Effects of DHRS3 in C2C12 Myoblast Differentiation and Mouse Skeletal Muscle Injury
Zhang Wen-yu, Xu Jia-hui, Zhang Chun-yu, Tong Hui-li, Li Shu-feng, and Yan Yun-qin
College of Life Sciences and Technology, Northeast Agricultural University, Harbin 150030, China
Abstract: Myoblast differentiation is an essential process during skeletal muscle development.C2C12 myoblast is a commonly used experimental model to study muscle cell differentiation in vitro.Dehydrogenase/reductase (SDR family) member 3 (DHRS3) is a highly conserved member in short-chain alcohol dehydrogenase/reductase superfamily and has been shown to be involved in the metabolism of retinol.Previous experimental results showed that the expression of DHRS3 increased significantly during the differentiation of myoblasts differentiation.However, the effect of DHRS3 on mouse muscle cell differentiation was unclear.The objective of current study was to determine if DHRS3 affected muscle cell differentiation, and if DHRS3 was involved in muscle regeneration.Protein expression was determined by western blot and immunofluorescence analysis.The activation and inhibition of DHRS3 increased and decreased C2C12 myoblast differentiation respectively, which indicated that DHRS3 could affect C2C12 myoblast differentiation.DHRS3 expression was significantly changed during muscle regeneration, with the regeneration of muscle injury, the expression of DHRS3 tended to increase first and then decrease.It suggested that DHRS3 might be involved in muscle regeneration.In summary, this study confirmed the involvement of DHRS3 in C2C12 myoblast differentiation and mouse skeletal muscle regeneration and provided a theoretical basis for further elucidating the molecular mechanism of muscle development.
Key words: DHRS3, C2C12, cell differentiation, mouse skeletal muscle injury
Introduction
Skeletal muscle development is a complex and multistage process, including the recruitment of integrated myoblasts from myoblast precursors, myoblast proliferation, cell cycle arrest and fusion of myoblasts into multinucleated muscle tubes.Muscle tubes further differentiate into muscle fibers and eventually develop into mature muscle tissues.These processes during muscle formation are mainly regulated by the myogenic regulatory factor (MRF) family, including myogenic determination factor 1 (MyoD) and myogenin (MyoG) (Duet al., 2016).Dehydrogenase/reductase (SDR family) member 3 (DHRS3) is a highly conserved member of the short-chain alcohol dehydrogenase/reductase superfamily.DHRS3 is first demonstrated to have similar activity to all-trans retinol dehydrogenase; subsequently, it is demonstrated that DHRS3 reduces the conversion of retinal to retinol during the visual cycle of cones (Chen and Li, 2016).As retinol reductase, DHRS3 and retinol dehydrogenase 10 (RDH10), these two related membrane-binding proteins activate each other functionally, mediating the transformation between retinol and retinal (Shannonet al., 2017).During the inflammatory process, a decrease in DHRS3 expression in the liver disrupts the systemic retinol metabolism (Lamarcheet al., 2015).Studies have shown that DHRS3 can prevent the excessive formation of retinoic acid during embryonic development.When there is too much retinoic acid, the expression of DHRS3 is up-regulated, while when the synthesis of retinoic acid is blocked, the expression of DHRS3 is down-regulated (Metzler and Sandell, 2016).DHRS3 is involved in maintaining the balance of retinoic acid signals, and thus regulates the formation of body axis during embryonic development (Kamet al., 2013).DHRS3 is regulated by p53 and p63 tumor suppressor proteins (Kirschneret al., 2010), which promote the storage of lipid droplets in HepG2 cells, which is consistent with the previously proved view that DHRS3 is an endoplasmic reticulum protein (Deisenrothet al., 2011).Although there are many studies on the effect of DHRS3 on retinol metabolism, the regulatory function of DHRS3 in skeletal muscle cell differentiation has not been previously demonstrated.
The objective of current study was to determine if DHRS3 affected muscle cell differentiationin vitro, and if DHRS3 was involved in muscle regenerationin vivo.The results of this study provided a theoretical basis for further elucidating the molecular mechanisms of muscle development.
Materials and Methods
C2C12 cell culture and treatment
C2C12 myoblast cells were a common model to study muscle differentiation (Nozhenkoet al., 2015).The C2C12 cell line used in this study was an immortalised mouse myoblast cell line that was purchased from Shanghai Shenggong (Shanghang, China).After resuscitation of cells, C2C12 cells were cultured in growth medium containing 10% foetal bovine serum (Biological Industries, Beijing, China).When the cell density reached 70%-80%, the growth medium was replaced with low-serum differentiation medium containing 2% horse serum (Biological Industries) to induce C2C12 cell differentiation.
Transfection with small interfering RNA (siRNA)
DHRS3 siRNA and negative control (NC) RNA sequences were purchased from Sangon Biotech (Shanghai, China).The siRNAs for DHRS3 were siDHRS3-475 and siDHRS3-942, and their sequences were as the followings: sense strand 5'-CCUCUGCAAAUGAUCUAUUTT-3', antisense strand 5'-AAUAGAUCAUUUGCAGAGGTT-3',and sense strand 5'-CCAUAUUGUGUGCCUCAAU TT-3', antisense strand 5'-AUUGAGGCACACAAU AUGGTT-3', respectively.The sequence of NC for DHRS3 siRNA was as the followings: sense strand 5'- CCUCUGCAAAUGAUCUAUUTT-3', and antisense strand 5'-ACGUGACACGUUCGGAGAATT-3'.C2C12 cells were transfected with Lipofectamine 2000 (Invitrogen, California, USA).
Construction of a DHRS3 overexpression vector
The full-length DHRS3 cDNA was amplified from C2C12 total cDNA by polymerase chain reaction using the following primers: 5′-TGTCCTCCCCTTAAA GGTGTTCATACAGGTG-3′ and 5′-ATGGTGTGG AAATGGCTGGGC-3′.The cDNA of DHRS3 was ligated into the pcDNA3.1(+) vector (Beyotime, Shanghai, China), and the reconstructed vector was sequenced by Sangon Biotech to confirm its fidelity.
Clustered regularly interspaced palindromic repeats (CRISPR) interference screening vectors
To explore the effect of DHRS3 on the differentiation of C2C12 cells, bioinformatics website ZiFit (http://zifit.partners.org/ZiFiT/) was used to predict DHRS3 promoter target site (Gene ID: 20148).Four target sites were selected and a single-stranded oligonucleotide single-directed RNA targeting different sites of the gene was designed.The synthesised oligonucleotides were digested withBbSI and ligated with the pSPgRNA expression vector.The resulting ligation product was transformed intoEscherichia coli.The sequencing results showed that the recombinant vectors pSPgRNA-DHRS3-1 (S1), pSPgRNA-DHRS3-2 (S2), pSPgRNA-DHRS3-3 (S3) and pSPgRNA-DHRS3-4 (S4) were successfully constructed (Table 1) and these were transfected using polyethyleneimine (PEI; Sigma-Aldrich).The cells were co-transfected with the recombinant plasmid and SP-dCas9-VPR plasmid (AddGene) or dCas9 plasmid (AddGene) for 48 h, and western blot analysis was performed to determine the expression level of DHRS3.
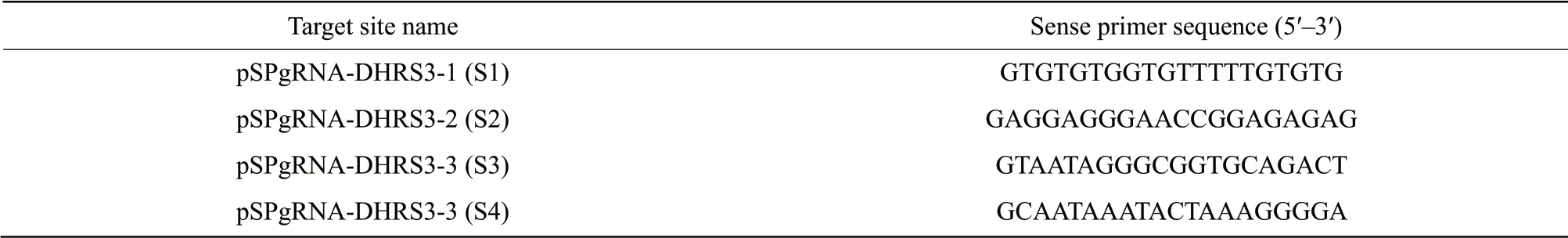
Table 1 Four sgRNAs sites specific to DHRS3 gene region
Western blot analysis
Proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Watford, UK) for western blot analysis.The membrane was first incubated with the primary antibodies against DHRS3 (1:500), MyoG (1:500) and GAPDH (1:2 000) and then with a goat anti-rabbit immunoglobulin G secondary antibody.Proteins were visualised using the Super ECL Plus Detection Kit (Applygen Technologies, Beijing, China).The film was exposed in a Mini-Chemiluminescence Imaging and Analysis System (MiniChemi 500; Sage Creation Science, Beijing, China) to obtain an image.
Immunofluorescence analysis
Previously, C2C12 cells were fixed in cold methanol for 20 min and placed in a −20℃ refrigerator.Next, cells were blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS)-Tween (PBST) for 2 h at 37℃ and incubated with a specific anti-desmin antibody (1:50; Santa Cruz Biotechnology) with 5% BSA in PBST and an anti-DHRS3 antibody (1:50), under agitation overnight at 4℃.The nuclei were stained with 4, 6-diamino-2-phenylindole (DAPI) for 3 min, the cells were washed three times with PBST, mounted with an anti-fluorescence quencher, and visualised by fluorescence microscopy (Olympus, Beijing, China).The rate of myoblast fusion was assessed by counting the number of nuclei (>2) in the differentiated myotubes as a percentage of the total number of nuclei.
Construction of muscle injury repair model
Male ICR mice were purchased from the Changchun Yisi Experimental Animal Technology Company (Jilin, China).Mice were used for the muscle injury experiment.To establish a mouse model of muscle injury, 50 μL of 0.5% bupivacaine hydrochloride (Fluka, Shanghai, China) was injected into one tibialis anterior muscle, and the same amount of normal saline was injected into the other tibialis anterior muscle to serve as a control.The samples were collected on 0 day, 1, 3, 5, 7 and 14 days, and each sample was divided into two parts, one for western blot analysis and the other for haematoxylin and eosin (HE) staining and immunohistochemistry.All the procedures carried out in studies involving animals were in line with the approval of the Experimental Animal Ethics Committee of Northeast Agricultural University in Harbin, China.The number of the approval document was neauec20190101.
Statistical analysis
Data were presented as means ± standard deviations.The statistical significance of differences was assessed using a Student′st-test.Differences were considered statistically significant with ap<0.05.All the statistical tests were performed using Prism software (GraphPad Software Inc., La Jolla, CA, USA).Each experiment was repeated at least three times.
Results
Expression of DHRS3 during differentiation of mouse C2C12 cells
Immunofluorescence results showed that DHRS3 expression was significantly increased in differentiated myotubes as the number of days of C2C12 cell differentiation increased (Fig.1A).Western blot analysis showed that DHRS3 expression was relatively low in the early stages of differentiation.From the third to the seventh day of differentiation, protein expression increased significantly and MyoG showed similar expression changes (Fig.1B–D).The results indicated that the expression level of DHRS3 increased linearly with the number of days of differentiation of C2C12 cells.
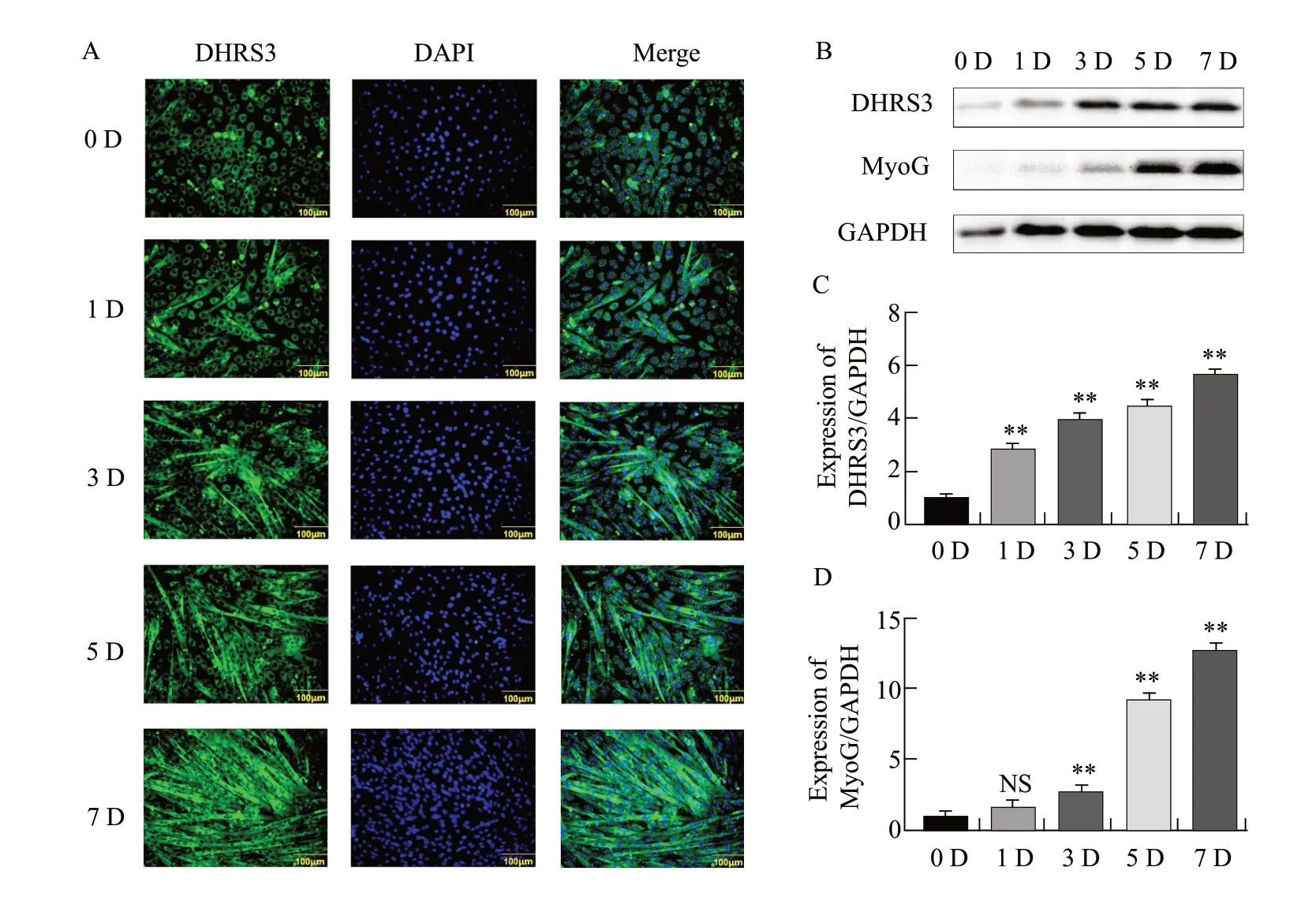
Fig.1 Expression of DHRS3 during differentiation of mouse C2C12 cellsA, Immunofluorescence staining is performed to detect expression of DHRS3.Green indicates distribution of DHRS3 protein and blue indicates presence of nuclei.Scale bar, 100 μm.B, Analysis of DHRS3 and myogenin (MyoG) protein expression levels by western blot.C, Quantification of relative DHRS3 protein level shown in panel B.D, Quantification of relative MyoG protein level shown in panel B.Parallel experiments are repeated three times; NS, No significant difference.**p<0.01.
Effect of activating DHRS3 protein expression on differentiation of C2C12 cells
To further investigate the effect of DHRS3 on the differentiation of C2C12 cells, four activation vectors that activated DHRS3 expressionviaCRISPR technology were constructed.Activation vector S3 with the best activation was screened by western blot.The expression of DHRS3 was the highest in the treatment group (Fig.2A, B).
Western blot analysis showed that the expression of MyoG was respectively increased by 49.50% (p=0.0006) and 51.32% (p=0.0015) when DHRS3 expression was activated relative to that with the respective controls (Fig.2C–E).The immunofluorescence results showed when the expression of DHRS3 was activated, the myotube fusion rate was also increased by 53.51% (p=0.003; Fig.2F, G).These results accordingly indicated that the activation of DHRS3 promoted the differentiation of C2C12 cells.
At the same time, DHRS3 and pcDNA3.1(+)-DHRS3 overexpression vectors were constructed.Compared to that with control pcDNA3.1(+), western blot analysis showed when DHRS3 was overexpressed, the expression level of MyoG was respectively higher by 35.50% (p=0.0086) and 51.32% (p=0.0045; Fig.2H–J).The immunofluorescence results showed when DHRS3 was overexpressed, the myotube fusion rate of the cells increased by 67.40% (p=0.0023; Fig.2K, L).These results indicated that the overexpression of DHRS3 could activate C2C12 differentiation.
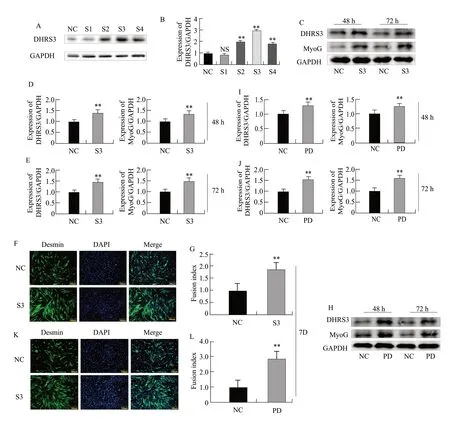
Fig.2 Effect of activation of DHRS3 protein expression on differentiation of C2C12 cells A, Expression of DHRS3 protein is detected after co-transfection of dCas9-VPR and pSPgRNA-S (1-4).NC represents cells transfected with pSPgRNA, and recombinant inhibitory vectors are represented by S1, S2, S3 and S4.B, Grayscale scan of DHRS3 protein in panel A.C, Expression of DHRS3 and MyoG protein is detected after co-transfection of pSPgRNA-S3 vector and dCas9-VPR into C2C12 cells and induction of differentiation for 48 and 72 h.D, Grey-scale scanning of DHRS3 and MyoG proteins at 48 h after cell differentiation in panel C.E, Grey-scale scanning of DHRS3 and MyoG proteins at 72 h after cell differentiation in panel C.F, Expression of Desmin is detected by immunofluorescence on seventh day of cell differentiation.G, Quantitative analysis of myotube fusion index based on panel F.H, Expression of DHRS3 and MyoG protein is detected after cotransfection of pCDNA3.1(+)-DHRS3 and pCDNA3.1(+) and induction of differentiation at 48 and 72 h.pCDNA3.1(+)-DHRS3 is represented by PD.pCDNA3.1 (+) (NC) is used as a control.I, Grey-scale scanning of DHRS3 and MyoG proteins at 48 h after cell differentiation in panel H.J, Greyscale scanning of DHRS3 and MyoG proteins at 72 h after cell differentiation in panel H.K, Expression of Desmin is detected by immunofluorescence on the seventh day of cell differentiation.L, Quantitative analysis of myotube fusion index based on panel K.Parallel experiments are repeated three times.NS, No significant difference.**p<0.01.
Effect of inhibiting DHRS3 protein expression on differentiation of C2C12 cells
To explore the effect of DHRS3 on the differentiation of C2C12 cells, four inhibitory vectors that inhibited the expression of DHRS3viaCRISPR technology were constructed.The best inhibitory vector S3 was screened by western blot.The expression of DHRS3 was the lowest in the treatment group (Fig.3A, B).
Western blot analysis showed that the expression level of MyoG was respectively decreased by 42.72% (p=0.006) and 51.30% (p=0.0019), when DHRS3 expression was inhibited relative to that with the respective controls (Fig.3C–E).Immunofluorescence results showed that with the inhibition of DHRS3 expression, myotube fusion rate was significantly reduced by 56.20% (p=0.0045) relative to that with the controls (Fig.3F, G).These results indicated that the inhibition of DHRS3 could suppress the differentiation of C2C12 cells.
SiRNAs that interfered with DHRS3 expression were also synthesized.siDHRS3-942 interference vector (si-D2) with the best interference effect was screened by western blot.The expression of DHRS3 was the lowest in all the treatment groups (Fig.3H, I).
Western blot analysis showed that the expression of DHRS3 was disturbed relative to that with the respective control, and the expression level of MyoG was also significantly decreased by 63.85% (p=0.0001) and 62.42% (p=0.0035; Fig.3J–L).Immunofluorescence results showed that when the expression of DHRS3 was disturbed, the rate of myotube fusion was also significantly reduced by 67.42% (p=0.0009) relative to that with the controls (Fig.3M, N).These results indicated that interfering with the expression of DHRS3 could inhibit the differentiation of C2C12 cells.

Fig.3 Effect of inhibiting DHRS3 protein expression on differentiation of C2C12 cells A, Screening of inhibitory fragments.NC represents transfection with pSPgRNA, and recombinant repressive vectors are represented by S1, S2, S3 and S4.B, Grey-scale scanning of DHRS3 protein in panel A.C, Expression of DHRS3 and MyoG protein is detected after co-transfection of pSPgRNA-S3 vector and dCas9 into C2C12 cells and induction of C2C12 cell differentiation at 48 and 72 h.D, Grey-scale scanning of DHRS3 and MyoG proteins at 48 h after cell differentiation in panel C.E, Grey-scale scanning of DHRS3 and MyoG proteins at 72 h after cell differentiation in panel C.F, Expression of Desmin is detected by immunofluorescence on the seventh day of cell differentiation.PSPgRNA (NC) is transfected as a control.G, Quantitative analysis of myotube fusion index based on panel F.H, Screening of interference fragments.siRNA-DHRS3-475: si-D1, siRNA-DHRS3-942: si-D2, NC, Control.I, Grey-scale scan of DHRS3 protein in panel H.J, Si-D2 is transfected into C2C12 cells, which are induced to differentiate at 48 h and 72 h to detect expression of DHRS3 and MyoG proteins.K, Grey-scale scanning of DHRS3 and MyoG proteins at 48 h after cell differentiation in panel J.L, Grey-scale scanning of DHRS3 and MyoG proteins at 72 h after cell differentiation in panel J.M, Expression of Desmin is detected by immunofluorescence on the seventh day of cell differentiation.N, Quantitative analysis of myotube fusion index based on panel M.Parallel experiments are repeated three times.* p<0.05; **p<0.01.
DHRS3 involved in mouse skeletal muscle regeneration
A muscle injury mouse model was next constructed through bupivacaine hydrochloride injection.HE staining was used to assess whether the model was successfully constructed.The results of HE staining showed that the structure of muscle cells on day 0 was clear and dense.The small amount of skeletal muscle fibres began to dissolve and break on day 1.On day 3, many skeletal muscle fibres were dissolved and the cells became dispersed.Different degrees of cell activation and proliferation were observed on day 5.Cell proliferation on day 7 was more obvious than that on day 5.Further, the newly added cells on day 14 gradually merged with the original skeletal muscles; the muscle cells were clear in shape and there was no significant difference relative to that on day 0 (Fig.4A).
Pax7 played an important role in muscle regeneration.When muscle was injured, activated satellite cells expressed Pax7.Then, when the satellite cells were committed to muscle differentiation, the expression of Pax7 was down-regulated, the expression of MyoG was down-regulated, and eventually myoblasts were formed.To further explore the relationship between DHRS3 and muscle regeneration after injury, the tissue sections were subjected to western blot analysis to detect the expression of DHRS3, MyoG and Pax7 during muscle regeneration.The expression of Pax7 was low on day 0 after injury but increased significantly on days 1 and 3.The expression of MyoG and DHRS3 was very low on day 0 after injury and gradually increased, reaching the maximum on day 5 and subsequently decreasing to basal levels on day 14 (Fig.4B–E).
To further elucidate the role of DHRS3 in the repair process, co-immunostaining of laminin was performed to observe the basal layer of muscle fibres.Immunostaining for laminin showed weak signals and clearer contours on day 0.On day 3, which showed the mode of maximum fracture, and faintly visible contours and strong signals were observed on day 7.This phenotype returned to its initial state on day 14 (Fig.4F).Surprisingly, the number of DHRS3-positive cells on day 3 was significantly higher than that on day 0 (Fig.4G).These results showed that DHRS3 was highly expressed during muscle regeneration after injury, indicating that it played a role in skeletal muscle regeneration in mice.
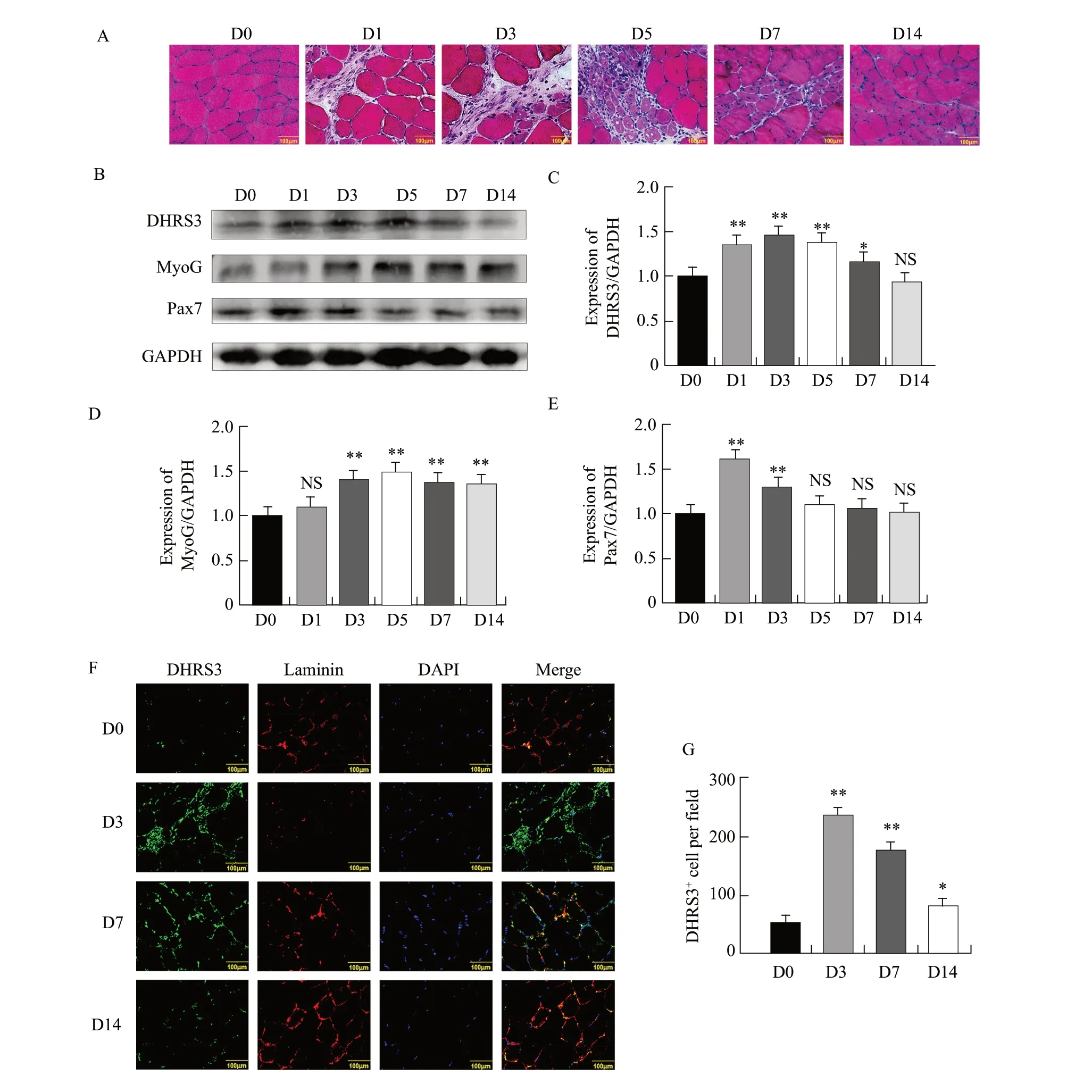
Fig.4 Expression of DHRS3 during muscle injury and repair in miceA, To induce muscle injury, 50 μL of 0.5% bupivacaine hydrochloride is injected into one of tibialis anterior muscles of mice, and the other is injected with the same amount of normal saline as a control.Mice are euthanized by injection on 0, 1, 3, 5, 7 and 14 days.Muscle injury is detected by haematoxylin and eosin staining.B, Mouse tibialis anterior muscle tissues are homogenised, and levels of DHRS3, MyoG and Pax7 are evaluated by western blot analysis.C, Grey-scale scan of DHRS3 proteins during cell differentiation in panel C.D, Grey-scale scan of MyoG proteins during cell differentiation in panel C.E, Grey-scale scan of Pax7 proteins during cell differentiation in panel C.F, Sections of tibialis anterior muscle from mice on days 3, 7 and 14 in A are immunostained for DHRS3 (green) and laminin (red).G, Quantification of DHRS3+ muscle fibres.Parallel experiments are repeated three times.*p<0.05; **p<0.01.
Discussion
The molecular mechanisms of skeletal muscle cell differentiation had always been an important part of life science researches (Osanaet al., 2020).DHRS3 was a short-chain alcohol dehydrogenase/reductase with key enzymatic functions in the regulation of the metabolism of retinol (ROL).Dhrs3a regulated the biosynthesis of retinoic acid through a feedback inhibition mechanism (Billingset al., 2013).Evidence supported the important role of DHRS3 in muscle physiology.DHRS3 was affected by the regulation of microRNA-223, which in turn affected osteoblast differentiation (Zhanget al., 2018).However, in the present study, the evidence indicated that DHRS3 might be involved in C2C12 myoblast differentiation and mouse skeletal muscle regeneration.Western blot showed that the expression of DHRS3 protein increased gradually with the number of days of differentiation.Its expression pattern was similar to MyoG, suggesting that DHRS3 might play a role in the differentiation of C2C12 cells.
In order to further verify the role of DHRS3 in cell differentiation, an overexpression vector was constructed and siRNA against DHRS3 was synthesized.Desmin was a protein that was specifically expressed in the sarcomere of skeletal muscle; therefore, it could be used as a marker for C2C12 myoblast differentiation.Myotube fusion rate was an important indicator of the degree of muscle cell differentiation (Amengualet al., 2018).Immunofluorescence results showed that the expression of Desmin increased significantly when DHRS3 was overexpressed, and the level of C2C12 myotube fusion increased.Interfering with DHRS3 expression Desmin expression decreased, myotubes atrophy, myotube number and fusion rate decreased.Western blot was used to detect the expression of MyoG, an important myoblast regulatory factor in muscle cell differentiation.When DHRS3 expression was modified, MyoG showed a similar expression pattern.When DHRS3 was overexpressed, MyoG expression was significantly increased; when DHRS3 expression was interfered, MyoG expression was significantly decreased.These results indicated that the expression of DHRS3 affected the expression of differentiation marker genes, which in turn provided favourable evidence for DHRS3 to affect the differentiation of C2C12 cells.
The skeletal muscle was a very complex organ that played an important role in injury, disease and regeneration (Mikiet al., 2019).Therefore, a muscle injury model was constructed through bupivacaine hydrochloride injection to explore the role of DHRS3 in skeletal muscle cell differentiation (Liet al., 2019).Pax7 was required to maintain satellite cell survival and played a role in self-renewal by inhibiting MyoD and causing cells to exit the cell cycle (Duester, 2013).In previous studies, PAX7 and MYOG were often used as markers of muscle regeneration (Cuiet al., 2018).In the present study, a small amount of skeletal muscle fibers began to dissolve on day 1 of injury.On day 3 of injury, a large amount of skeletal muscle fibers was dissolved, and the structure was unclear.These phenomena reflected muscle damage.On Day 5 of injury, different degrees of cell activation and proliferation were observed.On day 7 of injury, cell proliferation was more pronounced.On day 14 of injury, the newly added cells showed a stable fusion with the original skeletal muscle.Like other SDR family members, DHRS3 was a membrane-bound enzyme.In the current study, immunofluorescent staining of DHRS3 was consistent with this description, because the protein was mainly localized on the cell membrane of C2C12 cells.Western blot analysis showed an increased expression of Pax7 in injured muscle, suggesting that activation of skeletal muscle satellite cells induced muscle turnover.The expression level of MyoG gradually increased with the damage repair process to reach a peak on day 5.DHRS3 had the highest expression on day 5.It was worth noting that DHRS3 and MyoG showed similar expression patterns.The above results indicated that DHRS3 was largely involved in the development of mouse skeletal muscle.
Conclusions
In summary, the results indicated that DHRS3 was a new regulator of C2C12 myoblast differentiation and played a role in the regeneration process of mouse skeletal muscle injury, which would provide a new method for repairing human skeletal muscle injury.Future studies will further elucidate the signalling pathways and molecular mechanisms that mediate DHRS3 during C2C12 cell differentiation.
 Journal of Northeast Agricultural University(English Edition)2021年3期
Journal of Northeast Agricultural University(English Edition)2021年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Physiological Response of Spring Soybean Seedlings Under Hightemperature Stress
- Effects of Plant Protein Hydrolysate on Emergence and Growth of Rice Seedlings
- Journal of Northeast Agricultural University (English Edition)
- Effects of Carbon and Nitrogen Additions on Soil Microbial Biomass Carbon and Enzyme Activities Under Rice Straw Returning
- Effects of Lanthanum and Cerium on Seeds Fatty Acid Composition in Soybean
- Reversal Effects of Ivermectin and Moxidectin on Multidrug Resistance in C6/adr Cells in vitro
