Antioxidant,cytotoxic,and anti-venom activity of Alstonia parvifolia Merr.Bark
Maria Carmen S.Tan ,Mary Stephanie S.Carranza ,Virgilio C.Linis ,Raymond S.Malabed,3 ,Yves Ira A.Reyes ,Francisco C.Franco,Jr. ,Glenn G.Oyong
1Chemistry Department,De La Salle University,2401 Taft Avenue,Manila 1004,Philippines
2Biology Department,De La Salle University,2401 Taft Avenue,Manila 1004,Philippines
3Department of Chemistry,Graduate School of Science,Osaka University,Osaka 563-0034,Japan
4Molecular Science Unit Laboratory,Center for Natural Sciences and Environmental Research,De la Salle University,2401 Taft Avenue,Manila 1004,Philippines
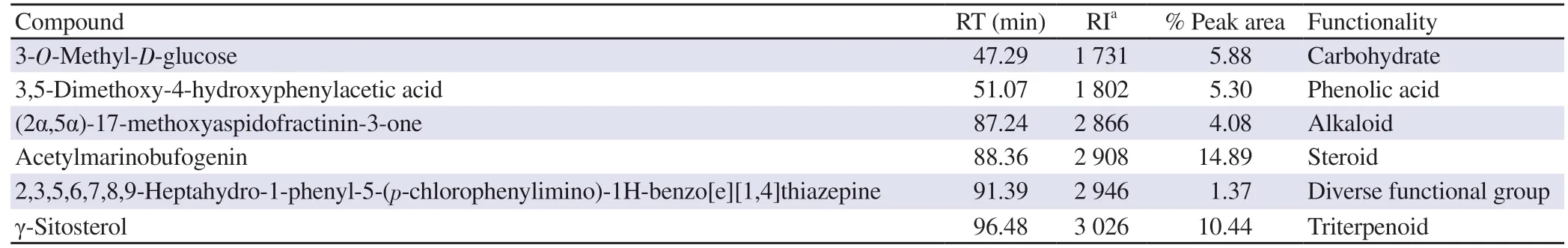
ABSTRACT Objective:To evaluate antioxidant,cytotoxic,and anti-venom capacity of crude bark extracts of Alstonia parvifolia Merr.Methods: Gas chromatography-mass spectrometry (GC-MS) and energy dispersive X-ray analyses were accomplished to characterize the chemical constituents of Alstonia parvifolia.Biochemical characterization was evaluated using an inhibitory phospholipase A2(PLA2) assay,DPPH,and cytotoxicity assays.Using the constituents listed in the GC-MS analyses,molecular docking was conducted to inspect the binding energies between the chosen compounds and selected PLA2 isoforms.Results: GC-MS analyses showed that the Alstonia parvifolia crude extract consisted predominantly of acetylmarinobufogenin (14.89%),γ-sitosterol (10.44%),3-O-methyl-D-glucose (5.88%),3,5-dimethoxy-4-hydroxyphenylacetic acid (5.30%),(2α,5α)-17-methoxyaspidofractinin-3-one (AFM) (4.08%),and 2,3,5,6,7,8,9-heptahydro-1-phenyl-5-(pchlorophenylimino)-1H-benzo[e][1,4]thiazepine (HPT) (1.37%).The principal elemental components of Alstonia parvifolia were Ca (4.012%)and K (1.496%),as exhibited by energy dispersive X-ray examination.Alstonia parvifolia showed significant free radical scavenging ability(IC50:0.287 mg/mL) and was non-cytotoxic to normal HDFn cells (IC50>100 μg/mL).Moreover,Alstonia parvifolia was favorably cytotoxic to MCF-7 (IC50:4.42 μg/mL),followed by H69PR,HT-29,and THP-1,with IC50 values of 4.94,5.07,and 6.27 μg/mL,respectively.Alstonia parvifolia also displayed notable inhibition against PLA2 activity of Naja philippinensis Taylor venom with IC50 of (15.2 ± 1.8) μg/mL.Docking and cluster analyses projected negative binding energies from AFM (−6.36 to −9.68 kcal/mol),HPT (−7.38 to −9.77 kcal/mol),and acetylmarinobufogenin (−7.22 to −9.59 kcal/mol).These calculations were for the particular interactions of Alstonia parvifolia constituents to PLA2 homologues where the utmost affinity was detected in HPT owing to the dipole interactions with amino acid residues.Conclusions:The bark extract of Alstonia parvifolia shows great potential as an anti-venom agent due to its low cytotoxic profile,remarkable PLA2 inhibition,and docking binding energies between its bioactive constituents and PLA2 homologues.
KEYWORDS:Alstonia parvifolia Merr.;Naja philippinensis Taylor;Gas chromatography-electron ionization-mass spectrometry;Secretory phospholipase A2;Cytotoxicity assay;Anti-venom
1.Introduction
In communities with underdeveloped health care systems,concoctions from recognized herbal sources such as the bark fromAlstonia
sp.have been employed as traditional medicine.Alstonia parvifolia
(A.parvifolia
) Merr.is a small tree (maximum height of 8 m) with glabrous branches and leaves in whorls of four[1].This species is endemic to the Philippines and is found in the few localities of the island of Luzon,in Negros island,and on Mount Kinabalu in the island of Borneo.A.parvifolia
occurs in scattered populations consisting of only a few trees of secondary growth in areas such as logged-over forests,rocky forested slopes,and along exposed ridges[2].Despite its close affinity to two medicinally important plants,Alstonia scholaris
(L.) R.Br.andAlstonia macrophylla
Wall.ex,A.parvifolia
has no reported or identified traditional medicinal use[3,4].From a 2019 report from the World Health Organization (WHO),nearly 5.4 million people are bitten by snakes annually.Statistics have shown that this corresponds to 2.7 million envenomed individuals,400 000 sufferers sustaining physical impairments,and 138 000 casualties[5].In 2013,WHO reinstated snake envenomation to the list of neglected tropical diseases due to the increasing number of deaths per year.The routine use of anti-venom serum or immunoglobulin (AVS) remains to be the first and foremost medical protocol for the disease;however,its use persists to be a critical health hazard for victims internationally[6].Conventionally,snake anti-venoms are produced by developing hyperimmune serum in animals (such as horses) as an agent against snake venoms.The extracted serum is then purified to isolate whole immunoglobulin G fractions.These isolates are then further fractionated and purified to attenuate unfavorable side effects and augment efficacy in envenomed patients[7].Granting that recent technology has led to the advancement of far superior commercially obtainable AVSs,undeniable scientific obstacles have yet to be overcome,such as lack of funding and government support in raising animal models,increasing the reliability of these hosts,and the accessibility/feasibility of such methods to produce the AVS.As a consequence,anti-venom research has trailed behind other researches and has been overlooked despite its life-long impact on victims and the alarming increase in deaths per year.
Particularly in the Philippines,the toxic venom of the cobra speciesNaja philippinensis
(N.philippinensis
) (Philippine Cobra)has beleaguered rice farmers from Bicol and Nueva Ecija.In these regions,the number of recorded cases of snakebites is high and is a great cause for alarm.Folk medicine has been a reliable alternative for locals in rural areas of the Philippines to treat snake envenomation.Previous studies have shown that the bark and leaves of medicinalAlstonia
species can hinder the effects of viper envenomation in animal experiments[8],which makes this plant more interesting to study as to its possible use as an anti-venom agent.In this research endeavor,we ascertained the antioxidant capacity,cytotoxic activity (HT-29,H69PR,MCF-7,THP-1 immortalized cell lines and normal HDFn primary culture cells),and anti-venom activity of crudeA.parvifolia
extracts.Chemical characterization was achieved using a gas chromatograph in tandem with a mass spectrometer and an energy dispersive X-ray (EDX) spectrometer.2.Materials and methods
2.1.Plant material
The bark ofA.parvifolia
Merr.was gathered from Mariveles,Bataan,Philippines and was authenticated by Virgilio Linis,a botanist from De La Salle University.The aforementioned plant was submitted to the De La Salle University Herbarium with a voucher specimen number:5804-17.2.2.GC-EI-MS analysis
A.parvifolia
bark (4.2 g) was pulverized and solvent-extracted with 50:50 methanol/dimethylsulfoxide (MeOH/DMSO,mass spectrometry grade) as stipulated by a previously described technique[9].The subsequent mixture was filtered using filter paper(Whatman 91) and then dried under nitrogen gas for 1 h.The crude extract afforded approximately 0.7 g and was reconstituted in 1 mL MeOH.The resultant sample was then filtered through a syringe with a 0.45 μm nylon membrane before commencing investigations.A.parvifolia
samples were studied employing an Agilent gas chromatograph-mass spectrometer (GC-MS) 7890B,an HP-5ms(5% phenyl methylsiloxane) Ultra Inert column (30 m × 250 mm× 0.25 mm),and ultra-pure helium gas as the mobile phase for the examination of vaporizable components.The constraints of helium gas were automated to the following parameters:flow rate of helium gas was at 1 mL/min,pressure fixed at 8.2 psi,with a mean velocity of 36.62 cm/s,and holdup time of 1.37 min.The splitless inlet was conserved at 250 ℃ at 8.2 psi,with an overall flow speed of 24 mL/min,and a septum purge flow speed of 3 mL/min.The injector temperature was retained at 250 ℃.The temperature gradient program began at 70 ℃ and the preset stepwise escalation of the temperature to 2 ℃/min was achieved until the maximum of 135 ℃was reached and this temperature was maintained for an additional 10 min.After the hold period,an additional temperature increment of 4 ℃/min was implemented to attain the required temperature of 220 ℃ after which this temperature was retained for 10 min.The temperature was then augmented to 270 ℃ at a rate of 3.5 ℃/min and this fixed temperature was maintained for 37 min.Compound identification was accomplished by means of the NIST library,2.0 and percent peak area average was managed from the subsequent total ion chromatograms.The consequent data was established by the assessment of the analytes according to their elution succession on a non-polar stationary phase.The retention indices were computed for the entirety of the compounds using a homologous series ofn
-alkanes.The experiments were undertaken in three replicates.2.3.EDX analysis
Dried samples ofA.parvifolia
bark were positioned in a 10 mm Mylar cup for elemental exploration using an EDX-7000.The experiments were achieved with the collimator set at 10 mm and the atmosphere was in vacuo.2.4.Free radical scavenging assay
The free radical scavenging capability ofA.parvifolia
was evaluated by an adjusted DPPH assay methodology[10].A total of 0.5 mg of ground bark biomass was mixed to 1 mL of 7.0 mL MeOH,2.95 mL HO and 0.05 mL HCl.The sample was incubated in the aforementioned solution for 3 h,and filtered (Whatman 91),and the eluents were then concentrated by drying under nitrogen gas for 2 h to yield 50 mg of crude extract.Afterwards,3.94 mg of 1,1-diphenyl-2-picrylhydrazyl (DPPH) in 50 mL of methanol was made to formulate a 0.2 mM DPPH solution.An original starting concentration of 4 mg/mL ofA.parvifolia
was made.The negative control consisted of 1 mL of DPPH solution with 1 mL of methanol.Reaction mixtures consisting of 1 mLA.parvifolia
extracts (17.5 to 0.27 mg/mL) and 1 mL of 0.2 mM DPPH solution were incubated at ambient temperature for 30 min and the absorbance of each trial was monitored at 515 nm (UV-Vis Hitachi spectrophotometer 2900).The ICvalue was deducted through linear interpolation from the regression equation.Inhibition of the DPPH free radicals in solution was established by the following equation:DPPH scavenging effect (%)=[(A–A)/A] × 100
Where Ais the absorbance of negative control and Ais the absorbance of sample.
2.5.Cell viability assay
The corresponding extraction procedure used for the GC-MS trials was also followed for the cytotoxicity experiments on the subsequent human cell lines (ATCC,Manassas,Virginia,U.S.A.):breast cancer(MCF-7),colon cancer (HT-29),small cell lung carcinoma (H69PR),human acute monocytic leukemia (THP-1);and a primary culture of normal human dermal fibroblast,neonatal (HDFn) (ThermoFisher Scientific,Gibco,USA).The immortalized cells were generously provided by the Cell and Tissue Culture Laboratory,Molecular Science Unit,Center for Natural Sciences and Environmental Research,De La Salle University.Culture and maintenance were performed in complete Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum and 1× anti-biotic,anti-mycotic (Thermo Fisher,Invitrogen,USA) and were incubated at 37 ℃ with 5%COand 95% humidity.The parameters concerning thein vitro
assays were reported in our previously published work and strictly followed in this work[11].Zeocin,an intercalating agent,was used as the positive control.Optical density (OD) was measured at 570 nm and was used to ascertain the percent cell viability following the equation: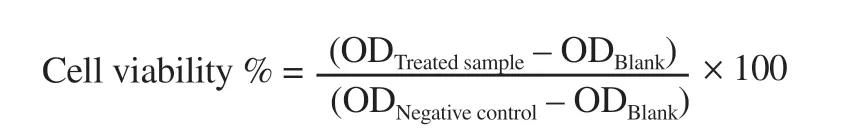
2.6.In vitro inhibitory secretory phospholipase A2 (sPLA2)activity
The anti-venom potential ofA.parvifolia
on sPLAofN.philippinensis
venom was determined through a sPLAtargeted commercially available kit (Secretory Phospholipase AAssay Kit Cat.No.ab133089) purchased from Abcam(Cambridge,United Kingdom).The reagents of the assay kit were formulated as indicated by the information sheet accompanying the kit.The protocol that was used for the experiments and resulting calculations were the same as those reported in our earlier published work[11].2.7.Molecular docking study
Autodock Vina was used for the initial screening of several protein candidates which are known to be present in theN.philippinensis
venom such as the following:phospholipases,neurotoxins,and cardiotoxins[12,13].Autodock 4.0 was used for the scrutiny of the top protein target candidates from the screening with PDB IDs:1LN8,1S6B,1PSH,and 1A3F. Lamarkian Genetic Search algorithm was used for the docking of (2α,5α)-17-methoxyaspidofractinin-3-one(AFM),2,3,5,6,7,8,9-heptahydro-1-phenyl-5-(p
-chlorophenylimino)(1H) benzo[e][1,4]thiazepine (HPT),and acetylmarinobufogenin(AMB) into the protein candidates.A grid box encompassing the entire protein structure for each target was established (blind docking).Independent 200 GA iterations with a population size of 150,2 500 000 assessments,and 27 000 maximum generations were executed for each target protein,respectively.The 200 docking conformations of the ligands,generated for each target,were scored based on the calculated binding energy and were clustered corresponding to the root mean squared deviation.2.8.Statistical analysis
Statistical assessments were performed using the software GraphPad Prism 7.01 (GraphPad Software,San Diego,CA,USA).Acquired data values were evaluated by one-way analysis of variance(ANOVA) as well as Tukey’s post-test for multiple comparisons among particular test sets.
3.Results
3.1.GC-EI-MS characterization of A.parvifolia extracts
Six constituents were identified inA.parvifolia
extracts.The identified components of the volatile compounds of the crude extract were verified by retention index and structural classvia
the NIST library generated from the resultant peaks in the chromatogram(Supplementary Figure 1).TheA.parvifolia
crude extracts were comprised of the detected constituents:one compound with diverse functional groups,HPT (1.37%);a steroid,AMB (14.89%);a triterpenoid,γ-sitosterol (10.44%);an alkaloid,AFM (4.08%);a carbohydrate,3-O
-methyl-D
-glucose (5.88%);and a phenolic acid,3,5-dimethoxy-4-hydroxyphenylacetic acid (5.30%).The compounds are itemized in Table 1 according to their elution succession through an HP-5ms column.3.2.EDX analysis of A.parvifolia extracts
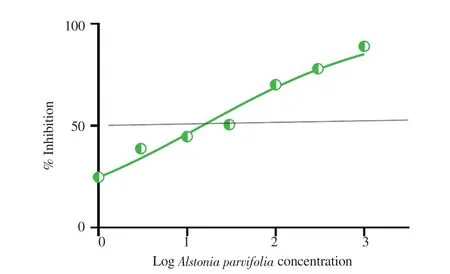
The elemental components obtained exhibited the following percentages:( A.parvifolia Figure 1. Free radical scavenging activity of MeOH/DMSO fractions of Alstonia parvifolia bark. A.parvifolia A.parvifolia A.parvifolia A.parvifolia A.parvifolia A.parvifolia A.parvifolia Napa Naja naja Figure 2. Dose-response curves showing the effect of Alstonia parvifolia,zeocin,and dimethyl sulfoxide (DMSO) on the cell viability of (A) MCF-7,(B)H69PR,(C) HT-29,(D) THP-1,and (E) HDFn.Each plot displays the effect of Alstonia parvifolia and controls against each cell line.Data are shown as mean ±SEM.GraphPad Prism 7.01 was used to perform extra sum-of-squares F-test to (A) evaluate the significance of the best-fit-parameter (half maximal inhibitory concentration) among different treatments,and to (B) determine the differences among the dose-response curve fits.MCF-7 F(Dfn,DFd)=(A) F (2,21)=28.58,P <0.001 and (B) F (2,48)=5 325,P <0.001;H69PR (A) F (2,21)=29.12,P <0.001 and (B) F (2,48)=5 707,P <0.001;HT-29 (A) F (2,21)=24.34,P <0.001 and (B) F (2,46)=9 518,P <0.001;THP-1 (A) F (2,21)=21.40,P <0.001 and (B) F (2,46)=3 697,P <0.001;HDFn (A) F (2,21)=26.79,P <0.001 and (B) F (2,48)=969.4,P <0.001. Figure 3. The inhibitory effect of Alstonia parvifolia against secretory phospholipase A2 activity. Table 1. Constituents of Alstonia parvifolia extracts. A.parvifolia Tabernaemontana catharinensis Alstonia A.parvifolia in vivo A.parvifolia Alstonia Raised concentrations of potassium (K) and calcium (Ca) indicated that the plant accrued an elevated quantity of mineral nutrient absorption from the soil.There have been reports on improved lipid blood chemistry,glucose metabolism,and blood pressure due to calcium ingestion in healthy elderly women[22,23].Assimilation of potassium-rich foods has been found to initiate regulation of blood pressure and cardiovascular anomalies,alleviate bone and renal disease,and prevent strokes[24]. A.parvifolia A.parvifolia t A.parvifolia A.parvifolia P A.parvifolia A.parvifolia A.parvifolia The anti-proliferative capacity of the extracts was linked to the high concentration of the alkaloids AFM and AMB.Though no activity has been reported with AFM,AMB was found to be cytotoxic to drug-resistant CEM/ADR5000 cells [IC:(1 090.0 ± 33.0) nM].This was reported with a subdued 1.2 degree of resistance relative to the cross-resistance of established anticancer drugs such as doxorubicin(<1 000 fold),vincristine (<400-fold),paclitaxel (<200-fold) and others[29]. A.parvifolia N.philippinensis A.parvifolia N.philippinensis N.philippinensis A.parvifolia Naja sagittifera Naja naja N.philippinensis in vivo A.parvifolia HPT was similarly docked to the four PLAhomologues.Pronounced negative binding energies were calculated for HPT relative to AFM.The presence of large and electronegative (Cl and S) atoms in the structure of HPT provided more dipole interactions with the amino acid residues.Supplementary Figure 5 depicts the best-docked poses which were oriented such that the said atoms were closer in proximity to polar hydrogen atoms of the amino acid residues.Consequently,the effect of the presence of Caions from the protein crystal structures was predicted to be less significant for the binding of HPT to PLAhomologues relative to that of AFM.Results also demonstrated that the binding energies of HPT to calcium-containing PLAhomologues were not favored.The bestdocked pose of HPT that gave the highest calculated binding energy,was with HPT to 1S6B,which incidentally was a PLAwithout Ca. Naja naja in vivo A.parvifolia A.parvifolia A.parvifolia A.parvifolia in vivo A.parvifolia in vitro N.philippinensis A.parvifolia A.parvifolia in vivo Conflict of interest statement The authors declare that there is no conflict of interest. Acknowledgments The authors deferentially recognize that the completion of this work was largely due to funding provided by the De La Salle University Science Foundation in coordination with the University Research Coordination Office (Project number:18 F U 2TAY16-3TAY17).The researchers would similarly like to acknowledge the vital assistance and provision that Professor Michio Murata of the Department of Chemistry,Graduate School of Science,Osaka University,munificently conferred to this endeavor. Funding This study is supported by the De La Salle University Science Foundation in coordination with the University Research Coordination Office (Project number:18 F U 2TAY16-3TAY17). Authors’ contributions Alstonia parvifolia3.3.Free radical scavenging activity of A.parvifolia extracts
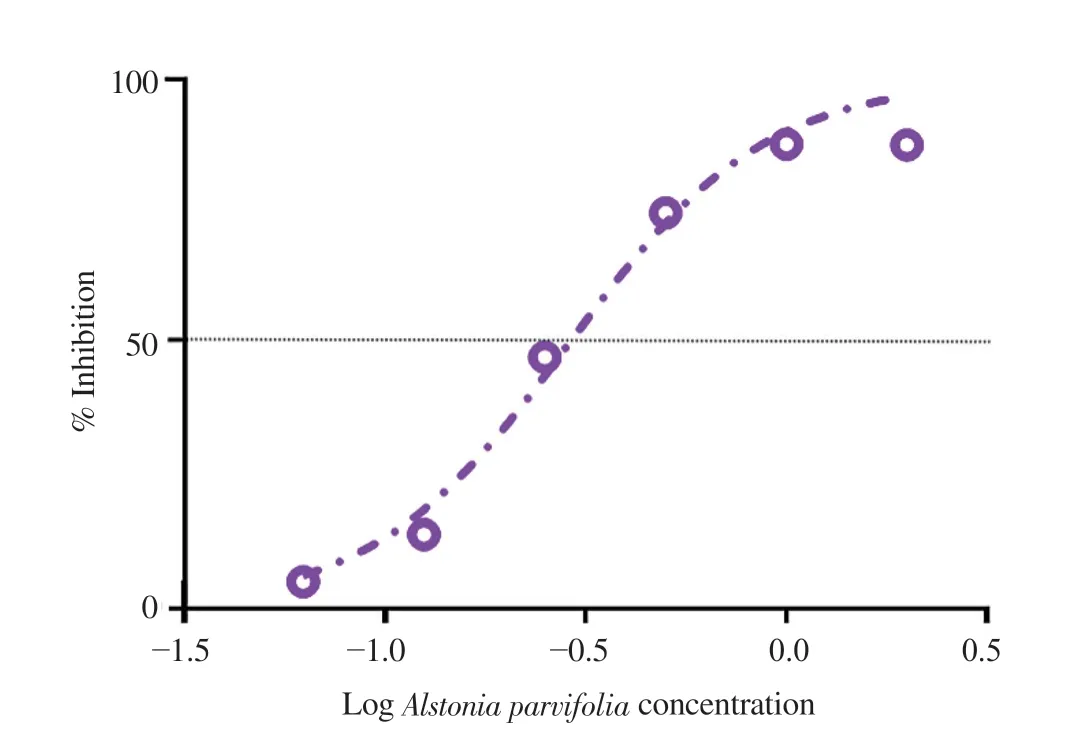
3.4.Cytotoxicity of A.parvifolia extracts
The anti-proliferative action of the crude extract of3.5. Inhibitory sPLA2 activity of A.parvifolia
The3.6.Molecular docking study
For this study,the best candidates were preliminarily screened on several PLAhomologues.Four structures:1LN8,1S6B,1PSH,and 1A3F were chosen for further docking analysis.The chosen crystal structures of PLAproteins are found in the venom of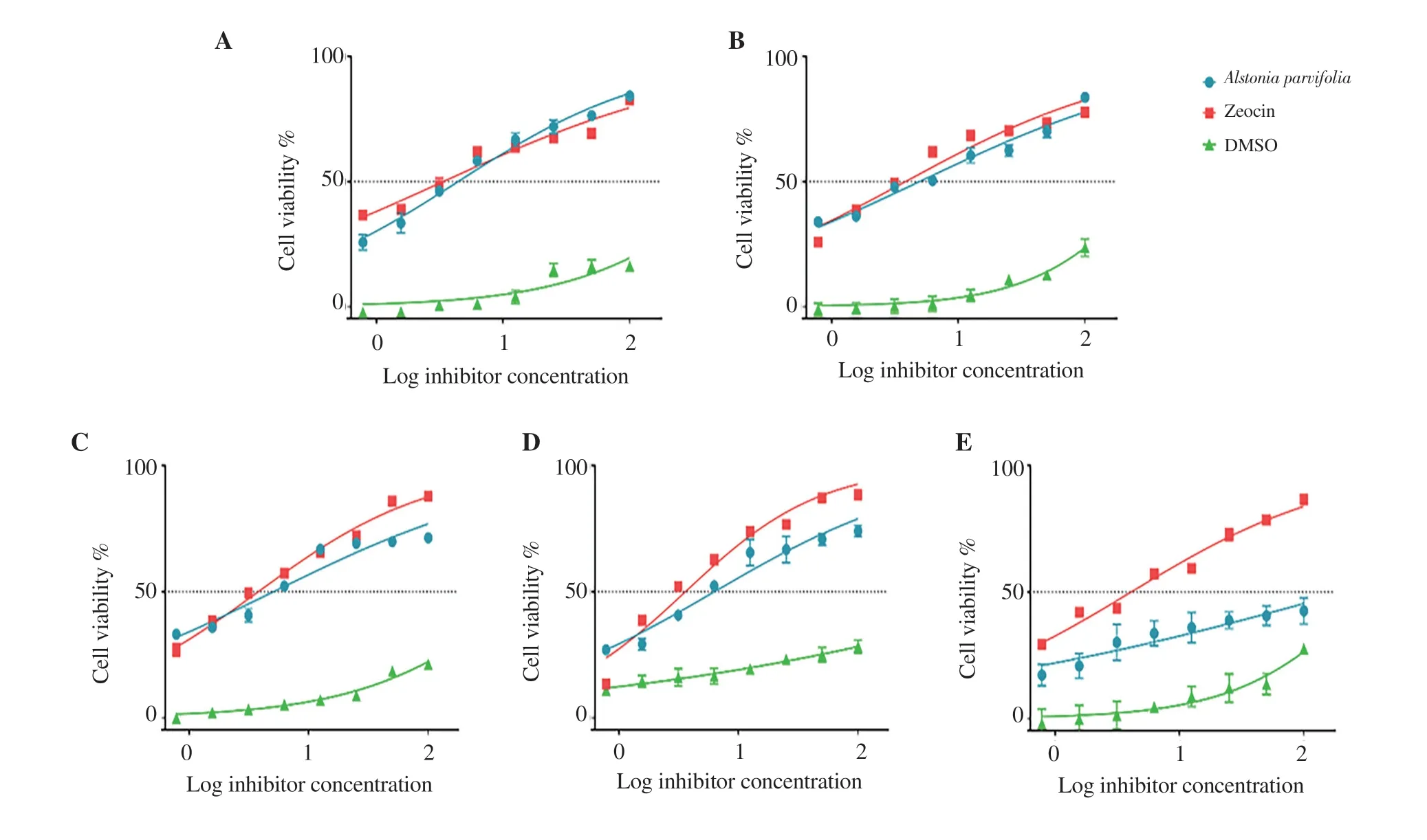
4.Discussion
Six bioactive compounds were present in the crude MeOH/DMSO bark extract of
 Asian Pacific Journal of Tropical Biomedicine2021年10期
Asian Pacific Journal of Tropical Biomedicine2021年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Ethanol extract of Chondracanthus tenellus (Harvey) Hommersand attenuates lipopolysaccharide-induced inflammatory and oxidative response by blocking the NF-κB,MAPKs,and PI3K/Akt signaling pathways
- Gracilaria fisheri oligosaccharides ameliorate inflammation and colonic epithelial barrier dysfunction in mice with acetic acid-induced colitis
- Effects of Sirt1 on proliferation,migration,and apoptosis of endothelial progenitor cells in peripheral blood of SD rats with chronic obstructive pulmonary disease
- Potential immunomodulatory role of sesamin in combating immune dysregulation associated with COVID-19
