Sexual dimorphism in the number of microglial cells isolated from neonate mice
Maryam Khajeh-Mobarakeh and Farshad Homayouni Moghadam
Abstract—Background: Microglia are brain resident macrophages originating from mesodermal/mesenchymal cells that migrate into the CNS as early as embryonic day 9 in mice (E9) prior to the emergence of neurons and other glia. They have variety of neuro-developmental and homeostatic functions during early embryonic central nervous system development and later in the adult brain. They may be responsible for mediating some gender-related structural and functional differences in the brain. Accordingly, identifying the microglia content and amount of migrated microglia in embryonic stage would be helpful to determine the function of microglia in induction of brain gender-related differences. Because of its complexity to determine the exact number of microglia in embryo, and since most studies harvest microglia form neonate mice, but it is not completely determined yet if there is any difference in the content of microglia in male and female neonates. Methods: Brains were extracted from male and female neonates to prepare mixed glia, then microglia were extracted from cultured mixed glia using shaking incubator. Isolated microglia were counted, cultured and gone under microscopic and flow cytometry assays. Results: The number of microglia harvested from male and female pubs is different and presumably male pubs have more microglia ab initio. Morphological assessments on presence of amoeboid, rod like and ramified types showed that both genders have similar ratios. Conclusion: Presence of a greater number of ab initio microglia in male neonates could indicate that gender-difference in the number of microglia may play a role in gender-dependent brain development from infancy.
Key words—Microglia, Gender, Brain, Sex, Neonatal, Mixed glia
INTRODUCTION
Microglia cells are resident immune cells of the brain [1]. Recent studies have shown that microglia originate from primitive yolk-sac-derived microglial progenitors (not hematopoietic), and respond to the pathological changes in the CNS such as inflammation, ischemia, trauma and neuronal defects [2]. Microglia have different morphological types: (i) rod like, (ii) amoeboid, and (iii) branched (resting microglia) [3]. In recent years, special attention has been projected toward studying the gender dimorphism in microglia function in healthy and diseased states [4]. According to these studies, there are gender differences in many processes of the brain and some part of them were attributed to microglia function [5, 6]. The number and morphology of microglia cells and expression of some of the immune-responsive genes depends on the age, gender and even each specific region of the brain [7]. It has also been shown by stereological cell counting methods that male rats have higher number of microglia than females on the fourth day after birth, and in contrast, females in P30 (post neonatal day 30) and P60 have higher number of microglia in some particular brain regions [7]. These data suggest there may be various types of microglia during functional development period: (i) early microglia (until E14), (ii) pre-microglia (from E14 to few weeks after birth) and (iii) adult microglia [8]. It is proposed that some of these differences may be attributed to the neurodevelopmental functions of the microglia [9].
Primary microglia or immortal microglia (BV2 and HMO6) can be used to study microglia. But according to some studies immortal microglia do not behave like primary microglia, as in one case it was reported that IL 1β release and NO production in BV2 and HMO6 cells differ from murine primary microglia. However, latest findings show that primary microglia are more similar to the brain microglia in terms of their transcriptome. Some studies also reported that this is possible to generate microglia from induced pluripotent stem cells using defined differentiation induction protocols [10], but still application of primary microglia is the common choice for most of the studies [11].
Microglia represent 5-10% of cells in the central nervous system and they can be isolated from brain tissue using different methods [12] and the most routine method is purification of microglia from cultured mixed glia with shaking [11].
To evaluate the gender-related differences in microglia function it is important to evaluate the yield and proliferation rate of male and female microglial cells in the time of isolation and after their expansion in culture. Also, it is important to know that at different stages of brain development, how many microglia are there in the male and female brains? And whether the process of microglia migration, colonization, and proliferation in the male and female brains follows a similar or different timing schedule? In present study, we explored the differences in cell number and morphological phenotypes of microglial cells harvested from male and female postnatal day 3 (P3) mice.
MATERIALS AND METHODS
Animal
Male and female postnatal day 3 (P3) C57BL6 mice (Male, n = 15, Female, n = 15) were used for this study. Animals were kept in the light/dark cycle (12/12 h), neonates were kept in cages with their mothers and had full access to breastfeeding, and their mothers had easy access to water and food. Care and maintenance of mice was carried out in accordance with the regulations approved by the ethics committee of the Royan Institute. Sex determination in neonates was performed by determining the anogenital distance (AGD), based on the fact that the AGD of male neonate is approximately twice longer than female neonate [13].
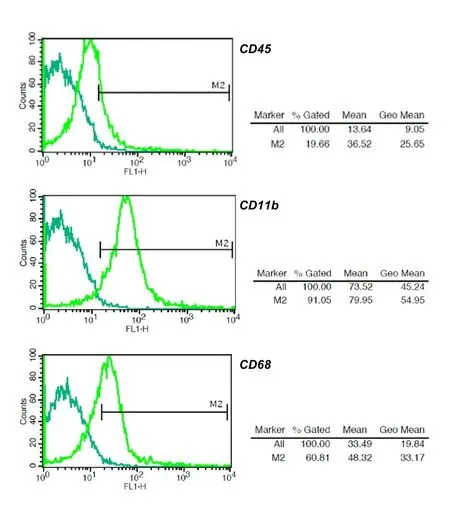
Fig.1 Flow Cytometry assay results for microglia stained against CD45, CD11b and CD68 markers, 20% of cells expressed CD45 marker, 60% CD68 marker and 91% CD11b marker.
Brain extraction and culture of primary mixed glia
All of the isolation, culture and counts of microglial cells were done on 15 male neonates and 15 female neonates. All of the isolation and culture conditions as well as culture media were same for both males and females. For this purpose, under sterile conditions each neonate brain was excluded from the head, and after washing with PBS containing 2% penicillin/streptomycin, the brain was sliced by surgical blade and slices were incubated with trypsin enzyme (0.05%) for 20 minutes, then trypsin enzyme was neutralized using culture medium (indicted in below) and afterwards the medium containing cells (mixed glia) were passed through the nylon mesh (70 micron) to separate the derbies. Next, mixed glia were collected by centrifugation and transferred to T75 flasks in the culture medium consisted of: Dulbecco's Modified Eagle's medium (DMEM, 32500-0350) + 10% fetal bovine serum (FBS, 10270) + 1% Penicillin/Streptomycin (15070), and incubated in cell culture incubator. All culture media and reagents provided from Thermo Fisher Scientific, USA.
Microglia isolation
Culture of mixed glia continued until cells reached to 90% confluency. For isolation of microglial cells from mixed glia, their culture medium was replaced with fresh one and flasks were placed for one hour in the shaking incubator (37 °C and 150 rpm) to mechanically detach microglia from underlying cells. The supernatant containing microglia cells were transferred into the centrifuge tube and were collected by centrifugation and after counting they were cultivated in 6-well plate coated with Poly-L-Lysine. The culture plates were coated with 0.01% Poly-L-Lysine (Sigma-Aldrich P4707) overnight at 37 °C. Then they were washed with sterile water three times and dried before plating the cells.
Cell counts were performed using hemocytometer slide and every count repeated for 3 times for cells extracted from each animal/flask. To determine the percent of rodlike, amoeboid and ramified microglia, 10 images were captured from each well of 6-well culture plate and their phenotypes were counted using Image j software according to their morphology.
Characterization of Microglial cells using flow cytometry technique
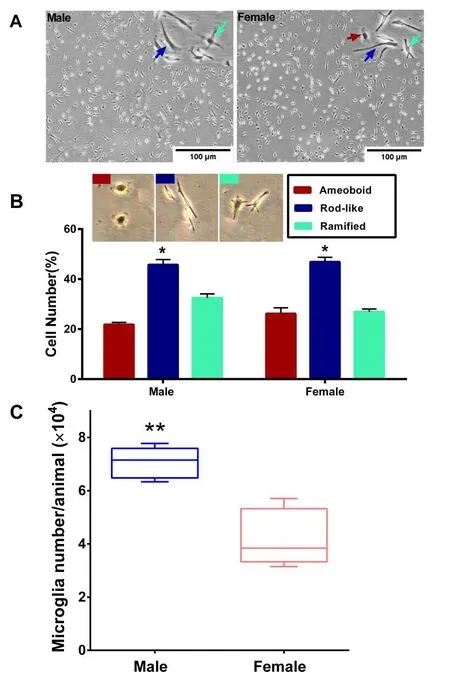
Fig.2 A) Primary microglial cells from male and female P3 mice brain in culture. B) Percentage of each type of cultured microglia based on their morphology (amoeboid, rod-like and ramified). C) Number of isolated primary microglia cells from male and female mice (*: P < 0.05, **: P < 0.01).
Cells were fixed using 4% paraformaldehyde (PFA)-PBS buffer for 20 minutes, then washed and incubated in permeabilization buffer (0.3% Triton™ X-100 in PBS) for 10 minutes, after washing, they were stained against CD11b, CD45 and intra-cellular marker CD68 using routine immunostaining methods. Antibodies were anti-CD11b (Abcam, ab133357), anti-CD45 antibody (Abcam, ab40763), and anti-CD68 antibody (Abcam, ab125047). Then cells were washed for three times and incubated in secondary antibody dilution buffer (0.5% bovine serum albumin (BSA)-PBS) containing goat anti-rabbit IgG (H&L, FITC, Abcam, ab6717), after three times wash, flow cytometry assay was performed.
RESULTS
Flow cytometry assay
Results of flow cytometry assay confirmed that isolated cells were microglia. According to the results, among the isolated cells, the CD45 marker was expressed in about 20% of cells, CD68 marker was expressed in 60% of the cells and CD11b markers in 90% of the cells which is the specific marker expression profile for microglial cells (Figure 1).
Microglial cell counts in male and female neonates
Results of cell counting showed that (Figure 2C) the men number of isolated microglia from male neonates were significantly higher than that in female ones (P< 0.01). This cell counts were performed in similar conditions and using similar procedures for both genders and were done before their culture in 6-well plates.
Gender-related differences in microglia phenotypes
After culture, microglia revealed variety of morphologies, including: Ramified (cells that had several branches around them), rod-like, and amoeboid (cells that have an almost round appearance and no branches). In both male and female mice, the number of rod-like microglia were significantly higher than ramified and amoeboid types (P< 0.05) (Figure 2B). The mean percentage of ramified cells was higher in male while in female the mean numbers of amoeboid cells were slightly higher, but both of them were not significantly different.
DISCUSSION
These days this is an accepted theory that females and males have structural and functional differences in their central nervous systems (CNS) [14]. And in the case of sex differences of microglia content in male and female brains it is currently unclear if more microglial progenitor stem cells may be enrolled into the male brain during early development (E8 to E9) or it is due to the more microglial expansion or less microglial apoptosis in male rodents (Han et al., 2021). The existing data indicates that the number and phenotype of microglia differ between genders in a region- and age-specific manner. The amygdala, cortex, hippocampus, and preoptic area are among the regions in which obvious gender-related differences of microglial numbers have been noted (Han et al., 2021). There are no significant differences in brain region-specific volume and microglial numbers between male and female rats at early embryonic day 17 (E17) [15]. Therefore, most of the changes in microglia number, type and function probably occurs near to the birth and later.
In this study, after extracting microglia cells from mixed-glia, their number and morphological types were evaluated. All of the isolation and culture experiments were repeated for fifteen times on each gender and the culture media and conditions were all the same for males and females. As all of the isolation and culture conditions were same in both sexes it can be concluded that these parameters could affect microglial cells in the same way.
Results of cell morphology assay revealed that male and female microglia show similar pattern and distribution of morphological types and among them in both sexes the rate of rod-like morphology was the highest one. Our findings showed that there is not significant gender-related difference in morphological types of microglial cells. However, even though it was not significant but the mean percent of amoeboid microglia were higher in female neonates, and ramified ones were higher in the male neonates. In accordance with our findings, it has been reported that the number of phagocytic microglia in threeday-old female neonates was higher than that in male ones [16]. During normal brain development, microglia undergo gender-specific changes over time, it has been reported by some studies that testosterone and estrogen can shift microglia phenotype toward amoeboid type in distinct brain regions such as preoptic area (POA), indicating that sex hormones can affect the function and number of male and female microglia [17].
In the case of number of microglial cells our findings showed that the number of primary microglia that can be harvested from P3 neonate male mixed glia was significantly higher than that from female ones. We used in vitro cell culture assays but interestingly in vivo studies already have reported same findings that male neonate P4 mice brain has more microglial cells compared to the female brain [17]. Schwarz et al. in their study by using the optical fractionator method have shown that the number of Iba1 labeled microglia were higher in the brain sections of male P4 mice neonates compared to females [15]. Since microglia have receptors for steroid hormones, and also a testosterone surge has been noted in male rodents around the time of birth [18], therefore this testosterone surge can affect the number and function of microglia in P3 male mice while in female mice this can be started later in life.
Present study shows that, at the time of isolation of microglia from mixed glia of P3 neonate mice, there is a significant difference between males and females in terms of the number of isolated microglia, which could be due to the presence of higher levels of testosterone in the brain of male neonates. Examining these differences and their causes can be important as a useful way to study microglial gender-related influences on male and female brain development and function in initial days after the birth and later.
ACKNOWLEDGMENT
The authors did not receive any funding for this study.
Ethical statements:The project was approved by the ethics committee of the Royan Institute.
Competing interests:The authors declare that they have no conflict of interest.
Citation:Khajeh-Mobarakeh M, Moghadam FH. Sexual dimorphism in the number of microglial cells isolated from neonate mice. Precision Medicine Research. 2021;3(3):13. doi: 10.53388/PMR2021070501.
Executive editor:Na Liu, Jin-Feng Liu.
Submitted:05 July 2021,Accepted:23 August 2021,Online:26 August 2021
© 2021 By Authors. Published by TMR Publishing Group Limited. This is an open access article under the CC-BY license (http://creativecommons.org/licenses/BY/4.0/)
 Precision Medicine Research2021年3期
Precision Medicine Research2021年3期
- Precision Medicine Research的其它文章
- Vitamin D has synergistic effect on the expression levels of SIRT1 and CYP24A1 in human breast cancer
- HPV prevalent types in a cohort of sexually active Nigerian women: implications for vaccination programmes
- Mesenchymal stem cells therapy and COVID19: a narrative review
