Reversal Effects of Ivermectin and Moxidectin on Multidrug Resistance in C6/adr Cells in vitro
Chen Chen, Liang Hong-sheng, Wang Li-wei, Wang Qing, and Gao Ai-li*
1 College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
2 Key Laboratory of Neurosurgery, College of Heilongjiang Province; The First Affiliated Hospital of Harbin Medical University, Harbin 150001, China
Abstract: Multidrug resistance (MDR) is a serious obstacle encountered in cancer treatment.This study was performed to explore the reversal MDR activity of ivermectin (IVM) from avermectin family and moxidectin (MOX) belonging to milbemycin family.The two compounds (5 μmol • L-1) showed strong potency to increase adriamycin cytotoxicity toward adriamycin-resistant rat glioma cells C6/adr with fold reversal (FR) of 31.02 and 13.40, respectively.In addition, the mechanisms of them on p-glycoprotein (P-gp)-mediated MDR demonstrated that the two compounds significantly increased the intracellular accumulations of adriamycin and Rh123 via inhibiting P-gp efflux.Based on the analysis of P-gp, MDR1 and MRP1 gene expressions by using immunofluorescence flow cytometry and RT-PCR, the results revealed that the two compounds could down regulate the expression of P-gp, and that MDR1 and MRP1 gene expressions were down regulated.These findings suggested that ivermectin and moxidectin probably represented potent agents for reversing MDR in cancer therapy, and especially ivermectin was a better modulator.
Key words: ivermectin, moxidectin, C6/adr, multidrug resistance, p-glycoprotein
Introduction
Chemotherapy is one of the major methods for treating malignant glioma.However, the clinical effectiveness of chemotherapy is often limited by the emergence of the resistance of human tumours to multiple anticancer drugs (multidrug resistance, MDR).A classic mechanism of MDR is overexpression of a 170 ku p-glycoprotein (P-gp), which is a member of the ATPbinding cassette (ABC) superfamily of membrane transporters (Clayet al., 2015; Thomas and Coley, 2003).
In tumour cells, P-gp acts as an efflux pump that extrudes chemotherapeutic agents such as adriamycin, vinblastine, teniposide, paclitaxel and mitomycin C out of cells, decreasing their intracellular concentration (Follitet al., 2017).ABC drug transporters also include the multidrug resistance proteins (MRPs) and the breast cancer resistance protein (BCRP or ABCG2) (Follitet al., 2017; Liuet al., 2010; Famuyiwa,2019).Other mechanisms play a part in MDR in addition to ABC drug transporters, including enhanced expression of glutathione-S-transferase or glutathione peroxidase, reduced expression of topoisomerase II and cell adhesion to extracellular matrix proteins (Zhouet al., 2009; Elliott and Sethi, 2002; Shabbitset al., 2001).
Reversing MDR is one of the major issues in the treatment of malignant tumors.In order to regain sensitivity of resistant tumour cells to chemotherapeutics, a number of compounds to reverse MDR have been explored for more than two decades, such as verapamil, PSC 833, trifluoperazine, prednisolone and LY335979 (Tsuruoet al., 2003).In addition, numerous compounds from Chinese herbal medicines have been reported as modulators that suppress transport of P-gp, for instance, natural lignans fromArctium lappa, hydro-alcoholic extract ofEclipta albaand cnidiadin (Bardelmeijeret al., 2004; Liet al., 2014; Shabbitset al., 2015; Chaudharyet al., 2013).
Although several chemosensitisers show potential for reversing MDRin vitro and in vivo, the results of clinical trials with these compounds are disappointing because of side-effects and/or weak potency.Therefore, development of new and more potent reversal agents with fewer side-effects is a priority for researches on MDR.Recently, some macrocyclic lactones (MLs) have been discovered to be effective on reversing MDR of tumor cells.MLs with lower toxicity are the most powerful agents used widely for fighting against ecto- and endo-parasites in livestock, pets and human (Barthomeufet al., 2005; Burget al., 1979).It has been reported that naturally occurring avermectins can modify the sensitivity of tumour cells to the substrates of MRPs.In some experimental systems, the most active avermectin is almost one order of magnitude more effective than the traditional inhibitor of multidrug resistance ciclosporin (Naraet al., 2009). In pervious studies, doramectin, nemadectin and milbemycins are found to reverse MDR of MCF-7/adr cells (Korystovet al., 2004; Gaoet al., 2010; Xianget al., 2010), and these compounds belong to macrocyclic lactones.On the basis, the macrocyclic lactones ivermectin and moxidectin are further evaluated for their abilities to reverse MDR of golima C6/adr.Ivermectin, a dehydrated derivative of avermectin B1a, is a member of the avermectin family and is a broadspectrum macrocyclic lactone endectocide.Millions of humans have been treated with ivermectin for the control of onchocerciasis and lymphatic filiarisis (Gaoet al., 2011).Moxidectin is a third generation macrocyclic lactone with potent endectocide activity, belonging to the milbemycin family.It differs from the avermectins mainly by the lack of a sugar moiety attached to the C13 of the macrocyclic ring (Fig.1).In this paper, the abilities of ivermectin and moxidectin were investigated to reverse MDR in adriamycinresistant golima cells (C6/adr) and the likely mechanism of this reversal.

Fig.1 Structures of ivermectin and moxidectin
Materials and Methods
Chemicals and reagents
Ivermectin and moxidectin were provided by Zhejiang Hisun Pharmaceutical Co., Ltd.(Taizhou, China), and purity>95%.3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and rhodamine 123 (Rh123) were purchased from Sigma-Aldrich Canada (Oakville, Ontario).R-phycoerythrin conjugated mouse anti-human monoclonal antibody against P-gp Mdr-1 (UIC2) was obtained from Santa Cruz Biotechnology, Inc (USA).
Cell lines and cell culture
Drug sensitive glioma cell line C6 was obtained from the Third Affiliated Hospital of Harbin Medical University (Harbin, China), and its adriamycinresistant counterpart C6/adr was derivated from C6 by stepwise exposure to increasing doses of adriamycin (ADR).Cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum and 1% penicillin/streptomycin at 37℃, humidity 95% and CO25%.
MTT assay
MTT assay was used to assess cytotoxicity of the drugsin vitro.The cells C6 and C6/adr were harvested in the exponential growth phase, and 100 μL aliquots were plated into 96-well plates.The cells were incubated for 24 h at 37℃ before the addition of modulators.After pre-incubation, the cells were treated with various concentrations of testing compounds for 48 h.Then 100 μL of 0.5 μmol•L-1MTT was added to each well after the withdrawal of the culture medium and was incubated for an additional 4 h at 37℃.After cells were centrifuged for 15 min at 3 500 r•min-1, 100 μL of MTT was carefully removed, and 150 μL of dimethyl sulfoxide (DMSO) was added.The absorbance was measured on a Bio-Rad Model/550 microplate reader (Hercules, CA, USA) with a test wavelength of 570 nm.The reversal effects of the two drugs were further investigated with the same method.C6 and C6/adr cells seeded into 96-well plates were treated with various concentrations of adriamycin in the absence and presence of the two compounds at 0.1, 1 and 5 μmol•L-1for 48 h, respectively.The inhibitory rate of cells growth was obtained through IC50values for adriamycin.Triplicate experiments with triplicate samples were performed.Control medium included equivalent amount of DMSO (as solvent control), but the applied dose did not exhibit modulation effects on the cell growth or drug sensitivity in these studies.In these experiments, verapamil (VRP) at 5 μmol•L-1was used as positive control drug.The fold reversal (FR) of MDR was defined as the ratio of theIC50for adriamycin alone (control) vs.theIC50for adriamycin in the presence of milbemycins.FR=IC50ADRalone/IC50ADR+modulator.
Adriamycin accumulation in C6/adr cells
C6 and C6/adr cells were harvested and seeded at 1.0×105cells per well in 24-well plates and cultured for 6 h.The cells were then treated with different modulators at 0.1, 1 and 5 μmol•L-1for 1 h.The cells were exposed to 15 μmol•L-1adriamycin and the control was treated with 15 μmol•L-1adriamycin alone.After incubation for 3 h, the cells were washed three times with ice-cold phosphate buffered saline (PBS, pH 7.2).The cells were resuspended in HCl 0.3 mol•L-1in 60% ethanol overnight.
The intracellular adriamycin concentrations were determined by high performance liquid chromatography (HPLC) (Agilent 1100, XDS-C18: 4.6×150 mm, 5 μm).The assay conditions were as the followings: mobile phase was 5 mmol•L-1phosphoric acid/methanol/2-propanol/acetonitrile (8 : 7 : 3 : 2, v/v) at 1 mL•min-1.Fluorescence detection was operated at excitation and emission wavelengths of 233 and 560 nm, respectively.Adriamycin concentrations were given by the adriamycin standard curve.Triplicate experiments with triplicate samples were operated.
Accumulation and efflux of Rh123
C6 and C6/adr cells were seeded at 1.0×105cells per well in 24-well plates and cultured for 6 h.At confluence, 400 μL fresh medium containing appro- priate concentrations of different modulators or VRP was added and incubated further at 37℃ for 1 h.Subsequently, 2.5 μg of Rh123 was added to each well, and the plates were incubated for another 1 h at 37℃.Cells were lysed with 0.1% Triton X-100 at room temperature after washing five times with icecold PBS, then the fluorescence of cell lysate was measured with F-7000 spectrofluorometer (Hitachi Seisakusho, Tokyo, Japan) at a wavelength of 485 nm for excitation and 525 nm for emission.
The efflux of Rh123 was also measured using cells incubated with 2.5 μg of Rh123, without modulators, for 2 h as described above.Cells were then washed twice with RPMI-1640, and incubated again with the medium in the presence or absence of modulators.After incubation for various times (15, 30, 45, 60 and 90 min), cells were then washed twice with an excess volume of ice-cold PBS, lysed with 0.1% Triton X-100, and fluorescence intensity was measured as described above.The experiments were carried out in triplicate.
RT-PCR
The total RNA from C6 and C6/adr cells treated with 5 μmol·L-1milbemycins for 24 h was isolated with the TRIzol reagent of TaKaRa RNAiso™ Plus, and RT-PCR was performed.The primers used for multidrug resistance protein 1 (MDR1), multidrug resistance-associated protein 1 (MRP1) andβ-actin genes were as the followings: MDR1, forward 5'-CCATCATTGCAATAGCAGG-3' and reverse 5'-AGTCCTCGTCTTCAAACTTG-3' for a 157 bp product; MRP1, forward 5'-GGTG CTTCCCACGGAGG-3' and reverse 5'-TCAACCAC AAAACTGCAGCC-3' for a 218 bp product; andβ-actin, forward 5'-CTACAATGAGCTGCGTGTGGC-3' and reverse 5'-CAGGTCCAGACGCAGGATGGC-3' for a 270 bp product.Amplification was performed for 35 cycles of sequential denaturation (94℃, 30 s), annealing (56℃, 30 s) and extension (72℃, 1 min).The amplified fragments were detected by 2% (w/v) agarose gel electrophoresis and stained with ethidium bromide.Each band was analyzed on image analysis system Tanon2500.The specific gene expression level was determined semiquantitatively by calculating the ratio of density metric value from specific genes expressed in relation to the internal standard (MDR1 orMRP1 gene expression/β-actin expression).Triplicate experiments with triplicate samples were performed.
Determination of P-gp
The cell surface P-gp levels were measured by immunofluorescence flow cytometry (Molyneuxet al., 2003).C6 and C6/adr cells seeded into 6-well plates at a density of 2×105cells per well were cultured for 24 h.Then the cells were exposed to the 5 μmol•L-1different modulators for another 24 h.After that the cells were harvested, washed twice with ice-cold PBS, counted and then labeled with R-phycoerythrin conjugated mouse anti-human monoclonal antibody against P-gp according to manufacturer's instruction.The fluorescent intensity was determined using flow cytometry (BD FACSAria, USA).Duplicate experiments with triplicate samples were carried out.
Western blot analysis
Firstly, after treating with indicated agents, C6 and C6/adr cells were suspended in RIPA lysis buffer (Beyotime, China) containing 1% phenylmethanesulfonyl fluoride (PMSF) on ice and the total protein was extracted by centrifugation.The total protein was mixed with 6× protein loading buffer (TransGen, China, J21020) and heated to 100℃ for 10 min.The protein concentration was determined by the BCA protein assay kit (Bio-Rad, Shanghai, China).The total protein was separated by different concentration sodium dodecyl sulfatepolyacrylamide gels and transferred electrophoretically and transferred onto a PVDF membrane (0.22 µm, Millipore, MA, USA).The membranes were incubated with 5% skim milk in TBST for 75 min and overnight at 4℃ with various antibodies (β-actin and P-gp).Then, the membrane was washed with TBST and incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h.At last, the membrane was allowed to detect the appropriate bands using the chemiluminescent HRP substrate.
Statistical analysis
All the results were presented as the mean±SD, and analyzed by one-way analysis of variance followed by Student-Newman-Keuls post hocqtest between difference groups.
Results
Intrinsic cytotoxicity of two drugs
The cytotoxic activities of ivermectin and moxidectin to tumor cells were first examined.TheIC50values of ivermectin for C6/adr and C6 were 28.97±0.70 and 23.01±0.20 μmol • L-1, respectively.TheIC50of moxidectin were 21.93±0.96 and 18.86±0.61 μmol • L-1for C6/adr and C6, respectively.The concentration ranges of these drugs used in the further experiments were below the individualIC10, that was, 0.1-5 μmol • L-1.At this concentration rangein vitro, ivermectin and moxidectin did not appear to affect cell survival.
MDR reversal efficacy of two drugs
C6/adr cells were approximately 41-fold resistant to adriamycin compared with C6 cells.Ivermectin and moxidectin significantly enhanced the sensitivity of C6/adr cells to adriamycin in dose-dependent manner, but had no such effects on the drug-sensitive parent C6 cells (Table 1).FRs of ivermectin, moxidectin and VRP at 5 μmol • L-1showed that the two experiment drugs were more effective than VRP on reversing MDR of C6/adr cells.

Table 1 Modulation by ivermectin and moxidectin of sensitivity of C6/adr cells and C6 cells to adriamycin
Effects on intracellular accumulation of adriamycin
The intracellular accumulation of adriamycin in C6 cells was approximately 3.46 times more than that in C6/adr cells.After C6/adr cells were treated respectively with 5 μmol • L-1ivermectin and moxidectin, the enhancement of adriamycin accumulation was approximately 2.51- and 1.81-fold, respectively compared to C6/adr cells untreated.However, the adriamycin accumulation in C6 cells was not remarkably affected by the two drugs (Fig.2).

Fig.2 Effects of ivermectin and moxidectin on intracellular accumulation of adriamycin in C6 and C6/adr cellsCells are pretreated with 0.1, 1 and 5 μmol • L-1 ivermectin and moxidectin.Adriamycin-associated mean fluorescence intensity (MFI) is measured as described in materials and methods.Each bar represents mean±SD from three independent experiments.*p< 0.05, **p< 0.01 vs.control group.
Effects of modulators on intracellular accumulation and efflux of Rh123
Determining intracellular Rh123-associated mean fluorescence intensity (MFI) in C6/adr and C6 cells were employed to study the effects of ivermectin and moxidectin on inhibiting P-gp function.As shown in Fig.3A, two drugs enhanced intracellular Rh123 accumulation of C6/adr cells in a concentration-dependent manner.Especially, when C6/adr cells were treated with ivermectin and moxidectin at 5 μmol • L-1, the intracellular MFI was much higher than that of untreated C6/adr cells (Fig.3B).The data also indicated that ivermectin had more statistically significant effects on intracellular Rh123 accumulation than moxidectin (p<0.05).On the contrary, no such increase in MFI was observed in modulators-treated C6 cells.

Fig.3 Effects of ivermectin and moxidectin on inhibiting P-gp functionA, Effects of ivermectin and moxidectin on Rh123 accumulation in C6 and C6/adr cells.Cells are pretreated with 0.1, 1 and 5 μmol • L-1 ivermectin and moxidectin.Each bar represents mean±SD from three independent experiments.*p< 0.05, **p< 0.01 vs.control group.B, Rh123 efflux in C6/adr cells and C6 cells.a, Untreated C6 cells; b, C6/adr cells incubated with 5 μmol • L-1 ivermectin; c, C6/adr cells incubated with 5 μmol • L-1 moxidectin; d, Untreated C6/adr cells.Data are expressed as means±SD of three independent experiments.**p<0.01, b and c vs.control group.
Down-regulation of MDR1 and MRP1 gene expressions
MDR1 andMRP1 genes of C6 and C6/adr cells were assessed using semiquantitative RT-PCR.As expected, there was no apparentMDR1 expression and a littleMRP1 expression in C6 cells, whereas the two genes expressions were clearly observed in C6/adr cells with acquired MDR.Compared with untreated C6/adr cells,MDR1 expression in C6/adr cells treated with 5 μmol•L-1ivermectin or moxidectin for 24 h was decreased by 43.54%±3.66% and 32.49%±4.16%, andMRP1 expression in C6/adr cells treated with 5 μmol•L-1ivermectin or moxidectin was decreased by 62.61%±6.66% and 72.14%±4.36%.
Decreased expression of P-gp by two drugs
To further confirm whether ivermectin and moxidectin could down regulateP-gpexpression, cells were exposed to 5 μmol L-1ivermectin and moxidectin.The expression level ofP-gpin C6 and C6/adr cells was analyzed by flow cytometry.C6 cells showed virtually fluorescent intensity labeled by anti-Pglycoprotein monoclonal antibody as the blank.C6/adr cells exhibited a strong fluorescent area corresponding toP-gp.After 24 h incubation with ivermectin and moxidectin, the expression level ofP-gpwas decreased by 66.72%±1.43% and 57.08%±1.78% in C6/adr cells, respectively in comparison to untreated C6/adr cells (Fig.4A).The protein expression ofP-gpwas examined using western blot after C6/adr cells were treated with ivermectin and moxidectin.P-gpexpression levels were reduced both in C6 and C6/adr cells treated with ivermectin and moxidectin compared to C6/adr cells (Fig.4B).These results indicated that they could decrease expression ofP-gpin C6/adr cells.
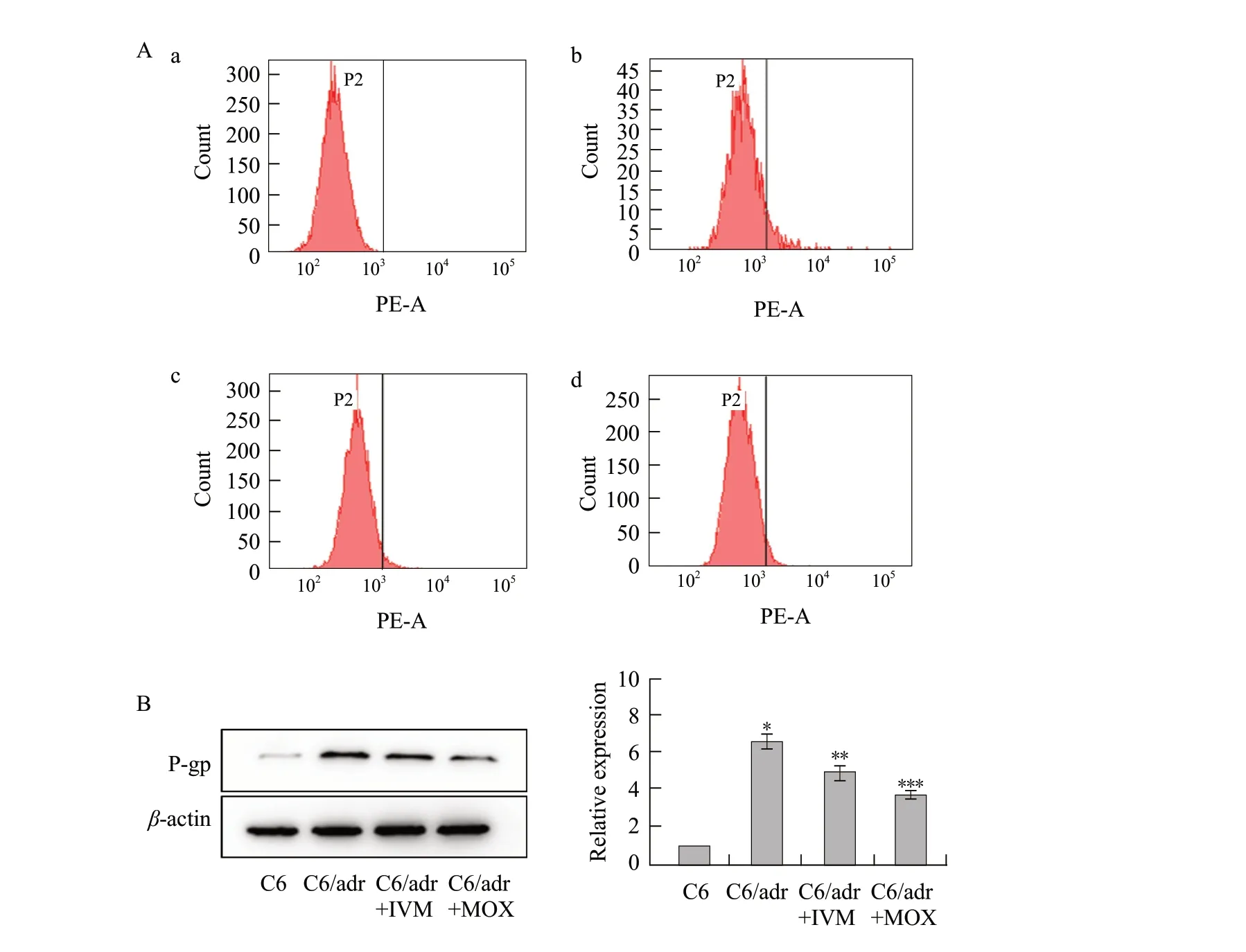
Fig.4 P-gp expression in C6 cells and C6/adr cellsA, After 24 h incubation with ivermectin and moxidectin, expression level of P-gp.a, C6 cells show no fluorescent signal; b, C6/adr cells show notable fluorescent signal; c, C6/adr cells incubated with 5 μmol • L-1 ivermectin; d, C6/adr cells incubated with 5 μmol • L-1 moxidectin.P-gp is determined by flow cytometry using R-PE-conjugated mouse anti-human monoclonal antibody against P-gp.Area in right frame indicates relative quantity of P-gp expression; B, Expression of P-gp proteins and semi-quantification by western blot.*p<0.05, **p<0.01, ***p<0.001.
Discussion
MDR of tumour cells is one of the major cuases for chemotherapeutic failure, so searching for effective and clinically applicable MDR modulators has been given the highest priority for enhancing the sensitivity of MDR tumor cells to chemotherapeutics.In the present work, the studies showed the effects of ivermectin (from the avermectin family) and moxidectin (from the milbemycin family) on reversal of MDR in C6/adr cells for the first time.
In this study, adriamycin and Rh123 were used extensively as indicators of P-gp transport function since they were good P-gp substrates with an autofluorescence capacity (Shiet al., 2008; Kimet al., 2003; Ponce and Barrera-Rodríguez, 2005).The results indicated that ivermectin and moxidectin (0.1-5 μmol•L-1) could concentration-dependent enhance the accumulation of adriamycin and Rh123 in C6/adr cells, but similar treatment of C6 cells showed no modulating effect.In the efflux assay, the two compounds could inhibit the efflux of Rh123 from treated C6/adr cells in a time-dependent manner.These results demonstrated that ivermectin and moxidectin as substrate competed with the cytotoxic agent for transport by the pump P-gp and inhibited the transport activity of P-gp.
This study found that ivermectin was more potent than moxidectin in reversing MDR of C6/adr cells.Lespineet al.(2007) reported that the chemical structures of macrocyclic lactones influenced their affinity for P-gp.The presence of a sugar moiety affected the hydrophobicity of the molecule, and this hydrophobic moiety seemed to be important for the interaction of macrocyclic lactones with P-gp.The hydrophobic characteristics of the two compounds were investigated in preliminary experiments by comparing their retention times on reverse-phase HPLC (data not shown).As expected, ivermectin had a longer retention time than moxidectin, as it possessed a disaccharide moiety at the C13 of the macrocycle.It was therefore more hydrophobic and had stronger affinity for P-gp, which might be the main reason why ivermectin more effectively reversed MDR of C6/adr cells than moxidectin, consistent with earlier findings (Ji and He, 2007).The high affinity for P-gp was one of mechanisms proposed for reversal of MDR by ivermectin and moxidectin (i.e.inhibition of P-gp efflux).
In addition, expressions of relative mRNA and P-gp were analyzed by RT-PCR and immunofluorescence flow cytometry, as some studies had proposed that suppressing the expression of ATP-binding cassette (ABC) transporters was a key mechanism for certain modulators or agents that reversed MDR.For example, Chunet al.(2015) found that Lapatinib significantly inhibited MDR1 and BCRP mRNA expressions.Xueet al.(2014) demonstrated that a novel compound RY-10-4 reversed MDR partially by down-regulation ofP-gpandMDR1 expressions in MCF-7/ADR cell line.Guet al.(2014) suggested that bufalin could effectively reverse MDRviadown-regulation ofMRP1 gene expression.It was shown here that ivermectin and moxidectin decreasedMDR1 gene,MPR1 gene andP-gpexpressions in C6/adr cells in different degrees.The results suggested that ivermectin and moxidectin might reduce the expression ofP-gpat both the transcriptional and translational levels, reversing MDR phenotype of C6/adr cells.
The concentrations of ivermectin and moxidectin used (0.1, 1 and 5 μmol•L-1) was similar to other macrcyclic lactones (Ji and He, 2007; Guet al., 2014; Dider and Loor, 1996), and the two MLs normally showed limited toxicity in most mammals because of effective drug efflux at the blood-brain barrier by P-gp (Lespineet al., 2006; Lespineet al., 2006).Furthermore, the lower neurotoxic potential of moxidectin was explained by its lower binding affinity or lower intrinsic activity at the relevant central nervous system receptors compared with ivermectin (Kiki-Mvouakaet al., 2010; Janko and Geyer, 2013).This study found that 1 μmol•L-1ivermectin and moxidectin could reverse MDR (12.64- and 2.33-fold reversal, respectively).Therefore, the further work is needed to determine a suitable concentration for usingin vivo.
Conclusions
Taken together, the findings demonstrated that ivermectin and moxidectin could both potently reverse MDR of C6/adr cells and that ivermectin was more effective than moxidectin and verapamilin vitro.
Acknowledgement
Chen Chen and Liang Hongsheng contributed equally to this manuscript.
 Journal of Northeast Agricultural University(English Edition)2021年3期
Journal of Northeast Agricultural University(English Edition)2021年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Water Consumption Processes of Different Planting Models in Rice Production of Northeast China
- Optimum Combination Test of Erosion Gully in Black Soil Regions by Coal Gangue Reconstruction Soil
- Effect of Chilling Stress on Synthesis of Antioxidant Enzymes, Osmotic Adjustment Substances and Membrane Lipid Peroxide Levels in Two White Clover Cultivars
- Complete Genome Sequencing and Analysis of Rehmannia Mosaic Virus Isolate from Shanxi Province
- Effects of DHRS3 in C2C12 Myoblast Differentiation and Mouse Skeletal Muscle Injury
- Effects of Lanthanum and Cerium on Seeds Fatty Acid Composition in Soybean
