cover the lower layer.From the observation of the high resolution images,it is revealed that the morphology of the film formed at 55°C is similar to that in Fig.2b,displaying a typical lamellar structure of LDH.The result is in accordance with the XRD and EDS analysis,since the composition of these two films is basically Mg–Fe LDH,which is similar.
The images of films obtained after different reaction times in the 70°C solution with pH value of 11 are shown in Fig.4 and Fig.2c–d.From the low-resolution images,it is observed that the discontinuous particles accumulate more and more with the increase of treatment time.At higher resolution,the film morphology in Fig.4b is absolutely different,which is interwoven and piled up by very fine wormlike materials,not like the typical hydrotalcite lamellar structure in Fig.2d and Fig.4d.It indicates that the structure of the film has not transformed into LDH compound when the treatment time is 12h,which is consistent with the XRD result.
3.1.3.Corrosion behavior of the films formed under differentprocesses
In addition,electrochemical and immersion tests were also carried out to investigate the corrosion resistance of the films formed under various process conditions.Fig.5 displays the polarization curves of the MgCa alloy with the films formed by different processes in Hank’s solution.The electrochemical parameters are shown in Table 2.The corrosion current density(icorr)of the films formed in the 70°C solutions with various pH values of 10,11 and 11.5 for 18h is about 29.9,16.6 and 26.3μA/cm2,respectively.Theicorrfirstly decreases and then increases,viz,theicorrof the film formed at pH 11 is the smallest.In Fig.5a,the hydrogen evolution rate in the cathodic side of the films formed in the solutions with high pH values decreases,indicating that the cathodic process of the corrosion reaction is effectively restrained.According to the SEM morphology,the defect of micro-cracks in the film formed at pH 10 must be the cause of fast corrosion rate.As we know,the micro-cracks are the channels for the corrosion medium passing through to the substrate.
From the data of Table 2 it can be seen that,temperature has a great influence on the open circuit potential(OCP)of the films.The higher the temperature is,the more negative the OCP is.Based on the results of XRD and electrochemical test,it is inferred that the OCP of the film is related to the composition of the film.When the content of hydroxides is high in the film,the OCP of the film is negative.It may be because that the Mg–Fe LDH is more stable than the hydroxides.It is in accordance with the viewpoint of other researchers,that in LDH all the cations coordinate octahedrally with hydroxyl groups by a common bridge type to form a closely packed network structure,hence Mg(OH)2and Al(OH)3are less stable than LDH[34,36].Theicorrof the three films formed at various temperature is close,only slightly increases with the increase of temperature.According to Fig.5b,in the case of the film formed at 85°C,there is a passivation trend in the anodic side in comparison with the other two films,of which the anodic sides show faster dissolution reaction.This may be because that the film is much thicker when the temperature is 85°C.However,the uniformity of this film is not good,there is a stratification phenomenon.Hence,the protection effect of the thick film is not very ideal.

Fig.4.SEM morphologies of the films formed in the 70°C solution with pH value of 11 for various times:(a–b)12h and(c–d)24h.
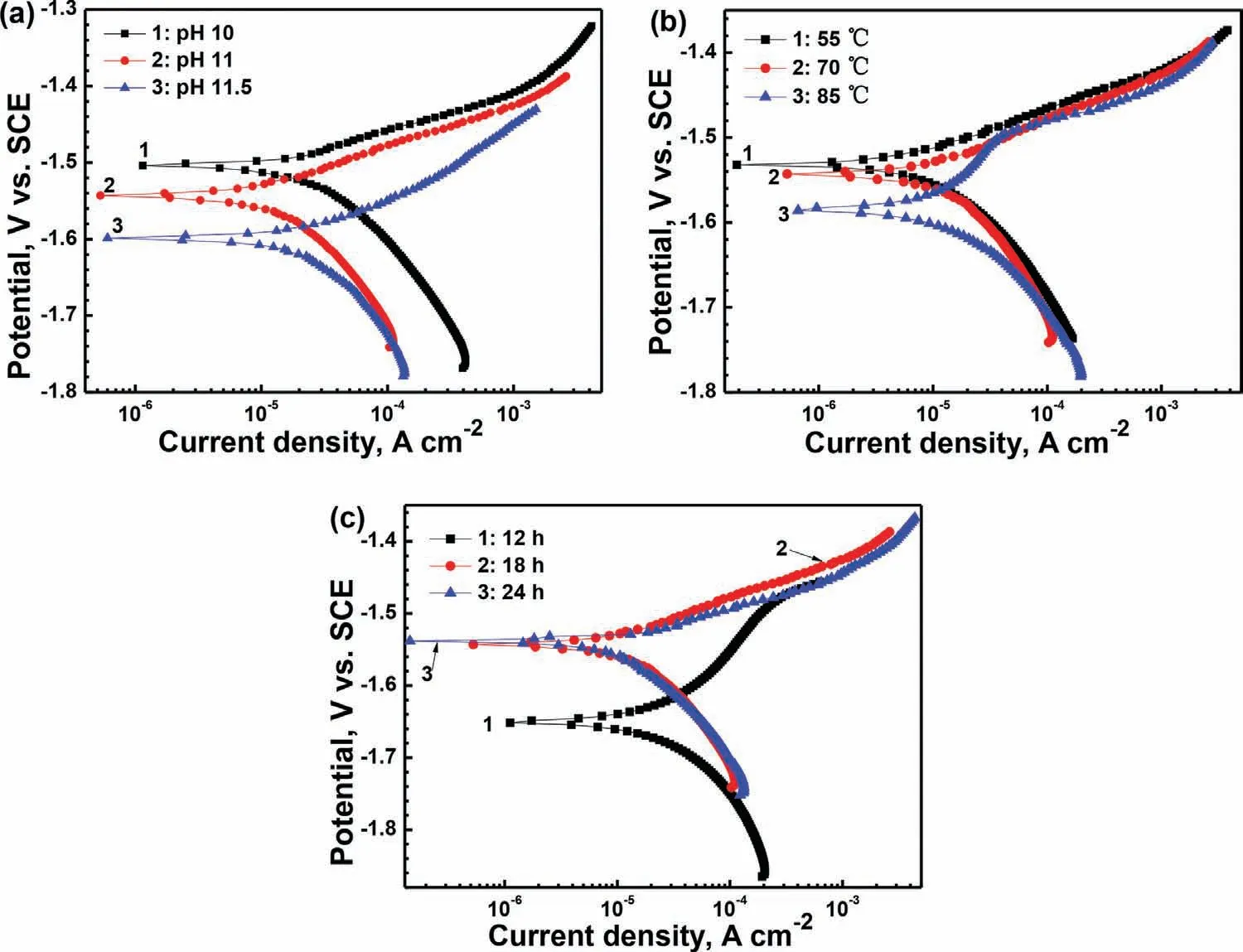
Fig.5.Polarization curves of the MgCa alloy with the films formed by different processes in Hank’s solution at 37±1°C:(a)in the 70°C solutions with various pH values for 18h,(b)in the solution with pH value of 11 for 18h at various temperatures and(c)in the 70°C solution with pH value of 11 for various times.
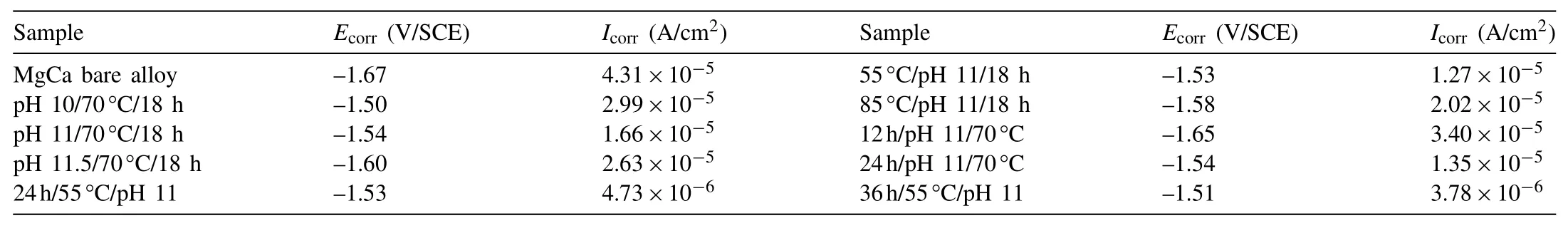
Table 2Electrochemical parameters of the MgCa alloy substrate and with the films formed under different process conditions in Hank’s solution at 37±1°C.

Fig.6.Optical corrosion morphology of the samples after immersion tests for 24h in Hank’s solution at 37±1°C:(a–c)in the 70°C solutions for 18h with pH values of 10,11,11.5,respectively,(d–e)in the solution with pH value of 11 for 18h at 55°C and 85°C,respectively,and(f–g)in the 70°C solution with pH value of 11 for 12h and 24h,respectively.
Polarization curves of the MgCa alloy with the films formed in the 70°C solution with pH value of 11 for various time in Hank’s solution are shown in Fig.5c.Fitting results in Table 2 reveal that,with the time prolonging,theicorrof the coated samples decreases.But the decreasing rate slows down after 18h.When the treatment time is only 12h,the hydrogen evolution rate in the cathodic side is faster,especially the OCP of which is about 0.1V(vs.SCE)lower than that of the other two films.It may be because that the film synthesized for 12h is mostly consisted of hydroxides,which have not transformed into LDH yet.In addition,it is also much thinner than the other films.Hence,the corrosion resistance of the film treated for 12h is much worse.
The optical corrosion morphology of the samples coated with the films formed by different processes after immersion tests for 24h in Hank’s solution is shown in Fig.6.The corrosion of the sample prepared at pH 10 is the most serious,that most of the surface area has been corroded.While the corrosion of the sample prepared at pH 11 is the lightest,that most of the film remains intact,only small pitting pits appear locally.The result is consistent with those of electrochemical test.According to the SEM morphology,the defect of microcracks in the film formed at pH 10 must be the cause of the fast corrosion rate.It indicates that the corrosion property of film changes with the film quality.Therefore,the determination of cracks is the key factor to optimize preparation techniques and control film quality.Thus,pH 11 is the best choice for the treatment.Compared Fig.6b,d and e to investigate the influence of temperature,it is observed that the sample prepared at 55°C has not been corroded,while the sample prepared at 85°C with rough surface and poor uniformity is corroded more severely.Associated with the XRD result,it can be seen that the film formed at 55°C condition has the best quality with highest purity and best corrosion resistance.Among all samples,the sample treated for 12h is corroded most seriously(in Fig.6f).The samples treated for 18h and 24h have no obvious corrosion.It shows that the treatment time has a great influence on the corrosion resistance of the films.From the color comparison,it is found that the color of the film formed at 85°C is quite different from that formed at 55°C before immersion corrosion test.The color of the latter is yellow–gray,which is similar to that of the sample showed in Fig.6a.It is also consistent with the XRD results,because the composition of the samples prepared at pH 10 and temperature of 85°C is the similar.Whereas,the sample prepared at 85°C is gray-white,similar to that of the sample in Fig.6f.This is mainly due to the deposition of a large amount of hydroxides on the film surface at high temperature,and the composition of the up layer on the sample prepared at 85°C and the sample treated for 12h is similar.Therefore,combined with results of XRD,SEM,polarization curve and immersion test,the optimum temperature is 55°C,and the treatment time is at least 18h.

Fig.7.XRD spectrums of the film formed in the 55°C solution with pH value of 11 for 24h(□-Mg,☆-Mg6Fe2(OH)16CO3•4H2O,°-Fe(OH)3,●-Mg(OH)2,★-CaCO3).
3.2.Characterization of the films formed under the optimum condition
3.2.1.XRD patterns of the films formed under the optimum condition
According to the above results,we prepared a film formed under the optimum condition,viz.,pH 11,55°C,treating for 24h.The XRD spectrum of this film in Fig.7 shows that,it is mostly consisted of Mg6Fe2(OH)16CO3•4H2O,but the content of hydroxides is much less compared to the films in Fig.1,and there is also a small amount of CaCO3.It can be seen that,most of the hydroxides have been transformed into LDH at the present process.Moreover,the peak strength of the LDH component is strong,indicating that the film is thick.Thus,the condition of 55°C,pH 11 and 24h treatment time is suitable for synthesizing a high purity Mg-Fe LDH film.However,it is unknown whether the duration of 24h is an optimum time for the corrosion property of the film.Hence,the treatment time was prolonged to 36h to synthesis a film for comparison,and the corrosion resistance of the two films was evaluated by electrochemical test as follows.
3.2.2.Corrosion comparison of the substrate and films formed under the optimum condition
Fig.8 displays the polarization curves of the MgCa bare alloy and with the films formed in the solution with pH 11 at 55°C for 24h and 36h.With the time prolonging,the curve shape and the electrochemical parameters of the two coated samples are similar.It indicates that the increase of the treatment time after 24h does not markedly affect the corrosion resistance of the samples.Hence,24h is the most suitable treatment time,because it can not only simplify the process and save energy,but also can achieve the commensurate corrosion resistance.Compared to the bare MgCa alloy,OCP of the coated sample greatly increases from−1.67 to−1.53V(vs.SCE).The LDH film slows down the corrosion rate of MgCa alloy mostly by inhibiting the cathodic hydrogen evolution reaction.Theicorrof the substrate and the coated sample is approximately 43.1 and 4.7μA cm−2,respectively.The positive shift of the OCP and the decrease oficorrindicate that the Mg–Fe LDH film can effectively improve the corrosion resistance of the MgCa alloy in SBF.
Based on the above results and analysis,the step 2 process has been settled as follows:temperature of 55°C,pH value of 11,and 24h treatment time.
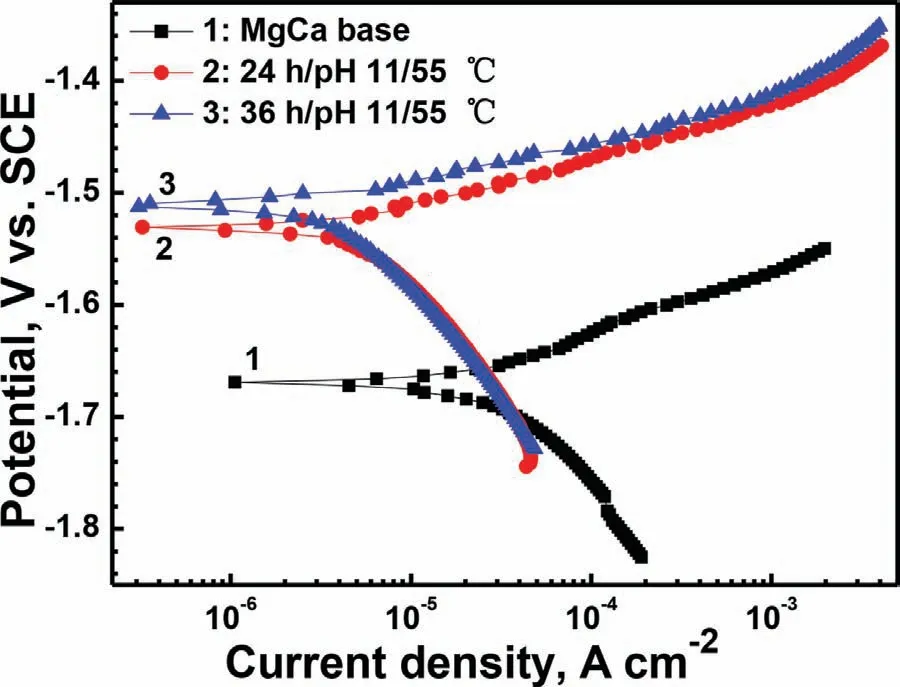
Fig.8.Polarization curves of the MgCa bare alloy and with the films formed in the 55°C solution with pH value of 11 for various times in Hank’s solution at 37±1°C.
4.Conclusions
Mg–Fe LDH chemical conversion film is a new research area for the corrosion protection of Mg alloys in the physiological environment.The effect of the synthesis parameters on the properties of the Mg–Fe LDH film has been summarized.
1.When the pH is low,the dissolving of Mg and hydrogen resolution has not stopped,which lead to the obviously cracking of the film.As a result,the corrosion resistance of the film formed at pH 10 is the worst.
2.Temperature has a great effect on the film formation.When the temperature is elevated,the amount of hydroxides deposition is increased obviously,which are less stable than LDH.In addition,the film is much coarser than that formed at low temperature.Hence,the corrosion property of the films is deteriorated with increasing of the temperature.Therefore,comprehensive judgment from the viewpoint of energy saving,as well as the quality of the film,55°C is chosen as the optimum temperature.
3.The transformation of the precursor film into LDH film is greatly related to the treatment time:adequate immersion time(>12h).Finally,an optimum process for in situ growth of Mg–Fe LDH film on MgCa alloy has been developed by two steps.This film is mainly consisted of Mg6Fe2(OH)16CO3•4H2O,which can provide good protection to the Mg alloy in SBF.
Acknowledgments
The authors thank the financial support by the Sichuan Science and Technology Program(No.2020YFG0165),the Projects in Sichuan Province Education Office(No.18ZA0453),the National Natural Science Foundation of China(No.51501156)and the Sichuan Science and Technology Program(No.2019JDTD0024 & No.2019ZHCG0048).
 Journal of Magnesium and Alloys2021年3期
Journal of Magnesium and Alloys2021年3期
- Journal of Magnesium and Alloys的其它文章
- Modifying microstructures and tensile properties of Mg-Sm based alloy via extrusion ratio
- The effects of Ca and Mn on the microstructure,texture and mechanical properties of Mg-4 Zn alloy
- H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution—A large-scale and quick strategy to get hydrogen
- The slip activity during the transition from elastic to plastic tensile deformation of the Mg-Al-Mn sheet
- Rotational and translational domains of beta precipitate in aged binary Mg−Ce alloys
- Does acid pickling of Mg-Ca alloy enhance biomineralization?
