Nano-tribological behavior of graphene nanoplatelet–reinforced magnesium matrix nanocomposites
Mohmmd Shhin,Khurrm Munir,Cuie Wen,Yuncng Li,∗
aSchool of Engineering,RMIT University,Melbourne,VIC 3001,Australia
Abstract
Keywords:Coefficient of friction;Graphene nanoplatelet;Magnesium matrix nanocomposite;Wear.
1.Introduction
Medical devices such as dental implants,orthopedic implants,and cardiovascular stents are composed of various metallic biomaterials.Thein vivoperformance of metallic biomaterials in these applications is affected by their corrosion and wear resistance in the physiological environment.Implant wear results in unwanted degradation of its surface,which results in an adverse inflammatory response to wear particles and subsequent implant failure.Biodegradable implants are generally designed to degrade progressively over a period of time,to provide essential mechanical support as a scaffold,and to assist the healing of host tissues[1,2].Therefore,biodegradable implants are expected to naturally degrade at appropriate degradation rates in the body,and a high wear rate limits their use in orthopedic applications.Wear in metallic implants is multifactorial but mainly depends on the chemical composition of the material,induced stresses on the components of the implant,the hardness and roughness of the implants,and their corrosion resistance[3].In the implant’s wear process,the dense oxide film which naturally forms on the metallic implant surface is removed during sliding movement and friction between different components of the implant and surrounding tissues[4].As a result,early degradation may occur and may also increase over time before healing of the infected site,resulting in premature failure of the implant[3].Moreover,the wear debris which is generated due to excessive wear and a high coefficient of friction(COF)between metal–host bone interfaces can cause osteolysis and sepsis,which subsequently results in implant failure[5,6].In several studies,the release of wear particles from hip and knee joint replacements was disseminated to the surrounding tissues and critical organs including the liver and spleen[7].
In this context,although other important factors in orthopedic surgical procedures,such as poor initial fixation,inadequate alignment,fluid flow,infection,and the stressshielding effect due to a mismatch in elastic modulus between the implant material and natural bone,also contribute to the resorption of bones and premature failure of the implants[8,9],limited longevity due to inadequate wear resistance is one of the main challenges in orthopedic implants and load-bearing applications[10–12].Therefore,the wear resistance of biodegradable implant materials must be measured to ensure the materials meet the requirements in relation to mechanical properties and degradation rates.Adequate mechanical strength,high corrosion and abrasion resistance,and low friction are primary requirements for metallic biomaterials used in orthopedic applications[13].
Current research is primarily focused on the development of iron(Fe),magnesium(Mg),and zinc(Zn)based biodegradable alloys for orthopedic applications[14–18].However,there are still challenges in utilizing these biodegradable materials as biomedical implants due to the mismatches between their degradation rates and bone-healing periods,inadequate mechanical properties,and inadequate wear properties[19].Compared to Mg-and Zn-based alloys,Fe-based alloys exhibit higher mechanical strength;however,the high elastic moduli and lower degradation rates of these alloys impede their utilization in bone-tissue engineering[20].Zn-based alloys exhibit appropriate degradation rates for bone-tissue engineering;however,poor mechanical strength and ductility of Zn-based alloys have been reported in recent studies[21].In recent years,biodegradable Mg-based alloys and composites have gained great attention due to their excellent biocompatibility,biodegradability,yield strength,elastic modulus,and density close to those of natural cortical bone[19,22–24].Mg is an essential trace element and its natural ionic presence is associated with various biological functions in the human body including stimulation of bone-tissue growth[25,26].In addition,Mg is a cofactor for many enzymes[1,2,22,27,28].
However,pure Mg possesses low corrosion resistance and degrades rapidly in the physiological environment,and is also known to exhibit poor wear properties due to low abrasion resistance[29–32].Therefore,various surface-modification techniques including laser direct melting,anodization,microarc oxidation,plasma electrolysis,laser cladding,ion implantation,conversion hard coating,and ion implantation have been used to enhance the wear resistance of Mg alloys[33].However,the reliability of the modified surfaces and deposited coatings on Mg alloys is still questionable in terms of their surface chemistry,interfacial bonding strength,and coating morphologies[33],and the abrasion and wear resistance of Mg-based materials is still insufficiently investigated.It has been reported that nonhomogeneous thickness of coating materials and their weak bonding to the Mg substrates in conventional surface-modification techniques adversely affect their corrosion and tribological properties[34–36].
Nano-tribological behavior of Mg matrix nanocomposites(MNCs)containing different nanoparticulate reinforcements are not commonly reported in the literature.Mostly the tribological behaviors of Mg alloys and composites have been assessed under dry sliding condition using pin/ball-on-disk wear tests.The addition of nano-reinforcement materials such as carbon(C)nanomaterials to metal matrices can significantly enhance their hardness and wear resistance;for example,Mg matrices containing 1.11vol.% alumina(Al2O3)nano-particulates exhibited significantly higher(1.8 times)wear resistance as compared to monolithic Mg[37].Similarly,dispersion of 8.0vol.% of micron-sized(14μm)silicon carbide(SiC)particles in AZ91 matrix improved the wear resistance by 15–30% as compared to the bare AZ91 alloy[38].In another study,Jiang et al.[39]used 10vol.% titanium carbide(TiC)particulates as a reinforcing material in an AZ91 matrix and reported a reduction in the wear rate of the fabricated composites.A reinforced AZ91 matrix composite with feldspar particles(of sizes 30–50μm)also exhibited good wear resistance,although the feldspar-reinforced AZ91 composite revealed subsurface cracks at high loads and crack propagation during loading caused delamination wear[40].Manakari et al.[6]investigated the COF and wear behavior under a dry sliding condition of Mg/glass micro-balloon(GMB)foams synthesized by disintegrated melt deposition and their results indicated that 25wt.% GMB addition to Mg enhanced its wear resistance by 250% and reduced the COF by 13% as compared to pure Mg.These studies indicate that during abrasion and oxidation,the surfaces of Mg alloys are prone to wear.Further,nonhomogeneous dispersion of reinforcing particles in Mg matrices leads to delamination wear.
Nevertheless,a few studies have reported the nanotribological behaviors of Mg-based composites under wet sliding condition in the literature.Sun et al.[41]reported that the 3.5wt.% NaCl solution caused a decrease in the wear rates for the yttrium(Y)-reinforced MNCs as compared to their wear rates measured under dry sliding conditions.The lower wear rates in the wet environment were attributed to the lubrication effect by the solution,which also dissipated the heat generated due to friction between the pin and disk components.Lin et al.[42]investigated the wear rates of biodegradable Zn alloys under dry and Hanks’balanced salt solutions(HBSS)and observed no difference in the wear rates of these alloys while testing in both conditions.However,Turan et al.[43]reported higher wear rates in carbon nanotubes(CNT)-reinforced MNC in 3.5wt.% NaCl solution as compared to the wear rates under dry conditions and it was attributed to the insufficient dispersion of CNT in the Mg matrix due to re-aggregation of CNT in the Mg matrices,leading to galvanic effect at the surrounding of aggregated regimes in the salt solution.The corrosion and wear of Mg-based materials are multifactorial and may occur simultaneously in the physiological environment and therefore require more systematic investigations.
Carbonaceous nanomaterials generally possess a high thermal conductivity and damping capacity,good self-lubrication properties,and low coefficient of thermal expansion[19,44].Therefore,these nanomaterials are generally considered ideal reinforcing materials for improving the tribological properties of metal matrices[45,46].Graphene nanoplatelet(GNP)is one form of carbonaceous nanomaterials and used in many applications of various industries due to significantly a high surface area(2630 m2g−1)and good self-lubrication property similar to graphite[47].Moreover,the dispersibility of GNPs in metal matrices is better than that of tubular CNTs due to their characteristic two-dimensional(2D)sheet-like morphology and GNPs-reinforced metal matrix composites exhibited enhanced hardness and wear resistance[44,48].GNPs can provide a unique combination of mechanical and physical properties including ultrahigh tensile strength(130GPa)and elastic modulus(1 TPa)[49],extremely high thermal conductivity(5000Wm−1K−1)[50],and charge-carrier mobility(200,000 cm2V−1s−1)[51]as 2D monolayer of sp2carbon atoms densely packed in a honeycomb lattice structure of GNPs.The sp2carbon layers are stacked along the c-axis in a graphitic structure and maintain a distance of 0.34nm between successive layers[44].Each carbon layer in the characteristic sp2graphitic structure of GNPs exhibits strong covalent bonding between carbon atoms and the stacked layers of carbon exhibit weaker van der Waals forces between successive layers[49-51].These properties make GNPs a promising nano-reinforcement material in metal matrix composites.
It has been reported that a continuous layer of solid lubricant forms on the surface of GNPs-reinforced composites due to the shearing of individual graphite layers during the sliding of GNPs-reinforced metal matrix composites against H13 alloy tool steel[52,53].In addition,GNPs contain various types of defects including wrinkles,cracked edges,and other structural defects which are beneficial for anchoring the grain boundaries in metal matrices[54,55].The C atoms in GNPs can strongly interact with the Mg atoms and therefore enhance the resistance to dislocation motion during plastic deformation[55].Moreover,the repair of defects in GNPs can enhance the interfacial strength between GNPs and the surrounding Mg matrix[55,56].The embedded GNPs with multiple wrinkled structures in the Mg matrix straighten during the plastic deformation,which increases the resistance to dislocation motion and enhances the yield strength and hardness of these composites[57].Vahedi et al.[52]investigated the tribological performance of friction-stir processed(FSP)AZ31 alloys containing graphite and GNP reinforcements,and reported that the wear resistance of AZ31 composites reinforced with GNPs and graphite was measured to be 67% and 45% higher than that of bare AZ31,respectively;similarly,their COF measurements revealed lower values for AZ31 reinforced with GNPs(COF=0.25)and graphite(COF=0.40)as compared to that of a bare AZ31 alloy(COF=0.49).Generally,high COF values are indicative of the gradual removal or deformation of solid surfaces due to excessive sliding wear of metals[58].Also,a high variance in COF indicates the agglomeration and pile-up of wear debris in the wear tracks,which may be attributable to the weak bonding of particles and their nonhomogeneous distribution in the metal matrices[59].
From the biological perspective,addition of an appropriate concentration of GNPs in Mg matrices can enhance the adsorption of biomolecules on their surfaces due to their large surface area.The covalent crosslinking mechanism of GNPs also promotes cell adhesion on the surface of metal matrices[56].The macrophage mediated and catalyzed biodegradation of GNPs have been extensively reported in the previous studies[60–63].In a recent study[30],the release of Mg2+ions during the biodegradation of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC was investigated and the results indicated that compared to pure Mg and Mg0.5Zr,0.1wt.%GNPs-reinforced MNC exhibited lower release of Mg2+ions.In another study[56],the in vitro cytotoxicity of Mg0.1GNPs,Mg0.2GNPs,and Mg0.3GNPs MNCs was assessed using human osteoblast-like cells(SaOS2)and the results indicated that addition of GNPs did not significantly affect the cytotoxicity of the pure Mg;and the cell viability for the pure Mg and Mg0.1GNPs MNC were measured 101% and 113%,respectively,indicating an increase of 12% in cell viability with the addition of 0.1 wt.% GNPs in Mg matrices.Similarly,Saberi et al.[64]investigated the biocompatibility of MNC containing 0.5–2.0wt.% GNPs via in vitro tests using MG-63 cells and their results indicated that the MNCs containing 0.5–1.0wt.%GNPs showed a higher cell viability than that of the MNC containing a higher concentration of GNPs(2.0wt.%).It was also reported[64]that a suitable concentration of GNPs(1.0wt.%)in Mg matrix does not produce any toxic reaction rather it leads to enhanced adhesion and proliferation of MG-63 cells and the fluorescent microscopic image of MG-63 cell cultured on Mg-GNPs composites containing 1.0wt.% GNPs showed adequate interactions between adjacent cells and good cell attachment and proliferation of osteoblast cells accommodated to the composite.
Grain refinement in metals improves their hardness,which subsequently reduces the ploughing effect during wear and formation of wear debris[65].Zirconium(Zr)is generally added to achieve grain refinement in Mg alloys[25];however,higher concentrations(>5%)of Zr in Mg alloys lead to the formation of unalloyed Zr phases,which adversely affect their corrosion resistance[66].From the biological perspective of Zr,it is well established that Zr exhibits no ionic cytotoxicity in vitro[67],excellent biocompatibility in vivo without any evidence of mutagenicity or carcinogenicity[25,68].Furthermore,it was reported[69]that chemically stable Zrbased oxides diminished adherent bacteria of Staphylococcus aureus and enhanced cell adhesion and proliferation of mouse MC3T3-E1 pre-osteoblasts.The biocompatibility assessment of Zr-containing Mg alloys showed that addition of 0.5–0.6wt.% Zr did not adversely affect the biocompatibility of Mg alloys[66,70].Moreover,the commercially available biomedical grade Mg alloy,WE43(Mg4Y3RE0.5Zr),which has been used as orthopedic screws and cardiovascular stents,contains 0.5–0.7wt.% Zr[71],[72].Ding et al.[73]also reported that the Mg alloy containing 1wt.% Zr showed higher cell attachment of osteoblast-like cells(SaOS2)than pure Mg.Gu et al.[74]reported that Mg1Zr showed 80–118%cell viability of L-929,NIH3T3,MC3T3-E1,ECV304,and VSMC cells and over 30% of hemolysis during contact in whole blood for 1h.A few studies have reported the potential of GNPs reinforcement in improving the wear resistance of common Al-containing Mg alloys such as AZ31 and AZ91[75–78];however,the nano-tribological behavior of pure Mg containing Zr and GNPs reinforcements is rarely reported in the literature.This study systematically investigates the nano-tribological behavior of pure Mg,Mg0.5Zr,and GNPsreinforced MNCs fabricated via powder metallurgy.
2.Experimental procedure
2.1.Raw materials
Powders of Mg(average particle size=100μm,purity=99.9%),Zr(average particle size=40μm,purity=99.5%),and GNPs(average particle size=15μm,thickness=5nm)(Sigma-Aldrich,Australia)were used as the starting materials.Whereas,stearic acid(SA,C18H36O2)(purity≥99.9%)(Sigma-Aldrich,Australia)was used as the process control agent(PCA)during a high-energy ball-milling(HEBM)process.
2.2.Fabrication of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC
The GNP and Zr powders were dispersed in the Mg powders via HEBM process using a planetary ball-mill machine(QM 3SP2 model,China).This method has been described in our previous studies[30,56].In brief,stainless steel(SS)balls(diameter=7,10,and 15mm)were used as the milling media to achieve high impact energy.Different sizes of milling balls were used to promote fracturing over cold welding of the Mg particles.Firstly,SS balls and Mg powders were added to SS vials by maintaining a ball-to-powder ratio(BPR)to 20:1.The SS vials were then topped up with SA(0.5wt.%)as the PCA and purged with pure argon(Ar)gas to eliminate residual oxygen(O)from the vials.The HEBM process was conducted at 300rpm for 18h with a 30min interval in every 1h to avoid overheating the powders inside the vials.The ball-milled(BM)Mg powder was used to manufacture the pure Mg sample in a first batch.In the second batch,0.5wt.% Zr powder was added to the BM Mg powder and further milled for 4h with a 30min interval in every 1h.The powder mixture from the second batch was used to manufacture the Mg0.5Zr alloy sample.As for the third batch,0.1wt.% GNPs were introduced in the BM Mg0.5Zr powder and further milled for 4h with the same intervals.The powder mixture from the third batch was used to manufacture Mg0.5Zr0.1GNPs sample.Cold pressing of the BM powders was performed at 760MPa for 30min using SS molds to make a green compact with 16mm in diameter and 18mm in height from the three batches of powder mixtures.Finally,the pressed samples were sintered under an Ar atmosphere at 400°C for 1h to remove the PCA and then at 610°C for 2h to achieve the final metallurgical bonds between the mixed powders in pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC samples.
2.3.Microstructural characterization of powder mixtures and pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC
The morphology of as-received GNPs powders and the morphology and dispersion of GNPs in Mg matrix were observed using a transmission electron microscopy(TEM;JOEL 1010).Whereas,thermo K-alpha X-ray photoelectron spectroscopy(XPS;Thermo Scientific)was used to identify the atomic percentages of C and O in the GNPs.Scanning electron microscopy(SEM;FEI Nova NanoSEM)was used to observe the elemental dispersion in the Mg powders and sintered Mg0.5Zr0.1GNPs MNC samples.
Electrical discharge machining(EDM)was used to be machined as a disk sample(10mm in diameter and 2mm in thickness)for microstructural observation.The samples were progressively ground using silicon carbide(SiC)grinding papers with different grit sizes such as 800,1200,2400,and 4000.Mechanical polishing of the ground samples was carried out using a 50:50 volumetric ratio of an oxide polishing suspension(OPS,0.05μm colloidal silica)and ethanol.Then,etching of the samples was carried out in a solution of picric acid(3ml),ethanol(50ml),acetic acid(5ml),and distilled water(10ml).An optical microscope(Leica DM2500M,3.1MP CCD)was used to determine the grain sizes of etched samples according to ASTM E112–12.X-ray diffractometry(XRD;BrukerAXS D4 Endeavor)was used to identify the various phases in the sintered samples and the XRD patterns were recorded over 2θ=10–90° using Cu–K radiation(λ=0.154nm)at a scanning rate of 0.02 °/sec.Raman spectroscopy(Raman/PL HORIBA LabRAM HR)was used to assess the graphitic structure and defect accumulation in the GNPs during the HEBM process.The raw GNP powder,BM powder mixtures of Mg0.5Zr,and Mg0.5Zr0.1GNPs were scanned using 5mW laser power with 514nm laser in the 1000–3000 cm−1spectral range.
2.4.Evaluation of nano-tribological behavior of sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC
The nano-hardness,wear resistance,and COF of the sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC samples were determined by nano-indentation,nano-wear,and nano-scratch tests using a TriboIndenter(Hysitron TI-950)with cube-corner tip,and spherical tip(of 1μm in radius),respectively.Nano-indentation testing was described in a previous study[30]and the three-dimensional(3D)hardness maps and stiffness maps were plotted to observe the distribution of hardness and stiffness in the sintered compacts.
Nano-wear tests were carried out at different sliding passes and loads to investigate the effects of the applied load and the number of passes of the indenter on the wear rates of the sintered samples.An area of 100μm2was selected on the mirror-polished surfaces of the sintered samples and scraped by 1,3,5,7,and 10 sliding passes using 50μN,100μN,and 200μN loads.Post-wear scans were then conducted using a reduced load of 2μN on an area of 20×20μm2to obtain images of worn and unworn surfaces on each sample.The wear volume and wear rates of the samples were determined by analyzing the depths of the surfaces using a TriboView software,as described in the previous study[46].

Fig.1.(a)TEM image of a GNP with flaky morphology;(b)wrinkles at the edge of an individual GNP with insert showing selected area electron diffraction(SAED)of the pristine GNP;(c)XPS spectrum in C1s region of GNPs;and(d)atomic ratio of C and O in GNPs.
To measure the COF of the sintered samples,nano-scratch testing was performed within a 10μm travel distance under 50μN,100μN,150μN,and 200μN applied loads.After wear tests,the morphology and chemical composition of the worn surfaces of the samples were analyzed using SEM coupled with energy-dispersive X-ray spectrometry(EDX).
2.5.Statistical analysis
In this study,the experimental results are presented as mean±standard deviation.The statistical significance of the variations observed between groups was determined using 1-way analysis of variance known as IBM SPSS Statistics.p<0.05 was accepted as statistically significant.
3.Results
3.1.Morphologies of raw GNPs and ball-milled powders,and microstructures of sintered samples
Fig.1a shows a TEM image of an individual flaky GNP and Fig.1b shows the wrinkles at the edge of a GNP with an insert showing the selected area diffraction(SAD)from a pristine GNP.The SAD pattern clearly revealed the characteristic graphitic plane(002)in the GNPs.Figs.1c and 1d show the XPS spectra obtained from pristine GNPs.XPS spectra revealed a characteristic sp2C peak at a binding energy of 284.5eV,confirming the graphitic structure of the GNPs,consistent with a previous study[79].The atomic percentages of O and C measured from the relative intensities of the C1a and O1s peaks are shown in Fig.1d and the atomic ratio of O/C is 0.04.It was reported that GNPs with a low atomic ratio of O/C(≤0.12)possessed higher conductivity and outstanding thermal and chemical stability as compared to graphene oxide(O/C=0.41)[80].
The morphologies of the BM pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs powder mixtures are shown in Figs.2a-c,displaying an average particle size of 35μm for pure Mg powder,28μm for Mg0.5Zr,and 24μm for Mg0.5Zr0.1GNPs,respectively.In the BM Mg0.5Zr powder mixture,there were still large unreacted Zr particles(Fig.2b);while uniformly dispersed Zr particles were embedded in the BM Mg0.5Zr0.1GNPs powder mixture(Fig.2c).
Figs.3a,3b,and 3c show OM images of the sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPS MNC,respectively.The appearance of large grains in the pure Mg is very clear(Fig.3a),while the Mg0.5Zr alloy showed clear boundaries with a decreased grain size compared to the pure Mg(Fig.3b)and the Mg0.5Zr0.1GNPs MNC exhibited the smallest grain size among all the samples.The average grain size was measured at 37μm for pure Mg,21μm for Mg0.5Zr,and 18μm for the Mg0.5Zr0.1GNPS MNC,respectively.The decrease in the grain size of the Mg0.5Zr0.1GNPs MNC sample may be attributable to dispersed GNPs which exhibit the significantly thermally conductive(̴~3000Wm−1K−1)GNPs[81]and a lower coefficient of thermal expansion(1×10−6K−1)[19]as compared to Mg(160Wm−1K−1and 2.61×10−5K−1,respectively).It has been reported that accumulation of GNPs in the grain boundaries of Mg matrix provides a pinning effect that prevents the growth of grains during high-temperature sintering of the samples[30,82].
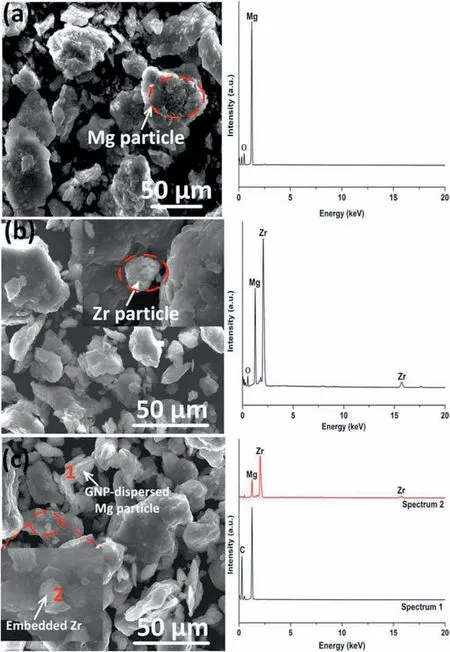
Fig.2.Morphology of ball-milled powders and corresponding EDX spectra:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs powder mixture.
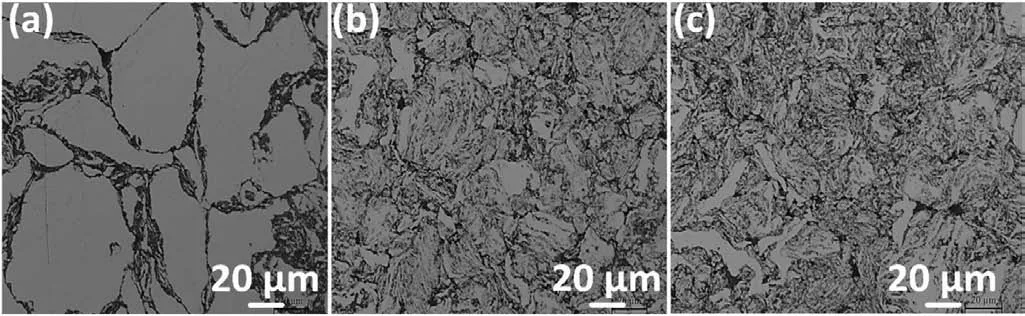
Fig.3.OM images of sintered samples:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.
3.2.Elemental dispersions in sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC
Figs.4a,4b,and 4c show surface morphologies and corresponding EDX maps showing the element distribution of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs samples,respectively,showing the elemental distributions of Zr,C,and O in the Mg matrix.It can be seen that the Mg0.5Zr showed a homogeneous distribution of Zr in the Mg matrix with refined grain size(Fig.4b)as compared to pure Mg(Fig.4a).Smaller grains form more grain boundaries and therefore enhance the strength and hardness of Mg matrices[65].Similarly,the Mg0.5Zr0.1GNPs exhibited uniform distributions of both Zr and C in the Mg matrices(Fig.4c),which can increase its strength and hardness.
The dispersion of GNPs in the Mg0.5Zr0.1GNPs MNC was analyzed using TEM and the results are shown in Fig.5.Interfaces between the GNPs and Mg matrix in the Mg0.5Zr0.1GNPs MNC are indicated by red-dotted lines(Fig.5a).The GNPs’edges are indicated by yellow arrows and the Mg matrix is indicated by white arrows in Fig.5b,which shows a uniformly dispersed GNPs in the Mg matrix.TEM analysis also revealed the formation of nanograins with a size of around 50nm in the Mg matrix(Fig.5c).The SAED pattern(inset of Fig.5d)obtained from the Mg–GNP interface revealed diffraction rings of a characteristic polycrystalline structure in the Mg0.5Zr0.1GNPs MNC[55].In Fig.5d,MgO(200)nanoparticles were fabricated in the inset,which confirms the interfacial reaction between the Mg and GNPs containing oxygen functional groups that were created during the powder processing of the Mg0.5Zr0.1GNPs MNC.The SAED pattern obtained from the Mg–GNP interface revealed GNPs(002),MgO(200),Mg(002),and GNPs(004),which is consistent with previous studies[55,83].The MgO nanoparticles formed in situ in Mg–GNP composites can increase the interfacial strength between the GNPs and Mg matrix,leading to good load transferability in the composite[83];however,they can also act as a brittle phase in Mg alloys[84].In addition,Raman analysis of the MNCs revealed a reduction shift of the 2D band due to the decreased number of layers of GNPs[30],which can promote uniform dispersion of GNPs in MNCs[85],leading to uniform load transfer during plastic deformation.Furthermore,the increased intensity of the 2D band in composite powder mixtures can enhance the interaction between GNPs and Mg during high-temperature sintering[86].
3.3.Nano-wear properties of sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC

Fig.4.SEM images and corresponding EDX maps showing distributions of Zr,C,and O in:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.

Fig.5.TEM images of Mg0.5Zr0.1GNPs MNC:(a)interfacial bonding between GNPs and Mg matrix;(b)dispersion of GNPs in Mg0.5Zr0.1GNPs MNC;(c)formation of nanograins in Mg0.5Zr0.1GNPs MNC;and(d)high-resolution TEM(HRTEM)image of nanograins with an inset showing a SAED pattern of the nanograins.

Fig.6.Wear rates as a function of sliding pass numbers for:(a)pure Mg;(b)Mg0.5Zr;(c)Mg0.5Zr0.1GNPs MNC.(d)Wear rates of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC under 50,100,and 200μN loads after 10 sliding passes.

Fig.7.Surface topographies after 5 sliding passes under 200μN applied load:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.(d)Average surface height profile of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC.
Figs.6a,6b,and 6c show the variation in wear rates of the sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC samples under different applied loads of 50,100,and 200μN.The wear rate of pure Mg(Fig.6a)showed a direct relationship with the number of sliding passes and the applied loads.However,the wear rates of both the Mg0.5Zr and Mg0.5Zr0.1GNPs MNC increased with the applied loads but decreased with the increasing number of sliding passes.Fig.6d shows the wear rates of the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC for 10 sliding passes as a function of the applied loads(50,100,and 200μN).The wear rates of the fabricated samples were found in descending order is pure Mg>Mg0.5Zr>Mg0.5Zr0.1GNPs MNC.This corresponds to their respective nano-hardness(H),asHMg=0.4GPa,HMg0.5Zr=0.6GPa,and HMg0.5Zr0.1GNPs=0.8GPa[30],as described by Archard’s rule[87]that material with high hardness exhibits a lower wear rate and increases at higher applied loads.The abrasion resistance of metallic materials is directly related to their hardness[52,59].In addition,GNPsreinforced MNCs also exhibit a self-lubrication effect due to the graphitic nature of the GNPs[88].It has been reported that the presence of C-based solid lubricant films prevents direct contact between the sliding surfaces[89].Moreover,grain refinement and improved dislocation density in the Mg matrix contribute to the overall wear resistance and load-bearing capacity[29,90].Mg0.5Zr0.1GNPs MNC containing GNPs showed better wear resistance than pure Mg and Mg0.5Zr samples because the dispersed nanoparticulate can offer obstacles to dislocation motion during the applied loads.
The interaction of dislocations and nanoparticles can be considered the main mechanism in improving the wear behavior of nanocomposites.The Mg0.5Zr0.1GNPs MNC exhibited the lowest wear rates at all applied loads among all the samples.Moreover,the wear rates of the Mg0.5Zr and Mg0.5Zr0.1GNPs MNC decreased by 89% and 92% at 200μN load,60% and 80% at 100μN load,and 94% and 93% at 50μN load,respectively,as compared to pure Mg.
Figs.7a,7b,and 7c show the typical surface topographies,with wear scars,pile-ups,and unworn surfaces,of the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC,respectively,after 10 sliding passes under 200μN applied load.The depths of the worn areas within the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC are shown in Fig.7d.It can be seen that the depth of wear scars and the pile-up height in descending order are pure Mg>Mg0.5Zr>Mg0.5Zr0.1GNPs MNC,and those of the Mg0.5Zr0.1GNPs MNC are significantly lower than those of the Mg0.5Zr and pure Mg,indicating considerably higher wear resistance of this material than those of the Mg0.5Zr and pure Mg samples.This may be attributable to various factors including(i)higher hardness of the Mg0.5Zr0.1GNPs MNC;(ii)lower delamination of the oxide tribo-layer(oxidation wear)due to uniform dispersion of the GNPs in the Mg matrices;(iii)self-lubrication by the GNPs;(iv)decreased contact area of counterface materials between the indenter and the Mg0.5Zr0.1GNPs MNC;and(v)high stiffness of the GNPs(EGNPs=~1TPa),which can reduce the tangential force during sliding by the counterface material[52,91–94].The average wear scar depths were measured as 78nm for pure Mg,52nm for Mg0.5Zr,and 6nm for Mg0.5Zr0.1GNPs MNC.
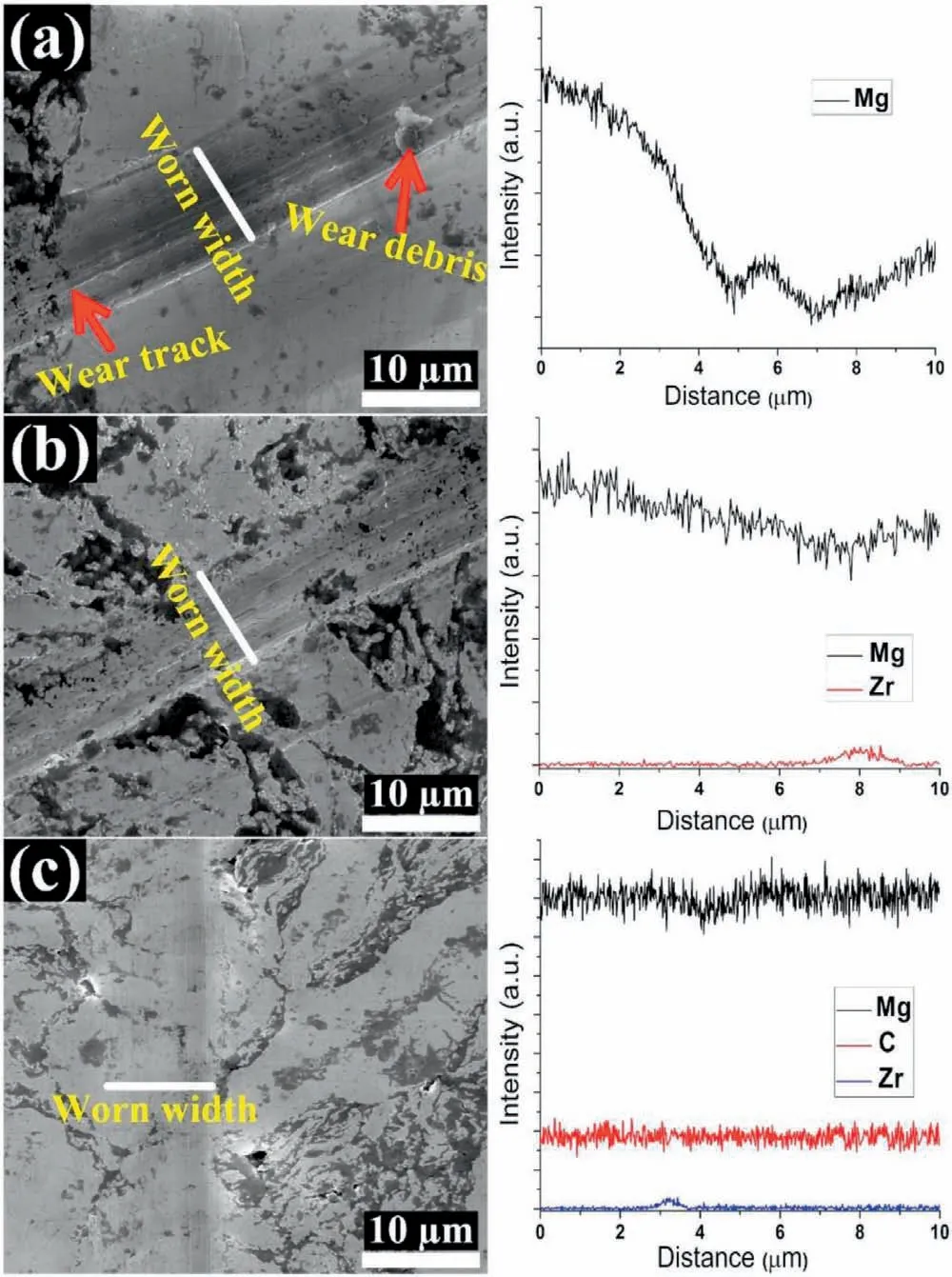
Fig.8.SEM images of worn surfaces and corresponding EDS line scans of samples after 10 sliding wear passes under 200μN applied load:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.
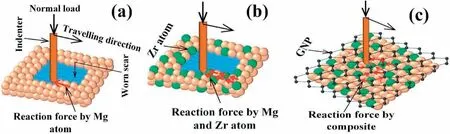
Fig.9.Schematic illustration of wear phenomena during sliding nano-wear tests for:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.

Fig.10.2D topographies of worn surfaces after 10 sliding wear passes under 200μN applied load along with corresponding 3D hardness and stiffness plots from the worn surfaces:(a)pure Mg;(b)Mg0.5Zr;and(c)Mg0.5Zr0.1GNPs MNC.
Fig.8 shows SEM images of wear tracks and EDS line scans within the worn areas of the sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC after 10 sliding wear passes under 200μN applied load.The line scan within the worn areas in the pure Mg showed uneven intensities due to the high wear scar depth,which resulted from the severe plastic deformation of the Mg matrix during sliding wear.The presence of wear debris in the worn area is consistent with the low wear resistance of pure Mg against plastic deformation(Fig.8a).The addition of Zr to the Mg matrix resulted in grain refinement of the Mg matrix,leading to enhanced wear resistance to plastic deformation during sliding wear in the Mg0.5Zr(Fig.8b),and the EDS line scan from the worn areas revealed uniform line scan intensity.The formation of grooves along the wear tracks in the pure Mg and Mg0.5Zr was greater than in the Mg0.5Zr0.1GNPs MNC.Furthermore,the Mg0.5Zr0.1GNPs MNC exhibited identical intensity of all elements during the line scan(Fig.8c),which confirms the lower plastic deformation in the Mg0.5Zr0.1GNPs MNC during wear than in the pure Mg and Mg0.5Zr samples.
Fig.9 shows a schematic illustration of the interaction between atoms in the Mg matrices and counterface material during the wear test.The low reaction force generated by Mg atoms against the tangential force during the sliding of the indenter produced high wear scars(Fig.9a).Fig.9b shows the effect of Zr atoms in the Mg matrix,which enhanced the reaction force against the tangential force using both Mg and Zr atoms in the Mg0.5Zr sample.The GNPs introduced a high amount of reaction force to the indenter against the tangential force generated in the Mg0.5Zr0.1GNPs MNC(Fig.9c).According to Hooke’s law[95],due to the high elastic modulus(1 TPa)of GNPs,they can regain their original shape once a load is removed,which led to a lower wear scar depth in the
Mg0.5Zr0.1GNPs MNC than in the pure Mg and Mg0.5Zr samples.

Table 1Tribological properties of Mg-based materials under different testing conditions.

Table 1(continued)
Fig.10 shows 2D wear topographies of the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC with corresponding 3D hardness and stiffness.Figs.10a,10b,and 10c show schematic illustrations of the 2D wear surface topographies and the corresponding 3D hardness and stiffness mapping of the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC,respectively.The Mg0.5Zr0.1GNPs MNC exhibited significantly lower wear depth(Fig.8c)compared to the pure Mg and Mg0.5Zr samples.In addition,the hardness and stiffness plots obtained from the nano-indentation studies revealed the uniform distribution on the Mg0.5Zr0.1GNPs MNC samples as compared to the pure Mg and Mg0.5Zr.
3.4.COF of sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC
Fig.11a shows the COF of the sintered pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC from nano-scratch tests under different applied loads.The COF at each applied load in descending order was COFMg>COFMg0.5Zr>COFMg0.5Zr0.1GNPs.The COF was 0.05 for Mg0.5Zr0.1GNPs MNC,0.10 for Mg0.5Zr,and 0.21 for pure Mg at 200μN applied load.Fig.11b shows the evolution of COF in the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC samples during the nano-scratch tests.The Mg0.5Zr0.1GNPs MNC revealed a lower COF,which may be attributable to the selflubrication characteristic of the oxide tribo-layers formed on its surface[52].Fig.11b shows a high friction force at the beginning of the nano-scratch tests in the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC.This may be attributable to the initial high adhesion between the sample and indenter,and the ploughing effect by the indenter,leading to the shearing of the Mg matrix[96,97].Once the shearing of Mg matrices began,the COF gradually reduced in these materials.

Fig.11.Coefficient of friction(COF)of pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC:(a)COF as a function of applied load;(b)COF as a function of time for 10μm travel distance under 200μN applied load.
Table 1 lists the tribological properties of various Mgbased materials under different testing conditions and environments and the wear rates of these materials were compared with those of the pure Mg,Mg0.5Zr,and Mg0.5Zr0.1GNPs MNC measured in the present study.However,due to the lack of standardized testing procedures,it is complicated to compare the wear behaviors of these materials under different wear testing conditions.The wear behavior and COF of Mg-based materials were mostly assessed under dry sliding conditions in the previous studies(Table 1).It can be seen that 0.1wt.% GNPs-reinforced MNC exhibited a lower wear rate(mm3/pass)under the dry condition at each of the applied loads than those of pure Mg and Mg0.5Zr in this study.Similarly,addition of various reinforcing particles such as Al2O3,Y,SiC,TiC,feldspar,and graphite(Gr)improved the wear resistance of Mg alloys(Table 1).Compared to the wear rates of Mg-based materials tested in dry conditions,wear testing of similar materials in tap water,3.5wt.% NaCl,and HBSS resulted in lower wear rates[41,98,99].The decrease in wear rates of these materials under wet environments was attributed to the lubrication effect by the medium which also dissipated the frictional heat generated during metal-to-metal contact in sliding wear tests.
4.Conclusions
In this study,the nano-tribological behaviors of powder metallurgically processed pure Mg,Mg0.5Zr alloy,and Mg0.5Zr0.1GNPs MNC were systematically investigated using nano-wear and nano-scratch testing under different loading conditions.The main conclusions are as follows:
The addition of 0.5% Zr to the Mg matrix effectively refined the Mg matrix grain structure,increased the hardness,and reduced the wear rate of Mg0.5Zr as compared to pure Mg.The addition of 0.5% Zr and 0.1% GNPs to the Mg matrix resulted in a further significant refinement of the Mg matrix grain structure,substantial increases in hardness and stiffness,and decreases in the wear rate,COF,and wear depth of the Mg0.5Zr0.1GNPs MNC as compared to the pure Mg and Mg0.5Zr.
The wear resistance of the fabricated samples was measured at different sliding passes.An increase in the number of sliding passes resulted in higher wear rates in pure Mg;however,wear rates showed inverse relationships with the number of sliding passes in case of Mg0.5Zr and Mg0.5Zr0.1GNPs MNC samples.
The wear rates of the Mg0.5Zr and Mg0.5Zr0.1GNPs MNC decreased by 89% and 92% at 200μN applied load,60% and 80% at 100μN applied load,and 94% and 93% at 50μN applied load,respectively,as compared to pure Mg.
Mg0.5Zr0.1GNPs MNC exhibited reductions in COF of 52% and 21% at 100μN applied load,57% and 43% at 150μN applied load,and 71% and 29% at 200μN applied load compared to the pure Mg and Mg0.5Zr,respectively.
Acknowledgments
The authors acknowledge the financial support for this research by the Australian Research Council(ARC)through the Future Fellowship(FT160100252)and the Discovery Project(DP170102557).The authors also acknowledge the scientific and technical assistance of RMIT University’s Microscopy and Microanalysis Facility(RMMF),a linked laboratory of the Australian Microscopy & Microanalysis Research Facility.
 Journal of Magnesium and Alloys2021年3期
Journal of Magnesium and Alloys2021年3期
- Journal of Magnesium and Alloys的其它文章
- Modifying microstructures and tensile properties of Mg-Sm based alloy via extrusion ratio
- The effects of Ca and Mn on the microstructure,texture and mechanical properties of Mg-4 Zn alloy
- H2 generation kinetics/thermodynamics and hydrolysis mechanism of high-performance La-doped Mg-Ni alloys in NaCl solution—A large-scale and quick strategy to get hydrogen
- The slip activity during the transition from elastic to plastic tensile deformation of the Mg-Al-Mn sheet
- Rotational and translational domains of beta precipitate in aged binary Mg−Ce alloys
- Does acid pickling of Mg-Ca alloy enhance biomineralization?
