Gracilaria fisheri oligosaccharides ameliorate inflammation and colonic epithelial barrier dysfunction in mice with acetic acid-induced colitis
Brenda Siringoringo ,Nawiya Huipao ,Chittipong Tipbunjong ,Jongdee Nopparat ,Santad Wichienchot ,Albert M.Hutapea ,Pissared Khuituan✉
1Division of Health and Applied Sciences,Faculty of Science,Prince of Songkla University,Songkhla,Thailand
2Gut Biology and Microbiota Research Unit,Prince of Songkla University,Songkhla,Thailand
3Trace Analysis and Biosensor Research Center,Prince of Songkla University,Songkhla,Thailand
4Center of Excellence in Functional Foods and Gastronomy,Faculty of Agro-Industry,Prince of Songkla University,Songkhla,Thailand
5Department of Pharmacy,Faculty of Science,Universitas Advent Indonesia,Bandung,Indonesia
ABSTRACT Objective:To investigate the effect of Gracilaria fisheri oligosaccharides (GFO) on inflammation and colonic epithelial barrier dysfunction in colitis mice.Methods: The animals were treated by oral gavage with distilled water,1 000 mg/kg inulin,100,500,or 1 000 mg/kg GFO for 14 d,or treated with 50 mg/kg mesalamine for 5 d after colitis induction (on day 10).Histopathology,inflammatory cytokines,colonic permeability,and tight junction proteins were investigated by hematoxylin and eosin staining,immunohistochemical staining,Ussing chamber technique,and Western blotting assays,respectively.Results: GFO ameliorated histological damage in colitis mice when compared to untreated colitis mice.Treatments with 100,500,and 1 000 mg/kg GFO reduced TNF-α expression,while IL-1β was significantly reduced in colitis mice treated with 500 and 1 000 mg/kg.Compared to untreated colitis mice,GFO increased transepithelial electrical resistance,reduced fluorescein isothiocyanate-dextran paracellular flux,and modulated tight junction proteins (occludin and claudin 2) in colitis mice.Conclusions:GFO has anti-inflammatory activity and could modulate colonic epithelial barrier dysfunction in acetic acidinduced colitis mice.Furthermore,GFO could modulate the expression of tight junction proteins that play important roles in colonic barrier function.
KEYWORDS:Ulcerative colitis;Cytokines;Gut permeability;Tight junction;Red algae;Gracilaria fisheri;Mice;Antiinflammation;Colonic epithelial barrier
1.Introduction
Ulcerative colitis (UC) is one type of inflammatory bowel disease(IBD).It mostly occurs at the large bowel and may start from the proximal colon to the rectum[1].A previous study reported that cytokines were triggered by the dysbiosis of the commensal gut microbiota in mice with IBD.These cytokines mediate the immune system and inflammation and contribute to colonic epithelial barrier dysfunction[2].The impairment of the intestinal epithelial barrier could increase intestinal permeability to luminal antigens.This process could stimulate further immune activation that releases proinflammatory cytokines and lead to acute mucosal inflammation[3].Cytokines,such as interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α),could induce dysfunction of epithelial tight junction proteins and then cause intestinal epithelial barrier dysfunction,including reduction of barrier integrity and tissue viability[4].When the integrity and viability of the intestinal epithelial barrier are reduced,ion transport across the intestinal epithelium could be altered,leading to ion disturbance and inflammatory modulators-mediated diarrhea[5].Intestinal barrier function could be assessed by electrophysiological parameters such as transepithelial electrical resistance (TEER),potential difference (PD),and shortcircuit current (I)[6].
Several drugs are used for the management of colitis,including anti-TNF-α therapies,immunosuppressive drugs,and 5-aminosalicylic acid (5-ASA):mesalamine or mesalazine.5-ASA is safe with few side effects for UC patients.However,its effectiveness is limited,and in some cases,prolonged treatment with high doses is needed[7].Therefore,a safer natural treatment could be a promising approach to colitis treatment.
Many recent studies have tried to improve host health through prebiotics.Prebiotics are a type of carbohydrate or dietary fiber,which the gastrointestinal system cannot digest.Prebiotics could increase the growth or activity of microbiota and byproducts that are beneficial to host health.The indigestible carbohydrates,such as inulin,human milk oligosaccharides,fructooligosaccharides,galactooligosaccharides,and other dietary fibers,are classified as prebiotics[8].The fermentation process of prebiotics produces beneficial byproducts,short-chain fatty acids (SCFAs).These SCFAs help to reduce pro-inflammatory cytokines and decrease mucosal inflammation[9].Oligosaccharides,such as agaro-oligosaccharides and neo-oligosaccharides,could be derived from agar or agarose of marine red algae or seaweed[10].In a recent study,Gracilaria fisheri
oligosaccharides (GFO) was produced from agarose ofGracilaria fisheri
,a major cultivated and consumed red seaweed in Thailand(known in Thai as“Pom Nang”).Another study reported that agarooligosaccharides processed through gut microbiota fermentation increased the abundance of lactobacilli and bifidobacteria[11].Fermented oligosaccharides derived from agar of red algae were provedin vitro
to reduce pro-inflammatory cytokines,such as TNF-α,IL-6,and IL-1β,thus showing anti-inflammation potential[12].In the present study,we aim to investigate whether GFO could ameliorate the inflammation and colonic epithelial barrier dysfunction in colitis mice.This study will provide new insight into the benefit of GFO in colitis mice specifically to ameliorate inflammation and colonic epithelial barrier dysfunction.Through this study,it will explain further the use of GFO,as a supplement,to treat inflammatory disease-related diarrhea such as colitis.Moreover,the analysis presented in this study will convey information for future studies that will explore further the medicinal benefits of GFO as a supplement.
2.Materials and methods
2.1.Reagents and drugs
Sodium chloride (AF507279,Ajax Finechem,Australia),potassium chloride (AF501339,Merck,Germany),magnesium sulfate(AF412352,Ajax Finechem,Australia),sodium bicarbonate (144-55-8,Merck,Germany),calcium chloride (1401251758,Ajax Finechem,Australia),glucose (AF501339,Ajax Finechem,Australia),and Tween 20 (P9416,Sigma-Aldrich,St.Louis,USA).Acetic acid(Glacial,J.T.Baker,Taiwan),normal saline solution (001369-B00,A.N.B.Laboratories,Bangkok,Thailand),and thiopental sodium(Anesthal,JPN3017002,Jagsonpal Pharmaceuticals Ltd.,Haryana,India).To measure the presence of blood in feces,ColoScreen ES Lab Pack fecal occult blood test was purchased from Helena Laboratories,Beaumont,Texas.As a positive control in this study,the following drugs were used:mesalamine (PHR-1060) and inulin(I2255) were purchased from Sigma-Aldrich,St.Louis,USA.The following reagents were used in the Ussing chamber experiment,H&E and immunohistochemistry (IHC) staining,and Western blot analysis:Fluorescein isothiocyanate-dextran MW:4 000 Da (46944,Sigma-Aldrich,St.Louis,USA),formalin (30525-89-4,Merck,Germany),100% ethanol (64-17-5,Merck,Germany),vector ABC kit for IHC staining (Vector Laboratories,Inc.Burlingame,USA),anti-TNF-α-antibody and anti-IL-1β antibody (ab6671 and ab9722,Abcam,Cambridge,MA,USA),and anti-occludin,anti-claudin 2,and β-actin (42-2400,32-5600,and MA5-15739,Thermo Fisher Scientific,Massachusetts,USA).
2.2.Extraction and purification of GFO from Gracilaria fisheri
Gracilaria fisheri
was dried,ground,and sieved by a 100 mesh sieve to obtain a powder with a particle size of about 150 microns.The raw powder was soaked in distilled water (DW) for 1 h and autoclaved at 130 ℃ for 90 min to obtain the extract.The extract was centrifuged and the supernatant was collected,filtered,and dried until agar containing a mixture of polysaccharides was obtained.To produce a mixture of poly-oligosaccharides,the agar was subjected to hydrolysis under acidified hydrothermal conditions.The partially hydrolyzed extract was purified by passing through an ultrafiltration membrane (molecular weight cut off:3 000 daltons) and the filtered compounds were collected as GFO.The GFO compounds were spray-dried to produce GFO in powder form with a high content of oligosaccharides 1-10 degrees of polymerization (DP).This GFO compound consists of 8.09% DP1,6.37% DP3,12.67%DP4-5,18.14% DP6,21.07% DP10,and 33.65% ≥ DP10,which has molecular weight around 540-1 800 daltons.GFO compound that was used contained 2.01% dry weight of sulfate contents andD
-galactose as monosaccharide contents.The voucher number ofGracilaria fisheri
was KW2.GenBank accession number and haplotype for therbcL
gene sequence were KY315314 and R1,respectively.GenBank accession number and haplotype for theCOX1
gene sequence were KY315274 and C1,respectively[13].2.3.Animals and experimental design
Male ICR/Mlac mice (6-8 weeks old) were purchased from the National Laboratory Animal Center,Mahidol University,Thailand.The animals were housed at Southern Laboratory Animal Facility,Prince of Songkla University,Thailand at (22±2) ℃,in proper humidity and a 12 h/12 h light/dark cycle.Mice were fed with standard mouse diet food (C.P.mice feed 082G,Perfect Companion Group,Thailand) and had free access to water.The mice were acclimatized for 2 weeks until 6 weeks of age.After acclimatization,the mice were randomly divided into 7 treatments groups (n
=6-10/group):normal control,colitis control,colitis+50 mg/kg mesalamine,colitis+1 000 mg/kg inulin,colitis+100 mg/kg GFO,colitis+500 mg/kg GFO,and colitis+1 000 mg/kg GFO.Groups were intragastrically pretreated with 6.67 mL/kg of DW,inulin,or GFO once a day from day 1 to day 14,except the colitis+50 mg/kg mesalamine group,which was intragastrically treated with mesalamine on days 10 to 14 after the induction of colitis.Colitis was induced on day 10 when the mice were anesthetized with 70 mg/kg thiopental sodium.A total of 100 μL of 5% acetic acid (AA) was slowly perfusedvia
PE-90 inserted into the anus to a depth of approximately 3 cm.The severity of colitis was assessed on day 3 after induction by recording disease activity index (DAI) scores (Supplementary Table 1),which consists of body weight loss (%),stool consistency,and the existence of blood in the stool,and is determined with an occult blood test[14].On day 15,colonic tissue was collected and colon length was measured.After tissues were collected,the animals were immediately euthanized by overdose anesthesia followed by heart removal.The summary of the timeline of the experimental procedure is presented in Supplementary Figure 1.2.4.Histopathological study
Once colonic segments were collected,they were directly fixed in 10% formalin for 24 h at room temperature (RT).The tissues were then processed,embedded in a paraffin block,and sectioned at a thickness of 5 μm.Colonic sections were then processed with hematoxylin and eosin (H&E) staining using standard procedures.Then,all slides were analyzed using an Olympus DP73 microscope equipped with cellSens software to observe the histological changes.A total of 27 fields per treatment group (n
=3 per group)were randomly selected and scored for the severity of colonic inflammation.Histological scoring was measured by evaluating crypt damage,the severity of inflammation,and the extent of inflammation of the colon[15].2.5.IHC staining
IHC staining was conducted according to the kit instructions.Briefly,the tissue sections were deparaffinized with xylene and hydrated with graded alcohol.Antigen retrieval was heat-induced using citrate buffer at pH 6.0 for 20 min followed by endogenous peroxidase blocking with 3% HOin darkness for 30 min.Slides were then incubated in blocking serum for 30 min and incubated overnight with mouse monoclonal anti-TNF-α-antibody and rabbit polyclonal anti-IL-1β antibody at a 1:200 dilution in Phosphate-Buffered Saline (PBS)+Tween 20 in the moist chamber at 4 ℃.After washing with PBS+Tween 20,the slide was incubated with biotinylated“universal”anti-mouse IgG/rabbit IgG (1 h) followed by incubation with ABC reagent (1 h).The tissues were then incubated with 3,3'-diaminobenzidine to precipitate brown staining and counterstained with hematoxylin.Stained tissues (3 fields per section,6-8 sections per mouse with 20 μm interval,3 mice per group) were randomly selected and the mean intensity in each field was recorded using NIH ImageJ software.The mean optical density of positive staining was calculated from log (max intensity/mean intensity),where max intensity=255 for 8-bit images[16].
2.6.Colonic permeability
Colonic permeability was assessed with an Ussing chamber(Physiologic Instruments,San Diego,US).Colonic segments were opened vertically and pinned to the tissue disk (surface area=0.3 cm),which was then inserted between the half-chambers.The tissue was incubated in 37 ℃ Krebs solution,composed (in mM) of 119 NaCl,2.5 CaCl,4.5 KCl,2.5 MgSO,25 NaHCO,1.2 KHPO,and 11.1D
-glucose,and continuously oxygenated with carbogen(95% Oand 5% CO).PD and TEER were measured by injection of external current pulses (3 μA) and calculation following Ohm’s law[17].Fluorescein isothiocyanate (FITC)-dextran flux assay was conducted by adding 0.3 mg/mL FITC-dextran at the mucosal side of the chamber and collecting the samples from the serosal side.Samples were loaded into a black 96-well plate and fluorescence was measured at an excitation wavelength of 485 nm and 538 nm as emission wavelength[18].The apparent colonic permeability coefficient (P app) was used to represent FITC-dextran flux[19].2.7.Western blot analysis
Colonic tissues were homogenized in radioimmunoprecipitation assay buffer containing protease inhibitor (1:1 000).The homogenate was centrifuged at 13 000 rpm at 4 ℃ for 30 min.The supernatant was collected and determined by a BCA protein assay kit.An equivalent amount of proteins (20 μg) were separated through SDS-polyacrylamide gel electrophoresis and then transferred onto the PVDF membrane.The membrane was blocked with blocking buffer [5% skim milk in Tris-buffer saline with 0.1% Tween 20(TBST)] for 1 h at RT.The membrane was shifted to incubate at 4 ℃overnight with the primary antibodies,anti-occludin,anti-claudin-2,and β-actin diluted in blocking buffer.The next day,the membrane was cleaned off the excess primary antibodies and incubated with appropriate HRP-conjugated secondary antibodies for 1 h at RT.The membrane was washed in TBST and the signal was visualized by chemiluminescence with hyperfilm exposure.Band intensity was measured using ImageJ software.
2.8.Statistical analysis
Results were presented as mean ± standard error of the means(SEM).Significant differences between groups were analyzed with one-way ANOVA followed by the Bonferronipost-hoc
test.Data were analyzed by GraphPad Prism (San Diego,US).The results were considered significantly different whenP
<0.05.2.9.Ethical statement
Animal experiments were performed following ethical guidelines from the International Council for Laboratory Animal Science[20]and all experiments were approved by the Animal Ethics Committee of Prince of Songkla University (MOE 0521.11/1069).
3.Results
3.1.GFO reduced severity of colitis in colitis mice
Compared to the normal control mice,the body weight (%) of mice significantly decreased after colitis was induced.However,body weight loss was significantly reduced or partially restored in mice in the 50 mg/kg mesalamine,100,500,and 1000 mg/kg GFO treatment groups (Figure 1A).DAI scores,which included %body weight loss,stool consistency,and the existence of blood in the feces,were significantly higher among colitis mice than normal control mice (P
<0.001).However,DAI scores of colitis mice were lower in the 50 mg/kg mesalamine,100,500,and 1000 mg/kg GFO treatment groups (P
<0.01).Although the DAI scores of the 1 000 mg/kg inulin group were not significantly lower compared to the colitis group,they were still reduced (Figure 1B).These results showed that the reference drug,mesalamine and the higher doses of GFO were better in lowering the DAI scores in comparison to the lower dose of 100 mg/kg GFO and the reference prebiotic,inulin.3.2.GFO improved the colon length in colitis mice
In this study,colon length was measured on day 15.The length was significantly shorter in colitis mice when compared to normal control mice (P
<0.001).On the other hand,colon length was longer in colitis mice treated with 50 mg/kg mesalamine,1 000 mg/kg inulin,100,500,and 1 000 mg/kg GFO (P
<0.05) than in untreated colitis mice (Figure 2).3.3.GFO attenuated histological damage in colitis mice
Histological damage increased in AA-induced colitis mice when compared to normal mice (P
<0.001).They showed severe inflammation that could extend from the mucosal layer to the submucosal or even the serosal layers.Inflammatory cells infiltrated the mucosa,and large areas of epithelial destruction could be observed,including crypt loss and columnar necrosis.Histological damage in colitis mice treated with 50 mg/kg mesalamine,1 000 mg/kg inulin,100,500,and 1 000 mg/kg GFO was attenuated by reductions in the severity of inflammation and the extent of inflammation and crypt loss (P
<0.01).The effects of GFO on histological damage scores that were comparable to the effects of mesalamine and inulin are shown in Table 1 and the representative data are shown in Figure 3.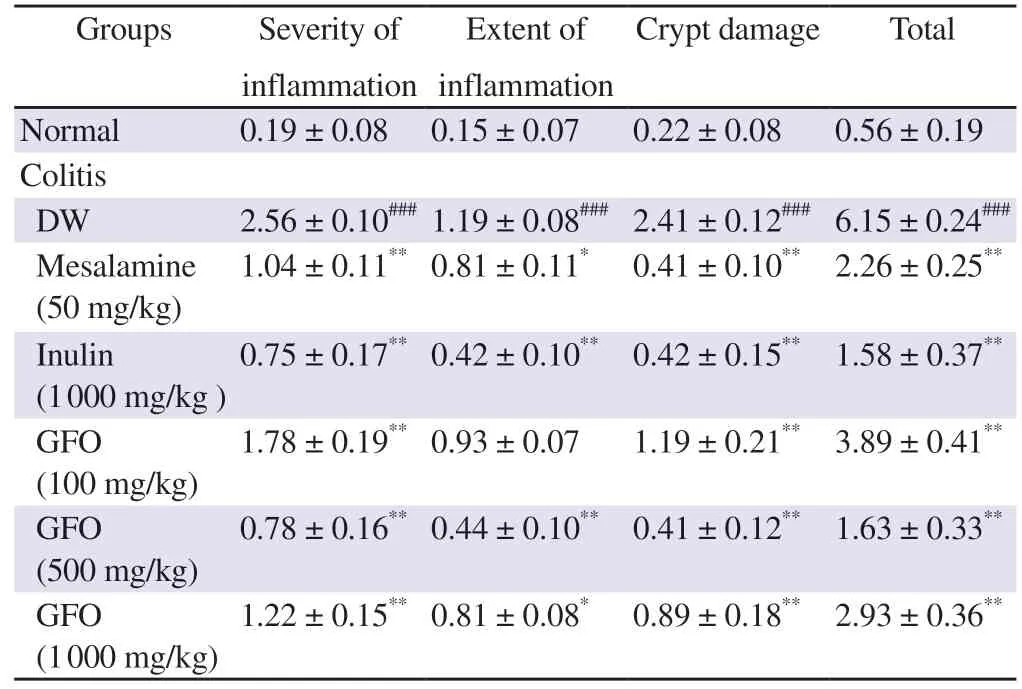
Table 1. GFO attenuates histological damage in colitis mice.
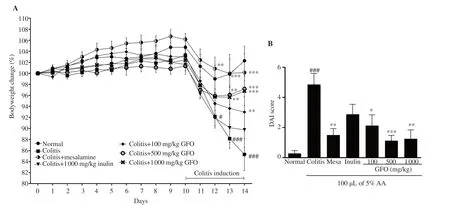
Figure 1.Gracilaria fisheri oligosaccharides (GFO) reduces the severity of colitis in mice.Bodyweight (A) was measured daily for 14 d.The data are expressed as mean ± SEM.###P<0.001 compared to the normal control group;*P<0.05,**P<0.01,and ***P<0.001 compared to the colitis group.Mesa:mesalamine,DAI:disease activity index,AA:acetic acid.
3.4.GFO reduced pro-inflammatory cytokines expression in colonic tissue of colitis mice
In this study,we measured the TNF-α and IL-1β expressions in the colonic tissue of colitis mice with IHC staining (Figure 3B,C).The results showed that TNF-α expressions in 1 000 mg/kg inulin,100,500,and 1 000 mg/kg GFO treatment groups were significantly lower than the expressions in the colitis group (P
<0.05).IL-1β expressions in 1 000 mg/kg inulin,500,and 1 000 mg/kg GFO treatment groups were significantly lower than the expressions in the colitis group (P
<0.05).On the other hand,50 mg/kg mesalamine,which was used as a drug reference,showed a reduction in TNF-α and IL-1β expressions but not a significant reduction.It suggests that the anti-inflammatory effects of GFO were comparable to inulin and better than 50 mg/kg mesalamine (Figure 4).3.5.GFO improved colonic epithelial barrier dysfunction in colitis mice
To evaluate epithelial integrity,TEER was measured and the results showed a significant reduction of TEER in colitis mice when compared to normal mice (P
<0.01).TEER in mice treated with 1 000 mg/kg inulin and 1 000 mg/kg GFO was significantly higher than the colitis mice (P
<0.05) (Figure 5A).PD was measured to represent epithelial cell health and the results showed that PD was significantly reduced in colitis mice when compared to normal mice(P
<0.01) but was significantly higher in the 1 000 mg/kg inulin,100,500,and 1 000 mg/kg GFO treatment groups than in colitis mice (P
<0.05) (Figure 5B).FITC-dextran was used as a paracellular permeability marker and FITC-dextran flux from the mucosal to the serosal sides was significantly higher in colitis mice when compared to normal mice (P
<0.01).However,mice treated with 50 mg/kg mesalamine,1 000 mg/kg inulin,100,500,and 1 000 mg/kg GFO (P
<0.05) showed significantly reduced FITC-dextran flux when compared to colitis mice (Figure 5C).These results showed that treatment with GFO ameliorated colonic epithelial barrier dysfunction in colitis mice,and this effect was comparable to 50 mg/kg mesalamine as a reference drug and 1 000 mg/kg inulin as a reference prebiotic.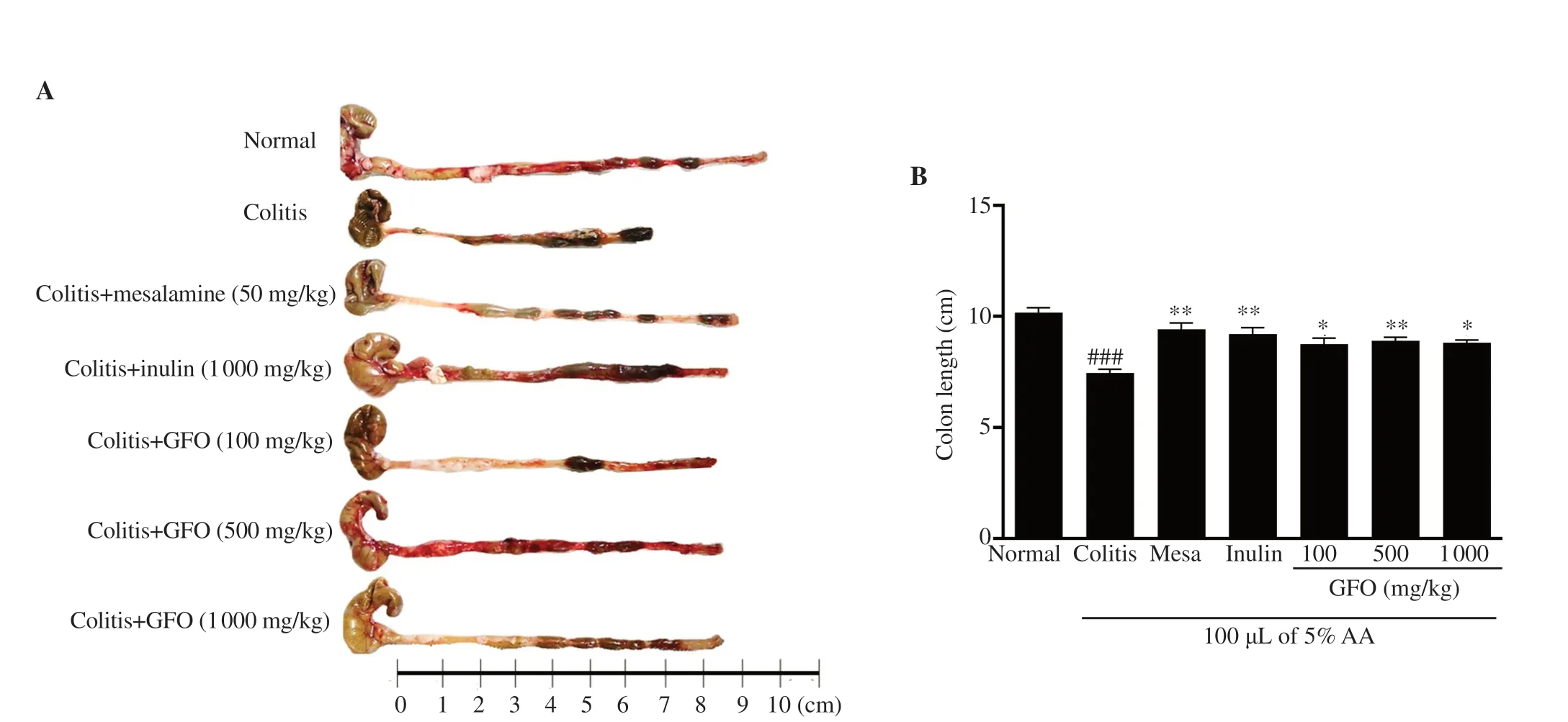
Figure 2.GFO inhibits shortening of the colon in colitis mice.(A) Representative colon length images.(B) Colon length differences.Results are shown as means ± SEM.###P<0.001 compared to the normal control group;*P<0.05 and **P<0.01 compared to the colitis group.
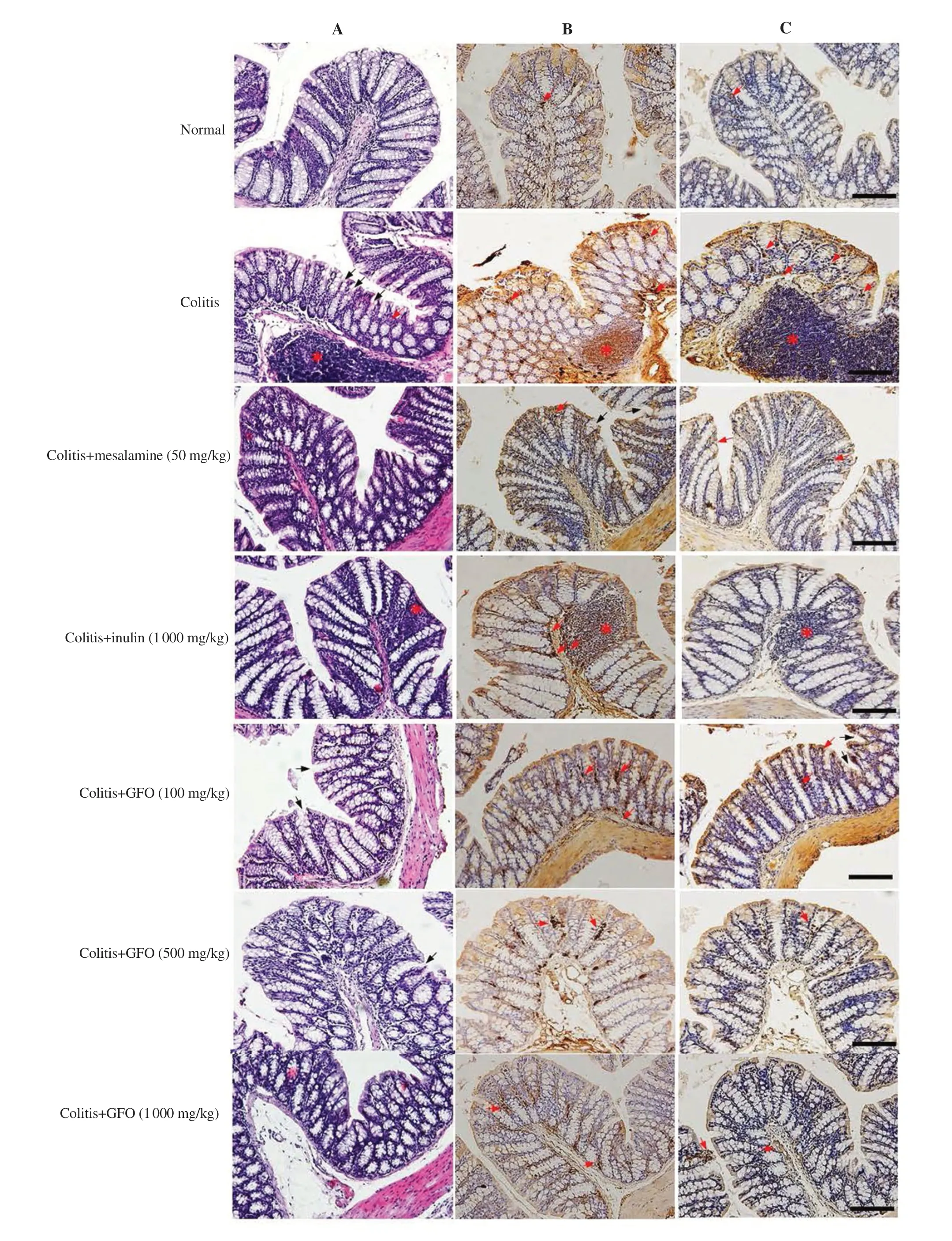
Figure 3.Representative H&E staining images of histopathology (A),immunohistochemistry of TNF-α (B),and IL-1β positive staining (C).Black arrows indicate crypt loss and distortion;red asterisks indicate inflammatory cell infiltration;red arrows indicate inflammatory cells.All images were taken at 200×magnification.Scale bar:50 μm.
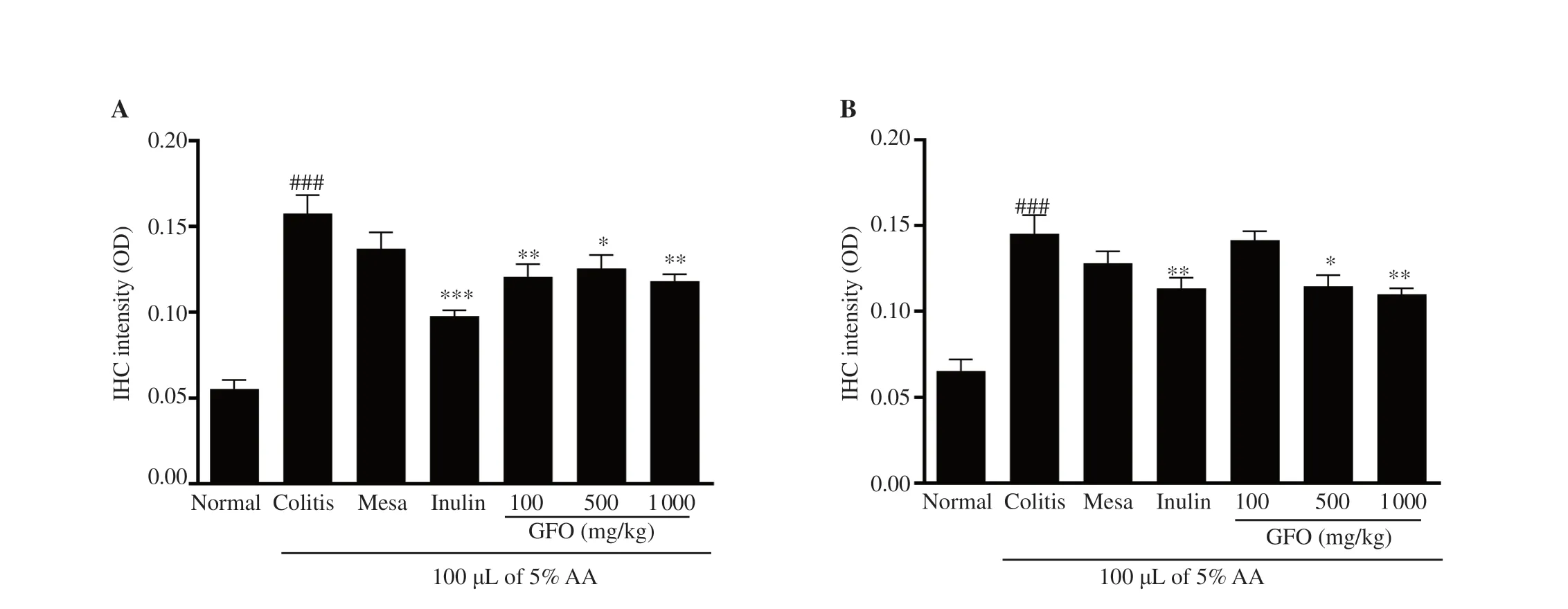
Figure 4.GFO reduces (A) TNF-α and (B) IL-1β expression in colonic tissue of colitis mice.Results are shown as mean ± SEM.###P<0.001 compared to the normal control group;*P<0.05,**P<0.01,***P<0.001 compared to the colitis group.IHC:Immunohistochemistry.
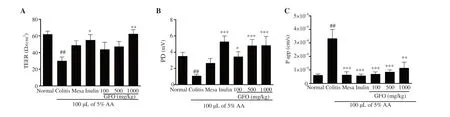
Figure 5.(A) Transepithelial electrical resistance (TEER),(B) potential difference (PD),and (C) the apparent colonic permeability coefficient (P app).All data represent mean ± SEM.##P<0.01 compared to the normal control group;*P<0.05,**P<0.01,***P<0.001 compared to the colitis group.
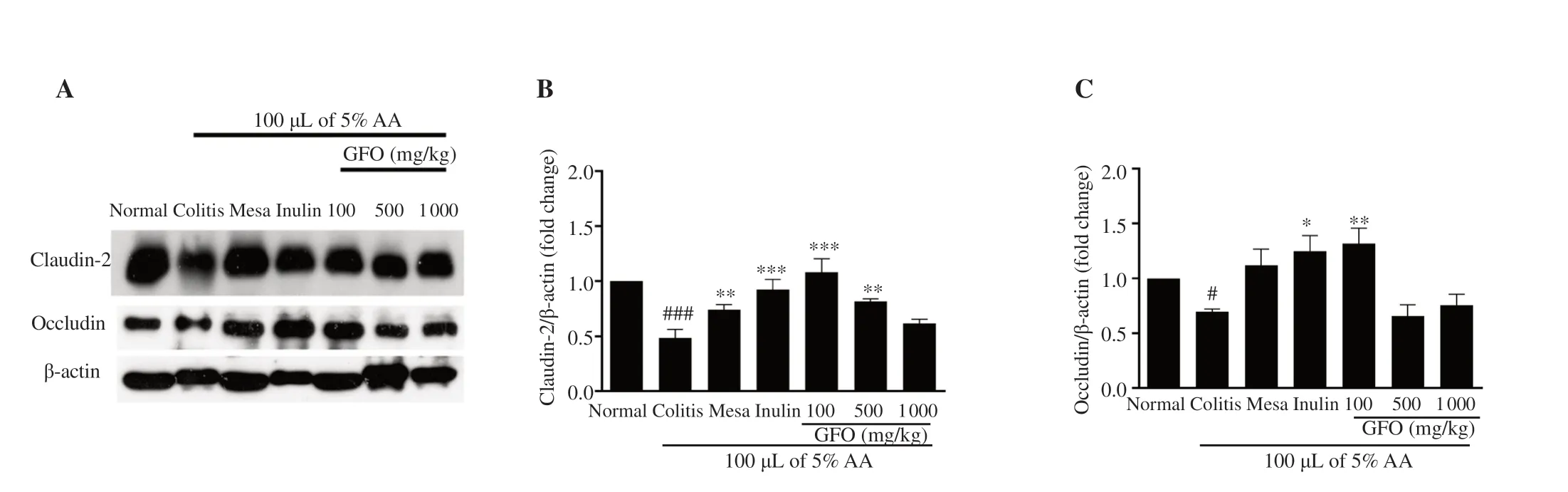
Figure 6.(A) Representative band intensity of claudin-2,occludin,and β-actin;(B) Claudin-2;(C) Occludin.Data are presented as mean ± SEM (n=3-5).#P<0.05 and ###P<0.001 compared to the normal control;*P<0.05,**P<0.01,***P<0.001 compared to the colitis group.
3.6.GFO modulated tight junction proteins dysfunction in colitis mice
To understand the effect of GFO administration on tight junction proteins in colitis mice,this study evaluated two tight junction protein expressions (claudin-2 and occludin) by Western blotting.The levels of both claudin-2 and occludin proteins in colitis mice were significantly lower than the expressions in normal mice (P
<0.05) (Figure 6).On the other hand,claudin-2 protein levels in mice treated with 50 mg/kg mesalamine,1 000 mg/kg inulin,100,and 500 mg/kg GFO were significantly higher than the levels in the colitis group (P
<0.05) (Figure 6A,B).Occludin protein levels in colitis mice treated with 1 000 mg/kg inulin and 100 mg/kg GFO were significantly higher than the levels in colitis mice (P
<0.05),but treatment with 50 mg/kg mesalamine,500,and 1 000 mg/kg GFO showed no significant effect on occludin levels in colitis mice (Figure 6A,C).4.Discussion
Excessive colonic inflammation and intestinal epithelial barrier dysfunction play a role in inflammatory disease-related diarrhea such as UC[2].This study aims to investigate the anti-inflammatory effect of GFO and its ability to improve the colonic epithelial barrier dysfunction in colitis mice.This study used 5% AA to induce UC.A previous study reported that symptoms of mice with AA-induced colitis are similar to UC in humans[21].Colonic shortening was a marker of inflammation in a rodent model of bowel diseases[22].The present study showed that DAI scores,colon length,and histological scores in mice of the colitis group were significantly altered when compared to normal mice.AA could be used to induce a UC-like disease model.Dietary supplementation with GFO could ameliorate mucosal inflammation in the colitis mice and improve colonic epithelial barrier dysfunction.The results of this study also showed that treatment with GFO for 14 d had protective effects with reduced DAI scores and histological damage as well as restoration of colon length in colitis mice.
Chemical induction of IBD or UC is commonly used in animal studies of IBD.It is hypothesized that the level of reactive oxygen species (ROS)will increase after AA induction and causes intestinal mucosal damage.Then luminal pathogens could pass through the intestinal epithelium and stimulate pro-inflammatory cytokines leading to further mucosal inflammation[23].ROS could also stimulate epithelial proliferation and result in colonic epithelial barrier dysfunction,which is assumed to induce colitis-associated colon cancer[24].Pro-inflammatory cytokines,such as TNF-α,IL-1β,IL-6,IL-13,and IL-17,are involved in IBD occurrence.These cytokines were found to increase in colitis patients[25,26].This study measured the expression of the pro-inflammatory cytokines in the colonic tissue with IHC.Measuring the pro-inflammatory cytokines with IHC provided an opportunity to directly observe the cells secreting the pro-inflammatory cytokines in affected colonic tissues[27].
In the colitis mice model,other than inflammatory modulators that lead to IBD,gut microbiota dysbiosis and reduction in gut composition were also observed[28].Harmful bacteria are known to play a role in inflammatory diseases.It was reported thatEscherichia coli
(E.coli
)in patients with UC can act as a pro-inflammatory agent.E.coli
has the mannose-resistant adhesive characteristic and can adhere to the epithelial cells with high affinity,which could promote the further release of inflammatory mediators[29].K-daet al
.reported thatE.coli
was increased in AA-induced colitis mice[30] and it was decreased after treatment with GFO.In anin vitro
study,24 hours of fermentation of agarose-derived agarooligosaccharides extracted from marine red algae increased the levels of beneficial bacteriaRoseburia
andBifidobacteria
[31].Fermentation of dietary oligosaccharides by beneficial gut microbiota produced SCFAs such as butyrate,acetate,and propionate[9].Butyrate can activate G-coupled-protein receptors and reduce histone deacetylase inhibition.Thus,the nuclear factor kappa-light-chain-enhancer of the activated B cells (NF-κB) signaling pathway was inhibited and the inflammation was finally controlled[32].This study also reported that in 24 h,the concentration of butyrate and acetate was increased in the culture medium of algae oligosaccharides fermented by pig fecal microbiota[31].Our previous study reported that the levels of SCFAs (propionate and butyrate) were significantly increased in colitis mice treated with GFO when compared to untreated colitis mice[30].The previous study and the present finding indicate that GFO derived fromGracilaria fisheri
could reduce TNF-α and IL-1β expressions in colitis mice by inhibiting disadvantageous bacteria and increasing the production of SCFAs.Tight junction proteins,which maintain the barrier function,could be altered and disrupted in the presence of pro-inflammatory modulators[33].A previousin vitro
study showed that Caco-2 cells incubated with TNF-α and IL-1β reduced TEER and increased mannitol flux through the paracellular junction[34,35].Barrier dysfunction makes luminal pathogens stimulate the production of inflammatory mediators and then leads to further inflammation.Colonic barrier dysfunction causes ion transport disturbance and results in the accumulation of fluids and electrolytes in the lumen,which is a general mechanism of inflammatory disease-related diarrhea[3,36].Therefore,colonic barrier function could be a potential target to manage inflammatory disease-related diarrhea.Results of the present study showed that treatments with GFO for 14 d help to increase TEER and PD as well as reduce FITC-dextran paracellular marker flux in colitis mice.Notably,restoration of TEER,PD,and FITC-dextran flux indicates improvement of colonic barrier functions,including barrier integrity and viability of the colonic tissue.The reduction of pro-inflammatory cytokines by GFO would contribute to improving colonic epithelial barrier dysfunction in colitis mice.A previous study showed that oligosaccharides from agar of red algae could induce heme-oxygenase 1 activity,effectively reduce ROS levels,and inhibit nitric oxide production during inflammation[37].In addition,a previousin vitro
study incubated RAW246.7 macrophage cells with agar oligosaccharides and found that these oligosaccharides could inhibit the expression of pro-inflammatory cytokines[12].Recent research revealed that SCFAs production from prebiotic fermentation could inhibit the adhesion of pathogenic bacteria to gut epithelium[38].Therefore,SCFAs could prevent mucosal barrier damage.A previousin vitro
study of agaro-oligosaccharides reported that these oligosaccharides increased the level of SCFAs byproducts[27].These byproducts,especially butyrate,helped to stabilize the hypoxia-inducible-factor 1,which is an important transcription gene and could strengthen barrier integrity[39].The maintenance of epithelial barrier integrity is partly dependent on proteins in the epithelial tight junction complex such as occludin and claudins[40].Tight junction disruption leads to increased epithelial permeability to luminal contents and pathogens.It is primarily caused by pro-inflammatory cytokines such as TNF-α,IL-1β,and interferongamma under acute inflammation conditions.The perfusion of AA into the colon of the mice reduces the expression of occludin and claudin-2.AA causes erosion and ulcers in gut mucosa leading to decreased tight junction levels.This chemical induction,in particular,increases TNF-α expression and activates the myosin light-chain kinase pathway.The consequent cytoskeleton contraction causes occludin and claudin disruption and endocytosis[32].This study shows that treatment with GFO could ameliorate the levels of IL-1β and TNF-α in colonic tissues of colitis mice.The anti-inflammatory property of GFO contributes to the modulation of occludin and claudin-2 protein expressions.The evidence of alteration of tight junction proteins by GFO helps to further understand its mechanism in improving colonic epithelial barrier dysfunction of AAinduced colitis mice.
In summary,GFO as a supplement could help to reduce the colitis severity of AA-induced colitis mice.GFO also shows anti-inflammatory effects.The ability of GFO to reduce pro-inflammatory cytokines contributes to the improvement of colonic epithelial barrier dysfunction.Furthermore,the effects of GFO on barrier function in colitis mice might be attributed to the ability of GFO to alter and restore tight junction disruption in inflamed mucosa.These findings support that GFO,as a supplement,could help to ameliorate inflammation and epithelial barrier dysfunction in inflammatory disease-related diarrhea such as UC.Future studies of the specific mechanism will help to develop novel treatments of UC diseases using natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
This work was supported by Prince of Songkla University (grant number SCI6202058S) and Thailand Education Hub-ASEAN Countries (TEH-AC) scholarship (grant number TEH AC 012/2017)Prince of Songkla University,Songkhla,Thailand.
Authors’ contributions
BS conducted the research,collected the data,performed data analysis,and contributed to the original and final versions of the manuscript.NH conceptualized the research design,conducted the research,performed data analysis,and contributed to writing and reviewing the manuscript.Both CT and JN contributed to conducting the research,performed data analysis,and contributed to writing and reviewing the manuscript.SW conceptualized the research design,supplied GFO,and contributed to writing and reviewing the manuscript.AH conceptualized the research design and contributed to writing and reviewing the manuscript.PK conceptualized the research design,conducted the research,performed data analysis,contributed to the original and final versions of the manuscript,and supervised the research.
 Asian Pacific Journal of Tropical Biomedicine2021年10期
Asian Pacific Journal of Tropical Biomedicine2021年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Antioxidant,cytotoxic,and anti-venom activity of Alstonia parvifolia Merr.Bark
- Ethanol extract of Chondracanthus tenellus (Harvey) Hommersand attenuates lipopolysaccharide-induced inflammatory and oxidative response by blocking the NF-κB,MAPKs,and PI3K/Akt signaling pathways
- Effects of Sirt1 on proliferation,migration,and apoptosis of endothelial progenitor cells in peripheral blood of SD rats with chronic obstructive pulmonary disease
- Potential immunomodulatory role of sesamin in combating immune dysregulation associated with COVID-19
