Preparation of Mo2C by MPCVD and Its Photocatalytic Properties
HAO Jianxin,CAI Kang,FU Qiuming,WENG Jun,XIONG Liwei,ZHAO Hongyang
(Hubei Provincial Key Laboratory of Plasma Chemistry and Advanced Materials,Wuhan Institute of Technology,No.206 Guanggu 1stRoad,Wuhan 430205,China)
Abstract:Mo2C was prepared by microwave plasma chemical vapor deposition(MPCVD)technique with the power of 800 W and pressure of 18 kPa.Compared with traditional preparation methods,MPCVD has faster growth rate and higher purity of the products.The influence of growth time on the morphology and structure of Mo2C was characterized by X-ray diffraction and Scanning Electron Microscopy.The photocatalytic performance of Mo2C was tested.It was found that Mo2C had good photocatalytic performance and the 6 h sample had the highest photodegradation rate,indicating the great potential of Mo2C as photocatalyst.
Keywords:microwave plasma chemical vapor deposition(MPCVD);molybdenum carbide(Mo2C);photocatalysis
0 Introduction
In recent years,the transition metal carbides(TMCs)have attracted much attention in the field of catalysis[1-4].TMCs are a class of metal compounds produced by inserting carbon atoms into the lattice gaps of transition metal elements.Based on their special structure,they are covalent solids,ionic crystals and transition metals at the same time,thus special physical and chemical properties could be found[5-8].At present,precious metals and their compounds were mostly used as promoters on photocatalytic production[9-11].However,precious metals are expensive due to their limited reserves,which greatly increases the industrial production cost and restricts permanent production[12].As a new type of material,molybdenum carbides show high melting point,high hardness,good mechanical stability,thermal stability and corrosion resistance[13-14].Moreover,because the introduction of C atoms changed the electron-carrying properties ofdordit of Mo,it became a kind of potential substitute material for noble metal catalyst[15].Because of the low price of Mo2C and its simple synthetic route,it is used as a substitute for precious photocatalyst.
At present,the main preparation methods of Mo2C include thermal decomposition[16],chemical vapor deposition (CVD)[14,17]temperature programmed reaction(TPR)[18-19]and mechanical alloying.However,the specific surface area of synthesized Mo2C by thermal decomposition method is generally small.For mechanical alloying method,its advantage is that it can be carried out at room temperature and Mo2C has a relatively high specific surface area,but it needs more energy.CVD and TPR methods are frequently used because of their relatively simple process.However,the reaction temperature is high and only high temperature Mo2C phase can be obtained[20-22].At the same time,the organic hydrocarbons contaminate in the catalyst is not easy to be removed in the subsequent hydrogenation reaction.Com-pared with the above methods,the preparation time of Microwave Plasma Chemical Vapor Deposition(MPCVD)method is shorter,and there is no aggregation of carbon and powder[23-24].We had synthesized high quality Mo2C by MPCVD[14].It is proved that MPCVD is an efficient way to synthesize Mo2C.
In this paper,Mo2C was prepared by MPCVD technique.The effects of different growth time on the composition,structure and morphology were studied by X-ray diffraction and Scanning Electron Microscopy.The degradation of Rhodamine B(RhB)was analyzed to investigate the catalytic performance of Mo2C.
1 Materials and methods
1.1 Experimental set-up
Mo2C was grown on 30 μm Mo substrate by MPCVD.All Mo substrates were cleaned by ultrasonic method with acetone,alcohol and deionized water for 30 minutes each.Then the Mo substrate was placed on the center of the substrate table and the vacuum pump was turned on to pump the chamber to 20 Pa.Hydrogen plasma was used to etch the surface oxide layer.Methane was used as carbon source and hydrogen was used as reductant.The gas flow ratio of methane to hydrogen was 1:200.Microwave power and working pressure are controlled at 800 W and 18 kPa respectively.The substrate temperature was stabilized at 950℃by adjusting the distance between the upper and lower substrate stages.The deposition time of Mo2C was 2,4,6,12 hours,respectively.
1.2 Characteristion
The phase formation was checked by X-ray diffraction(XRD)analysis at room temperature using CuKα radiation(λ=1.540 Å)in a wide range of Bragg’s anglesθ(10°<2θ< 90°)with a scanning step of 0.2°.Scanning electron microscopy(SEM)morphology of the samples was observed by a SU8010,Hitachi.In order to study the degradation rate and efficiency of thin film catalysts to pollutant solutions,the UV-vis spectrophotometer(Shanghai YK 723 N)was used to detect the absorbance of dyes.
2 Results and discussion
2.1 Structure and phase of Mo2C
Fig.1,illustrates the XRD patterns of the Mo2C samples at the growth time of 0 h ,2 h ,4 h,6 h and 12 h.The peaks in Fig.1(b),(c)and(d)are identified to be Mo2C(PDF 01-1188).The substrate peaks at 2θ=58°and 73°are indexed as Mo(PDF 42-1120).The peak intensity of Mo2C for 2 h is lower due to short reaction time and coexistence of growth and etching process.When the deposition time is 6 h,the main peak of Mo2C is higher and sharper because the amount of Mo2C increases with the increase of input content of the carbon source.When the growth time is extended to 12 h,extra peaks appear at 2θ=35.71°,48.49°and 75.91°.Compared with standard diffraction PDF 20-0748,it is confirmed that they are MoC(100),(101)and(200)peaks and diamond(111),respectively,which indicates that the best growth time is 6 h under the condition of power of 800 W and pressure of 18 kPa.
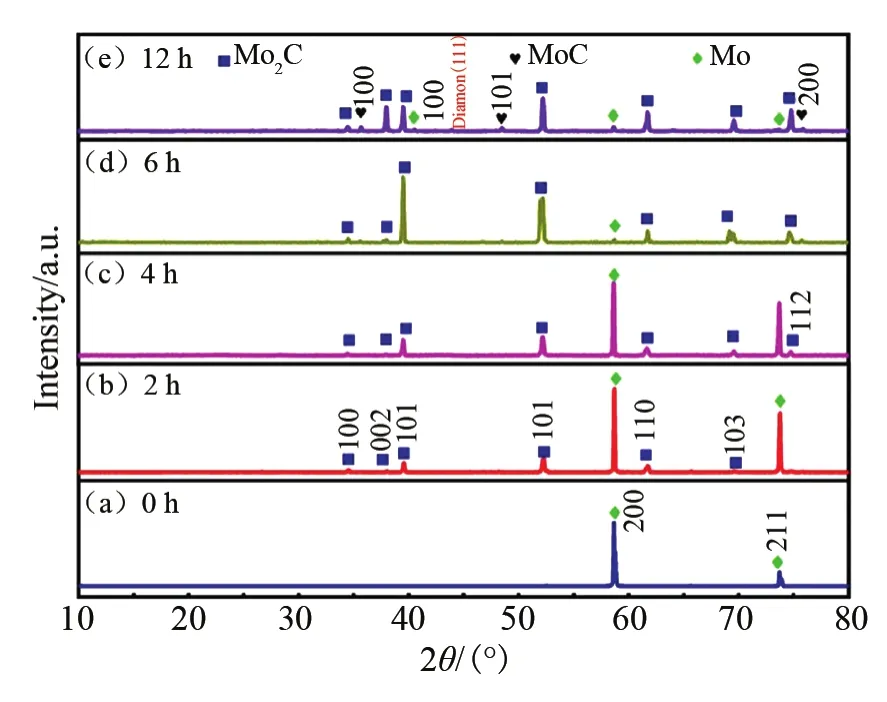
Fig.1 XRD patterns of Mo2C deposited at the same microwave power of 800 W and a pressure of 18 kPa
Fig.2 shows the SEM cross-sections of the asgrown samples.The results of XRD and SEM show that the carbon-containing groups in the gas phase are first adsorbed to the surface of the Mo substrate in the early stage of growth.When the concentration of carbon atoms adsorbed on the surface is relatively high,it will diffuse and penetrate into the Mo substrate to a certain extent,thereby combining with molybdenum atoms to form Mo2C.As the growth time increases,the ratio of molybdenum atoms and carbon atoms participating in the Mo2C growth on the substrate surface changes.In the early stage,the content of molybdenum atoms is relatively high,and with the growth of Mo2C,the propor-tion of the substrate surface that can participate in the growth of Mo2C decreases.The decrease of molybdenum atoms causes the change of composition,resulting in the MoC mixed phase and eventually even a diamond phase.The morphology of 6 h sample is most smooth which is in accordance with the XRD results.
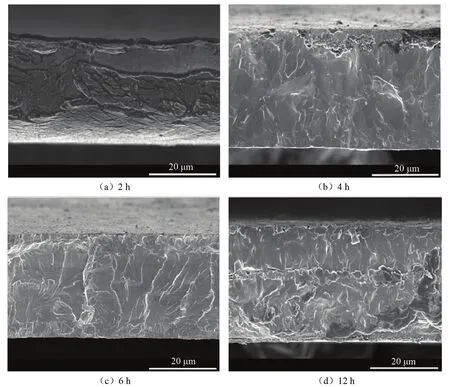
Fig.2 SEM cross-section of the three samples prepared for
2.2 Photocatalytic Activity analysis
Fig.3(a)shows the degradation curve of RhB by samples with growth time of 0,2 h,4 h,6 h and 12 h.RhB,as a conventional dye,has a stable structure and is of general significance for target degradation products.RhB will not degrade when pure molybdenum substrates are used as catalysts,which indicates that molybdenum substrates do not have photocatalytic properties.The degradation rate of RhB solution increases gradually from the samples prepared for 2 h,4 h and 6 h.After 1 h of illumination,each sample showes different degradation efficiency,among which the sample with 6 h preparation time is the best.Combining with the previous conclusions,Mo2C with 6 h preparation time has the best XRD and SEM results because of its pure phase,high intensity and smooth morphology.The degradation efficiency of RhB under the catalysis of 6 h sample is 94.2%,while the degradation efficiency of RhB under the catalysis for 2 h and 4 h samples is 65.9% and 84.2%,respectively.When the growth time is extended to 12 h,the trend of degradation curve is weakened,and the degradation efficiency of RhB is 90%,which is lower than that of 6 h sample.As mentioned above,when the growth time is longer,the ratio of carbon to molybdenum on the sample surface chan-ges.MoC and diamond phases grow on the surface of the samples.At this time,the photocatalytic performance of Mo2C will be affected to some extent.Therefore,the photocatalytic activity of the sample for 12 h is slightly lower than that of the sample for 6 h.
In order to compare the degradation rates of RhB by Mo2C under different preparation time,irradiation time and ln(C0/C)are used to study the degradation rate constants of RhB for each component.As shown in Fig.3(b),the trend of the curve shows that the degradation rate and degradation efficiency of the samples are basically the same,which indicates that Mo2C has excellent photocatalytic activity.The reaction rate constants of the samples at growth time of 2 h,4 h,6 h and 12 h are 0.0179 min-1,0.0308 min-1,0.0475 min-1and 0.0455 min-1,respectively.The rate constant of 6 h sample is 2.6 times of that of 2 h sample,which indicates that the photocatalytic activity of Mo2C changed with the composition.
Fig.3(c)shows the light absorbance of Mo2C with a deposition time of 6 h as a function of cycle time.The fatigue properties of Mo2C photocatalyst were studied by cyclic photodegradation experiments.Fig.3(d)shows that the cyclic experiments of RhB degradation for Mo2C which is deposited at 6 h,were carried out 4 times.In every cycle,94% of RhB is degraded within 60 min of sun-light irradiation,which indicates no observable loss of activity of catalyst.It still has good photocatalytic activity.
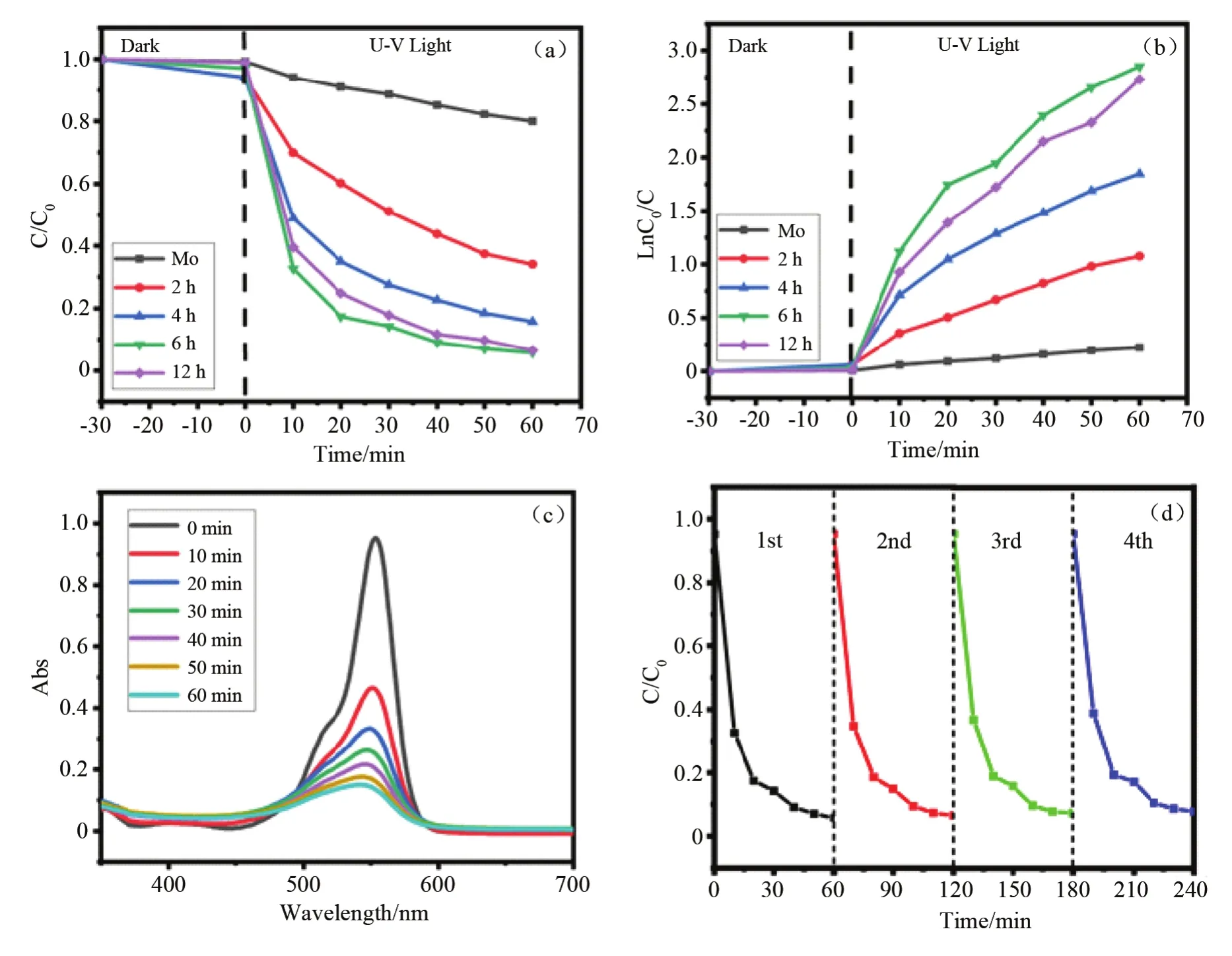
Fig.3 (a)Photocatalytic degradation of RhB with time by Mo2C in different deposition time under U-V light;(b)Plot of ln(C0/C)as a function of time by Mo2C in different deposition time under U-V light;(c)UV-vis absorption spectra of RhB as a function of irradiation time for Mo2C of 6 h;(d)Time profiles of RhB degradation for four successive cycles with Mo2C under U-V light irradiation
3 Conclusions
Mo2C was successfully prepared by MPCVD.It is proved that MPCVD is an effective way to obtain Mo2C.The prepared Mo2C was characterized by XRD and SEM.The best growth time is 6 h due to the pure phase of Mo2C and the relative smooth cross section morphology.The photocatalytic degradation of RhB by samples with different growth time showed that Mo2C had photocatalytic activity,and the degradation rate of 6 h sample was the highest.The photocatalytic activity remained 90% after four cycles of degradation.

