Ethanol extract of Chondracanthus tenellus (Harvey) Hommersand attenuates lipopolysaccharide-induced inflammatory and oxidative response by blocking the NF-κB,MAPKs,and PI3K/Akt signaling pathways
Da Hye Kwon ,Cheol Park ,Hyesook Lee ,Su Hyun Hong ,Gi-Young Kim ,Hee-Jae Cha ,Suhkmann Kim,Heui-Soo Kim,Hye-Jin Hwang,Yung Hyun Choi✉
1Anti-Aging Research Center,Dong-eui University,Busan 47340,Republic of Korea
2Department of Biochemistry,College of Korean Medicine,Dong-eui University,Busan 47227,Republic of Korea
3Division of Basic Sciences,College of Liberal Studies,Dong-eui University,Busan 47340,Republic of Korea
4Department of Marine Life Sciences,School of Marine Biomedical Sciences,Jeju National University,Jeju 63243,Republic of Korea
5Department of Parasitology and Genetics,College of Medicine,Kosin University,Busan 49104,Republic of Korea
6Department of Chemistry,College of Natural Sciences,Pusan National University,Busan 46241,Republic of Korea
7Department of Biological Sciences,College of Natural Sciences,Pusan National University,Busan 46241,Republic of Korea
8Department of Food and Nutrition,College of Nursing,Healthcare Sciences &Human Ecology,Dong-eui University,Busan 47340,Republic of Korea
ABSTRACT Objective:To investigate whether the ethanol extract of Chondracanthus tenellus (Harvey) Hommersand,a type of red algae,could exhibit anti-inflammatory potential in lipopolysaccharide(LPS)-stimulated macrophages.Methods: The ethanol extract of Chondracanthus tenellus was applied to 100 ng/mL LPS-stimulated RAW 264.7 cells,and cell viability,phagocytic ability,levels of pro-inflammatory factors,and the production of reactive oxygen species were measured.To identify the underlying mechanism of the ethanol extract of Chondracanthus tenellus,the expression of inflammation-regulated genes was estimated.Results: The ethanol extract of Chondracanthus tenellus had no cytotoxic effect at concentrations below 300 µg/mL,and reduced the LPS-induced production of inflammatory mediators including nitric oxide (NO) and prostaglandin E2.Furthermore,the extract markedly suppressed the expression of inducible NO synthase and cyclooxygenase-2,as well as the production of reactive oxygen species.The LPS-induced up-regulation of pro-inflammatory cytokines was attenuated by treatment with the ethanol extract of Chondracanthus tenellus,reducing their extracellular secretion.The Chondracanthus tenellus extract also inhibited LPS-mediated activation of nuclear factor-kappa B (NF-κB).In addition,the phosphorylation of mitogen activated protein kinases (MAPKs) and phosphatidylinositol 3 kinase (PI3K)/Akt was markedly increased by LPS,which was significantly abolished by the Chondracanthus tenellus extract.Conclusions:Our findings indicate that the ethanol extract of Chondracanthus tenellus exhibited potential anti-inflammatory and antioxidant effects through downregulating the NF-κB,MAPKs,and PI3K/Akt signaling pathways in LPS stimulated RAW 264.7 macrophages.
KEYWORDS:Chondracanthus tenellus;Inflammation;ROS;NFκB;RAW 264.7 cells
1.Introduction
A properly controlled inflammation is a biological response of the immune system,but uncontrolled excessive inflammation contributes to chronic inflammatory diseases such as rheumatoid arthritis,diabetes,atherosclerosis,pulmonary fibrosis,chronic hepatitis,degenerative diseases,as well as various cancers[1-3].Macrophages are potent innate immune cells that regulate immune homeostasis in the physiological and pathological stages.Lipopolysaccharide (LPS) is known to be a critical pathogenic stimulator in the innate immune system[4].Accumulated evidence suggested that LPS stimulates local or systemic inflammatory response[4].Among the pro-inflammatory mediators,nitric oxide(NO) and prostaglandin E(PGE) are synthesized by inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2),respectively[5,6].In LPS-stimulated macrophages,the expression of iNOS and COX-2 is closely correlated with the immoderate generation of proinflammatory cytokines,such as tumor necrosis factor (TNF)-α,interleukin (IL)-1β,IL-6,IL-10,and interferon-γ[5-8].There is well known that LPS-mediated inflammatory response is activated by the toll-like receptor 4-mediated signaling pathways,including nuclear factor-kappa B (NF-κB),mitogen activated protein kinases(MAPKs),and phosphatidylinositol 3 kinase (PI3K)/Akt[4,9].Meanwhile,reactive oxygen species (ROS) act as integral signaling molecules that regulate autoimmune response and immunemediated inflammatory diseases[1,10].In macrophages,ROS was stimulated by LPS,promoting the inflammatory response,and overgenerated inflammatory factors may accelerate to enhance ROS generation[1,11].
Recently,there has been increasing interest in using marine species as a resource for suppressing various inflammatory and oxidative diseases[12-14].Among them,seaweed has great potential to develop as a preventive agent for these diseases because of the abundance of physiologically active substances with various pharmacological actions[14-16].For example,several red algae extracts,includingSarcodia ceylanica
,Porphyra dioica
,Gelidium elegans
,andChondrus crispus
,have been reported to exhibit the anti-inflammatory effect in LPS-stimulated macrophages[17-21].In addition,edible red algaeGelidium elegans
extract has been reported to attenuate lipid accumulation and the generation of ROS and reactive nitrogen species in 3T3-L1 preadipocytes and RAW 264.7 macrophages[18,22].Moreover,Wijesingheet al.
[23] reported that the anti-inflammatory efficacy of a bioactive substance isolated fromLaurencia snackeyi
,a red seaweed was associated with inhibition of oxidative stress in a zebrafish embryo.Chondracanthus tenellus
(C.tenellus
) (Harvey) Hommersand is a species of red algae distributed throughout the coasts of East Asia and has long been consumed as an edible seaweed.AlthoughC.tenellus
is known to reduce ultraviolet B-induced cellular damage by scavenging ROS in human keratinocytes[24],research on its pharmacological action has been limited to date.Therefore,in this study,the effect of the ethanol extract ofC.tenellus
on LPS-stimulated RAW 264.7 macrophage cells was investigated and the related mechanisms were elucidated.2.Materials and methods
2.1.Preparation of the ethanol extract of C.tenellus
C.tenellus
was collected from offshore Jeju Island,Republic of Korea in March 2017,the ethanol extract ofC.tenellus
used in the current study was provided by Dr.DS Lee from National Marine Biodiversity Institute (Seocheon,Republic of Korea).A voucher specimen of the fruits (WECU-17-6) was deposited in the Department of Biochemistry,Dongeui University College of Korean Medicine (Busan,Republic of Korea).In brief,driedC.tenellus
was extracted five times with 70% ethyl alcohol (1:10w/v
) at 1 h interval by sonication.Subsequently,the extract was evaporated in vacuo,and then dissolved in dimethyl-sulfoxide (DMSO,Sigma-Aldrich Chemical Co.,St.Louis,MO,USA).The concentration of DMSO used in this study below 0.05% did not affect cytotoxicity on RAW 264.7 cells.2.2.Cell culture
The RAW 264.7 cells (ATCCTIB-71) were purchased from the American Type Culture Collection (Manassas,VA,USA).The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin,in atmosphere of 5% COat 37 ℃.All reagents for cell culture were obtained from WelGENE Inc.(Daegu,Republic of Korea).
2.3.Cell viability
To determine cytotoxicity of the ethanol extract ofC.tenellus
in RAW 264.7 cells,the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;Sigma-Aldrich Chemical Co.)reduction assay was conducted[25].Briefly,the cells were seeded in 96-well plates (1×10cells/wells),and were treated with various concentrations of the ethanol extract ofC.tenellus
or pre-treatment with the ethanol extract ofC.tenellus
for 1 h before exposure to 100 ng/mL LPS (Sigma-Aldrich Chemical Co.) for 24 h.The cells were incubated with 500 μg/mL of MTT solution for 3 h.Subsequently,the cells were lysed with DMSO,and the absorbance was measured using a microplate reader (Dynatech Laboratories,Chantilly,VA,USA).The cell morphology was observed under a microscope (Carl Zeiss,Oberkochen,Germany).2.4.Phagocytic activity
The phagocytosis was analyzed using a commercial phagocytosis assay kit (Cayman Chemical,Ann Arbor,MI,USA),as previously described[26].The extent of phagocytosis was determined using Cell Lab Quanta SC flow cytometry (Beckman Coulter,Brea,CA,USA).
2.5.The levels of NO,PGE2,and cytokines
The NO level in the supernatants was measured using Griess reagent (Sigma-Aldrich Chemical Co.)[27].The levels of PGEand cytokines were assayed using commercial enzymelinked immunosorbent assay (ELISA) kits (R&D Systems Inc.,Minneapolis,MN,USA) according to the manufacturer’s instructions,as previously described[28].
2.6.Western blotting analysis
The total proteins were lysed with lysis buffer as previously described[28],and proteins from the nucleus and cytoplasm were extracted using nuclear extraction reagents (Pierce,Rockford,IL,USA).Equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred into polyvinylidene difluoride membranes.The protein-transferred membranes were blocked and then reacted with primary antibodies(Table 1) overnight at 4 ℃.Subsequently,the membranes were incubated with secondary antibodies for 2 h at room temperature.Actin and lamin B were used as an internal control for cytosol and nucleus,respectively.Protein bands were visualizedvia
X-ray film.Densitometric analysis of the bands was performed using the ImageJsoftware (v1.48,NIH,Bethesda,MD).
Table 1. List of primary antibodies used for Western blotting analysis in the study.
2.7.Immunofluorescence staining for NF-κB
The cells were pre-treated with 300 µg/mL of the ethanol extract ofC.tenellus
for 1 h and then stimulated with or without 100 ng/mL LPS for 1 h.The cells were fixed,blocked,and then probed with anti-NF-κB at 4 ℃ overnight.The cells were reacted with the goat antirabbit IgG cross-absorbed secondary antibody conjugated to Alexa Fluor 594 (Thermo Fisher Scientific,Waltham,MA,USA) for 1 h.The cells were counterstained with 4’,6-diamidino-2-phenylindole(DAPI,Sigma-Aldrich Chemical Co.),which is a nucleus-specific dye,and observed under a fluorescence microscope (Carl Zeiss).2.8.Assessment of ROS levels
Cells (3×10cells/mL) were cultured with the ethanol extract ofC.tenellus
and/or LPS for 1 h and were stained with 10 μM DCF-DA for 15 min.The stained cells were visualized under a fluorescence microscope and analyzed by flow cytometry (BD Biosciences,San Jose,CA,USA) as previously described[29].2.9.Statistical analysis
All data were expressed as mean ± standard deviation (SD) of at least triplicate experiments and analyzed with GraphPad Prism software (GraphPad Software,Inc.,La Jolla,CA,USA).Data were compared with one-way analysis of variance following by Tukey’spost hoc
test.Differences were considered statistically significant if theP
value was <0.05.3.Results
3.1.Cytotoxic effect of the ethanol extract of C.tenellus
Figure 1A shows that the ethanol extract ofC.tenellus
had no cytotoxic effect on RAW 264.7 cells at concentrations below 300 µg/mL.However,remarkable cytotoxicity was observed in over 400 μg/mL-treated cells.Meanwhile,no cytotoxicity was observed when 100 ng/mL LPS-treated cells were treated with the ethanol extract ofC.tenellus
at concentrations of no more than 300 µg/mL.Moreover,a feature of activated macrophages such as polygonal spindle-shaped pseudopodia wasverified in LPS-stimulated cells,whereas the original round shape largely existed in the presence of the ethanol extract ofC.tenellus
(Figure 1C).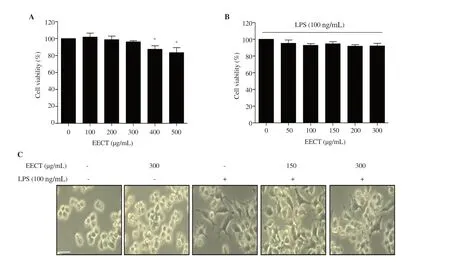
Figure 1. Effect of EECT on the cytotoxicity of RAW 264.7 macrophages.(A) Cytotoxic effect of different concentrations of EECT on RAW 264.7 cells.(B) Cell viability of LPS-stimulated cells with or without EECT treatment.Each value indicates mean ± SD (n=3).* indicates significant difference from untreated cells at P <0.05.(C) The cell morphology was observed using a microscope.Scale bar:200 μm.(magnification:× 200) EECT:the ethanol extract of Chondracanthus tenellus.LPS:lipopolysaccharide.
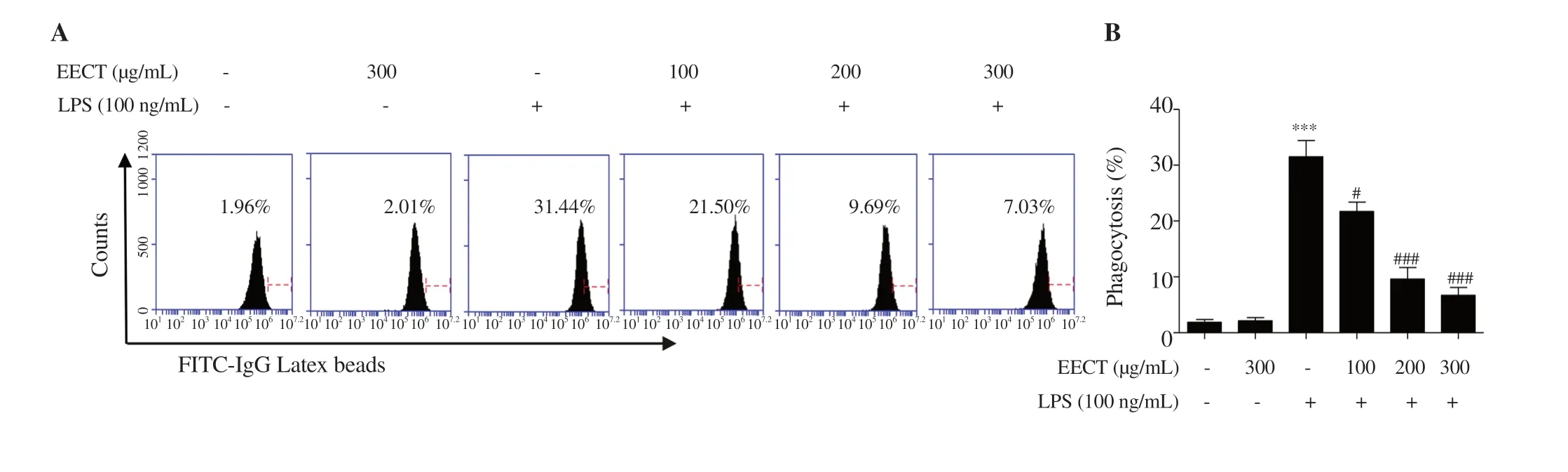
Figure 2. Effect of EECT on cell morphology and phagocytosis in LPS-stimulated RAW 264.7 macrophages.(A and B) The phagocytic uptake of FITClabeled dextran was measured by flow cytometry.(A) The representative plots.(B) The histogram results of phagocytic uptake of FITC-dextran.Data are expressed as mean ± SD (n=3).*** indicates significant difference from untreated cells at P <0.001.# and ### indicate significant difference from LPSstimulated cells at P <0.05 and P <0.001,respectively.
3.2.Prevention of phagocytosis by the ethanol extract of C.tenellus in LPS-stimulated RAW 264.7 macrophages
To evaluate the effect of the ethanol extract ofC.tenellus
on phagocytosis that is one of the key features of abnormally activated macrophages,we determined phagocytic activity using a commercial analysis kit.Figures 2A and B show that LPS greatly promoted phagocytosis in RAW 264.7 cells,whereas LPS-induced phagocytosis was markedly attenuated by the ethanol extract ofC.tenellus
in a concentration-dependent manner.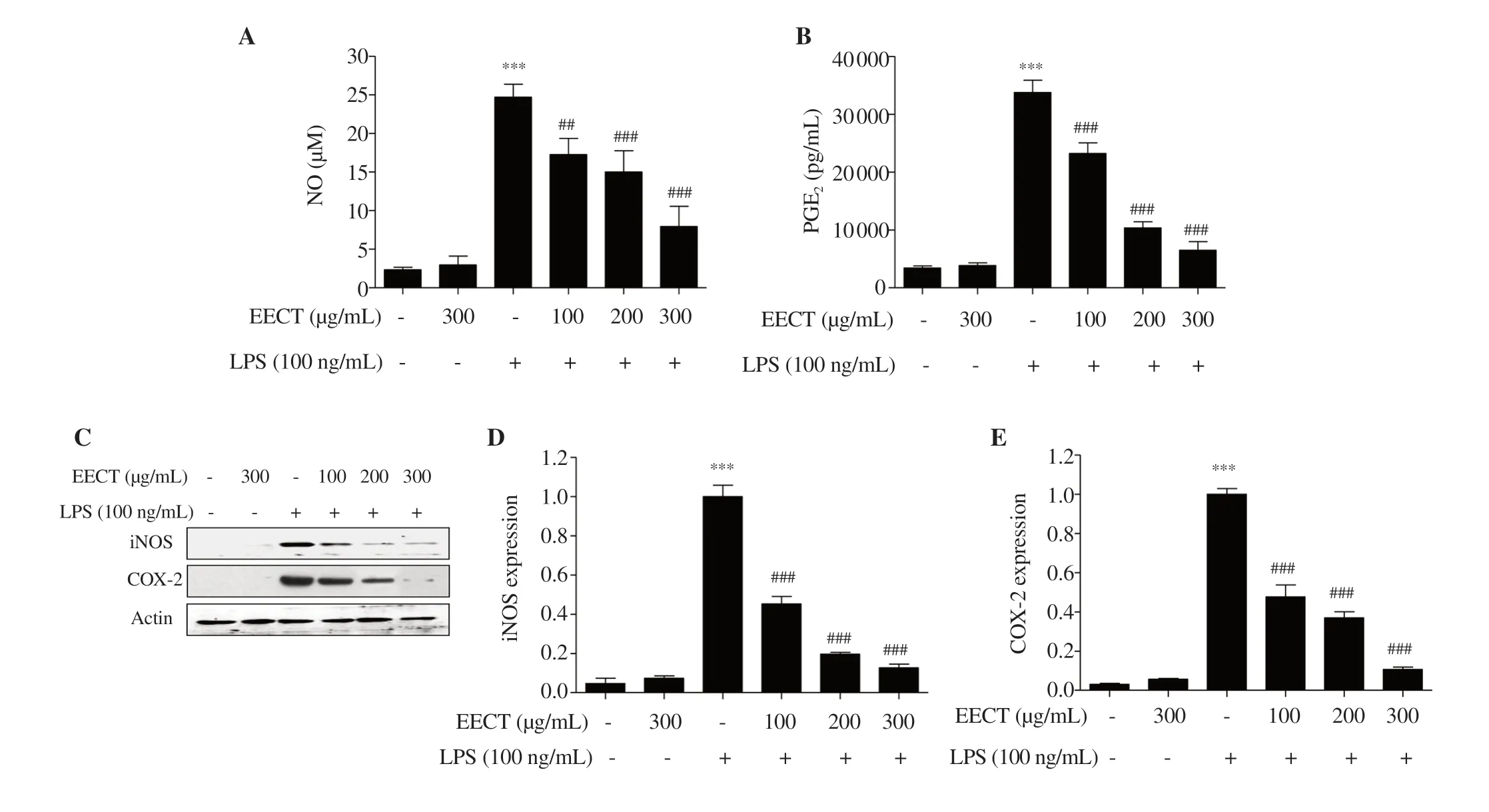
Figure 3. Effect of EECT on the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages.(A) NO concentration was measured by Griess reaction.(B) PGE2 concentration was determined using a commercial ELISA kit.(C) The expression of iNOS and COX-2 was determined by Western blotting.(D and E) Band densities were quantified using ImageJ and normalized against actin.Data are expressed as mean ± SD (n=3).*** indicates significant difference from untreated cells at P <0.001.## and ### indicate significant difference from LPS-stimulated cells at P <0.01 and P <0.001,respectively.NO:nitric oxide,PGE2:prostaglandin E2,iNOS:inducible NO synthase,COX-2:cyclooxygenase-2.
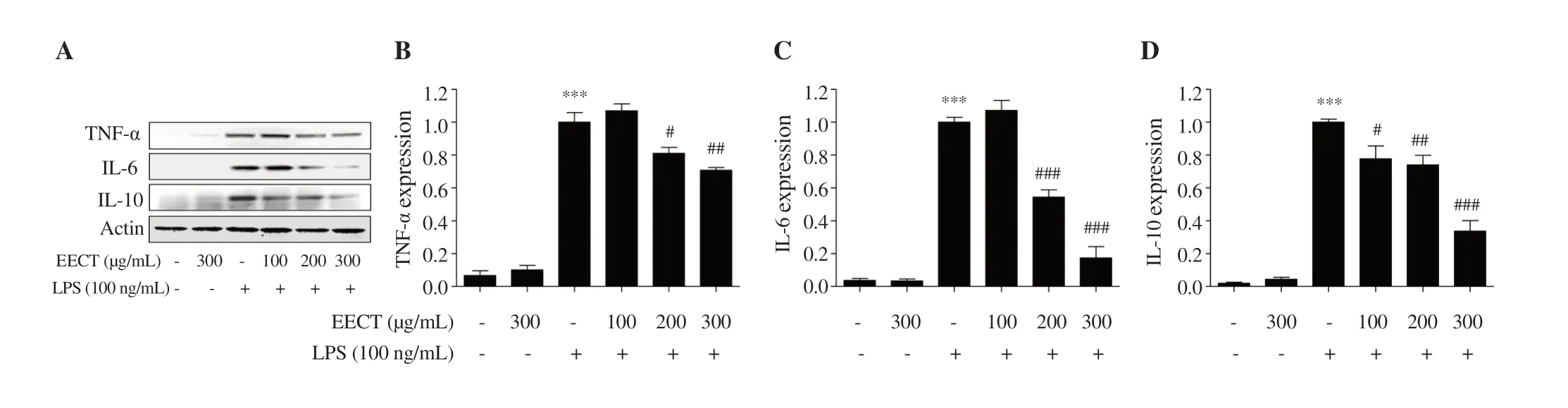
Figure 4. Effect of EECT on the production pro-inflammatory cytokines in LPS-stimulated RAW 264.7 macrophages.(A) The expression of cytokines (TNF-α,IL-6 and IL-10) was measured by Western blotting.(B-D) Bands were quantified using ImageJ and normalized against actin.Data are expressed as mean ± SD(n=3).*** indicates significant difference from untreated cells at P <0.001.#,## and ### indicate significant difference from LPS-stimulated cells at P <0.05,P<0.01 and P <0.001,respectively.
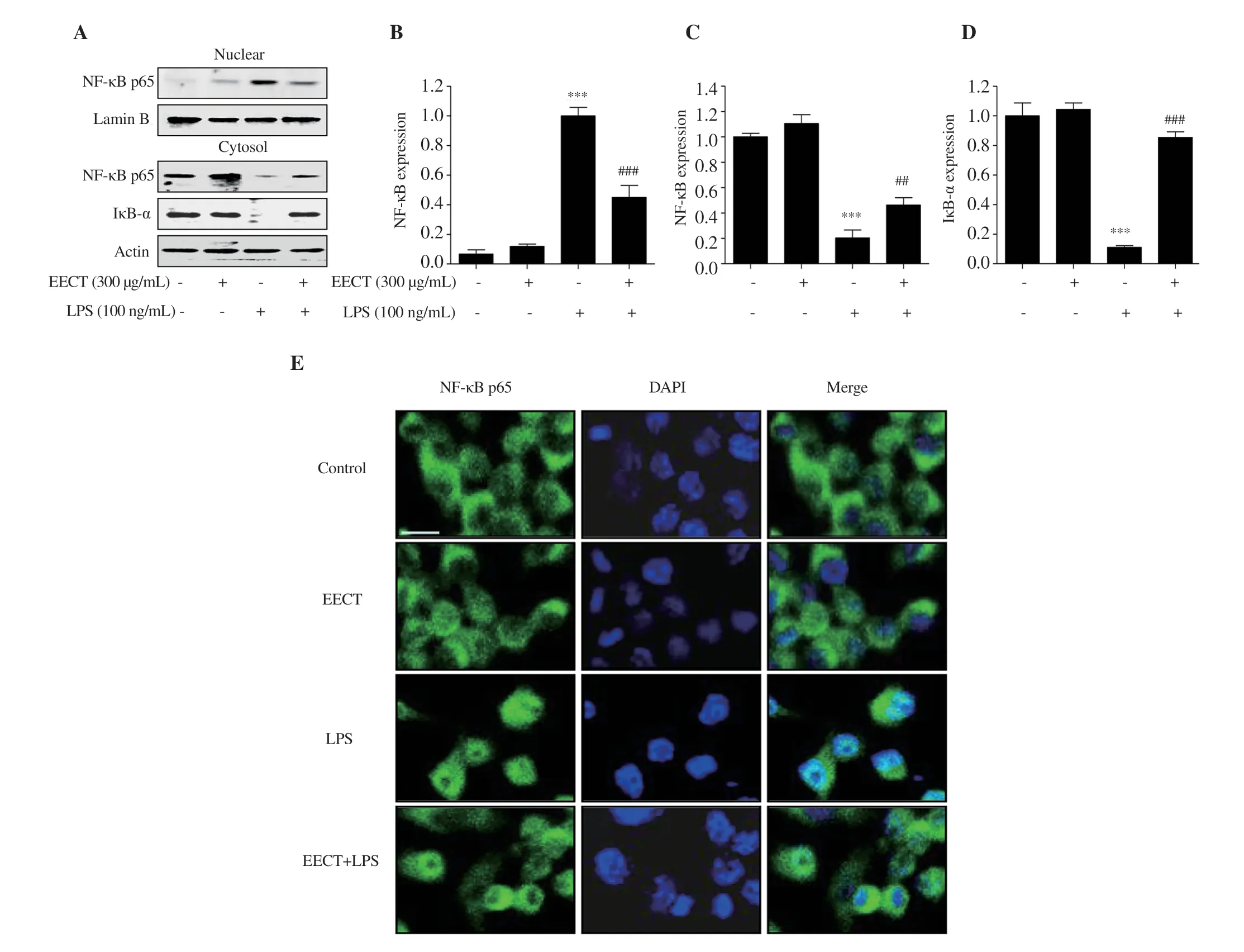
Figure 5.Effect of EECT on the activation of the NF-κB signaling pathway in LPS-stimulated RAW 264.7 macrophages.(A) The expression of NF-κB and IκB-α in the cytoplasm and nucleus.Bands were quantified using ImageJ and normalized against lamin B (B) or actin (C and D),and the ratio was determined.Data are expressed as mean ± SD (n=3).(E) The representative images of immunofluorescence staining with NF-κB p65.Green fluorescence indicates the localization of NF-κB p65.DAPI was used for nuclear counterstaining (blue).Scale bar:75 μm.*** indicates significant difference from untreated cells at P <0.001.## and ### indicate significant difference from LPS-stimulated cells at P <0.01 and P <0.001,respectively.
3.3.Inhibition of LPS-induced inflammatory mediator release by the ethanol extract of C.tenellus in RAW 264.7 macrophages
Figures 3A and B show that LPS treatment greatly promoted the levels of NO and PGEwhereas this increment was markedly inhibited by the ethanol extract ofC.tenellus
.Furthermore,according to the Western blotting results,the expression of iNOS and COX-2 was up-regulated by LPS,which was substantially down-regulated by pre-treatment of the ethanol extract ofC.tenellus
(Figures 3C-E).3.4.Inhibition of production and expression of proinflammatory cytokines by the ethanol extract of C.tenellus in RAW 264.7 macrophages
The levels of TNF-α,IL-6,and IL-10 were significantly enhanced by LPS stimulation.However,the increment of these pro-inflammatory cytokines by LPS was markedly reduced in the presence of the ethanol extract ofC.tenellus
.Moreover,LPS markedly elevated the expression of TNF-α,IL-6,and IL-10,which was suppressed by the ethanol extract ofC.tenellus
(Figures 4A-D).3.5.Amelioration of nuclear translocation of NF-κB by the ethanol extract of C.tenellus in LPS-stimulated RAW 264.7 macrophages
Next,we evaluated the effect of the ethanol extract ofC.tenellus
on LPS-mediated activation of NF-κB that is a critical transcriptional factor controlling inflammatory response.Figures 5A-D show that the expressions of NF-κB in the nucleus and inhibitor of nuclear factor-κB (IκB)-α in the cytoplasm were significantly enhanced and decreased by LPS stimulation,respectively.However,this activation of NF-κB by LPS was markedly reversed in the presence of the ethanol extract ofC.tenellus
.This result corresponded with the result from fluorescence staining that the increase in fluorescence intensity of NF-κB p65 observed in the nuclei of LPS-treated cells was markedly reduced by pre-treatment with the ethanol extract ofC.tenellus
(Figure 5E).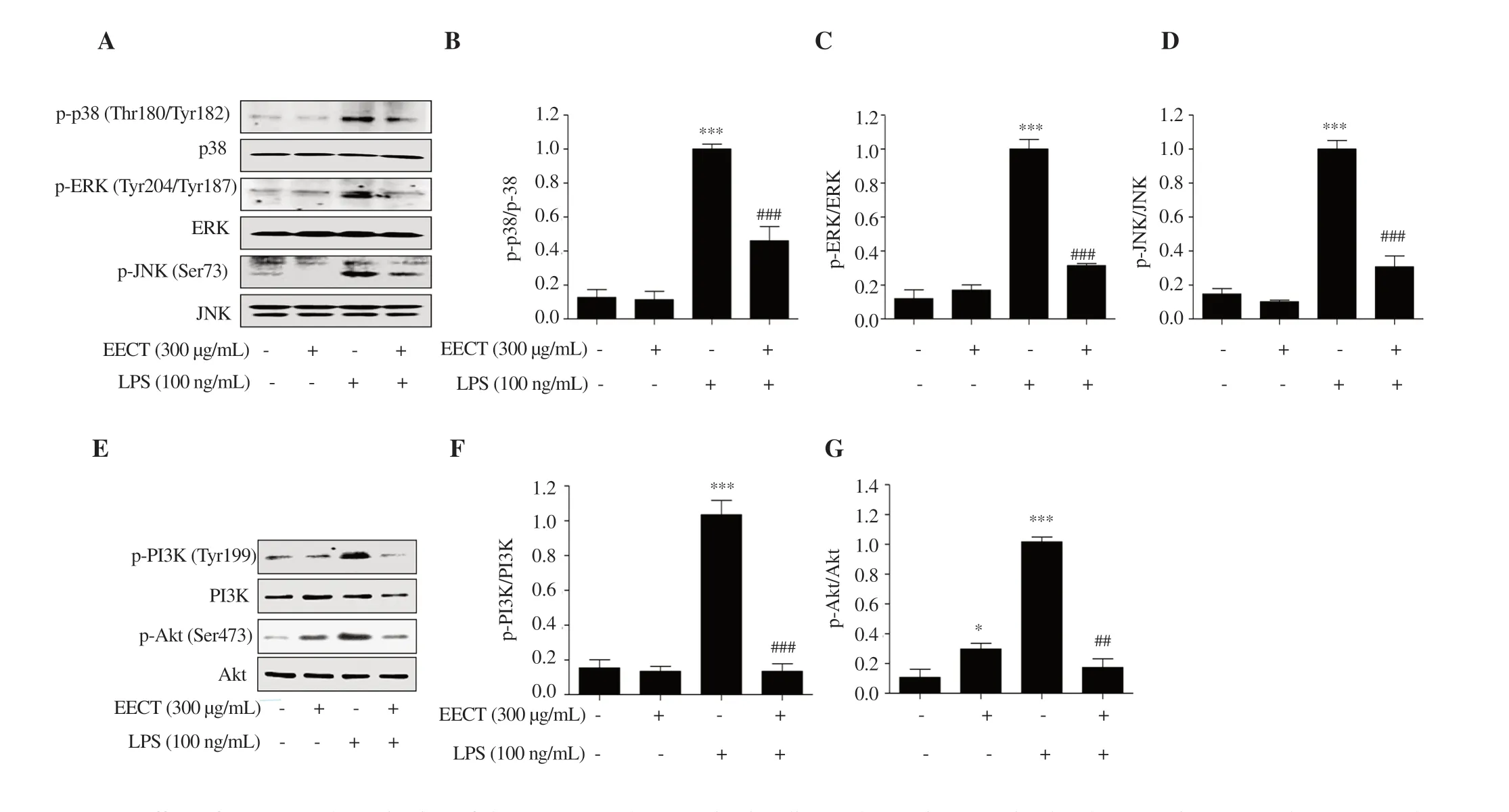
Figure 6.Effect of EECT on the activation of the MAPKs and PI3K/Akt signaling pathways in LPS-stimulated RAW 264.7 macrophages.(A and E)Expression of proteins involved in the MAPKs and PI3K/Akt signaling pathways.(B,C,D,F and G) Bands were quantified using ImageJ and normalized against lamin B or actin.Data are presented as mean ± SD (n=3).* and *** indicate significant difference from untreated cells at P <0.05 and P <0.001,respectively.## and ### indicate significant difference from LPS-stimulated cells at P <0.01 and P <0.001,respectively.
3.6.Inhibitory effect of the ethanol extract of C.tenellus on the activation of MAPK and PI3K/Akt signaling pathways in LPS-stimulated RAW 264.7 macrophages
To assess the regulation of the upstream signaling cascade associated with inhibition of NF-κB activity,we determined a family of conserved enzymes involved in the MAPKs and PI3K/Akt signaling pathways.Figures 6A-D show that the phosphorylation levels of three kinases belonging to MAPKs,including p38 MAPK,extracellular signal-regulated kinase (ERK),and c-JunN
-terminal kinase (JNK),were upregulated in LPS-stimulated cells compared with the control group.In addition,the expression of the phosphorylated form of PI3K and its downstream gene,Akt
,was increased by LPS treatment (Figures 6E-G).These results indicate that both the MAPKs and PI3K/Akt signaling pathways were activated in LPS-treated cells.However,LPS-induced phosphorylation of these enzymes was completely attenuated in the presence of the ethanol extract ofC.tenellus
.3.7.Reduction of LPS-mediated ROS generation by the ethanol extract of C.tenellus in RAW 264.7 macrophages
To evaluate the effect of the ethanol extract ofC.tenellus
on LPSstimulated oxidative stress that plays a critical role in the activation of macrophages,we measured the intracellular oxidative stress levels.Figures 7A and B show that the levels of intracellular ROS contents were increased with the stimulation of LPS,but the ethanol extract ofC.tenellus
significantly reduced DCF-DA-positive cells in LPS-stimulated RAW 264.7 cells.As shown in Figure 7C,the enhancement in the fluorescence intensity of DCF-DA by LPS stimulation was greatly weakened by the ethanol extract ofC.tenellus
,which was consistent with the result of flow cytometry.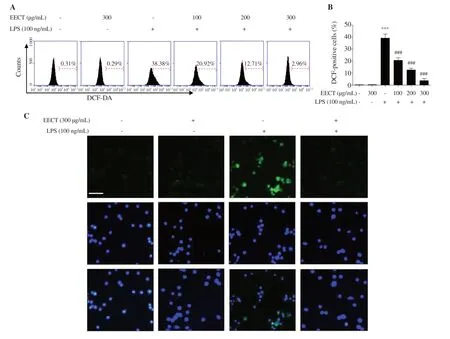
Figure 7.Inhibition of ROS generation by EECT in LPS-stimulated RAW 264.7 macrophages.(A) The representative histograms of DCF-DA-stained cells.(B)The percentages of DCF-DA-stained cells.Data are presented as mean ± SD (n=3).(C) The representative fluorescence micrographs depicting ROS generation.Green fluorescence indicates the intensity of ROS generation.Nuclei are visualized by DAPI staining (blue).Scale bar:200 μm.*** indicates significant difference from untreated cells at P <0.001.### indicates significant difference from LPS-stimulated cells at P <0.001.
4.Discussion
To estimate the anti-inflammatory efficacy of the ethanol extract ofC.tenellus
,we evaluated its effect on the production of NO and PGE[5,7,30,31].Among them,NO controls normal physiological functions,but under pathological conditions,the excessive NO produced by iNOS triggers the inflammatory response and oxidative stress[30,32].PGEis mainly synthesized by COX-2,and excessive PGEacts as an inflammatory key mediator[5,7,30,31].Therefore,suppressors against overproduction of these inflammatory mediators could be considered as potential therapeutic agents for inflammatory treatment.In this regard,our findings showed that the section of NO and PGEand the expression of iNOS and COX-2 were markedly increased by LPS,but up-regulation of these inflammatory mediators was greatly decreased in the presence of the ethanol extract ofC.tenellus
.Macrophages produce several pro-inflammatory cytokines that are required in the initiation and improvement of the inflammatory response and promote the inflammatory response by vicious cycle[5,7,8,32-35].Therefore,the level of pro-inflammatory cytokines has been considered as an indicator to assess anti-inflammatory efficacy in macrophages.In this study,our results indicated that the ethanol extract ofC.tenellus
decreased the secretion of TNF-α,IL-6,and IL-10 in LPS-stimulated RAW 264.7 cells by suppressing their expressions.NF-κB plays a key transcriptional factor involved in the control of inducible anti-inflammatory enzymes and cytokines in LPS-activated macrophages[4,9].Typically,NF-κB is bound to the inhibitory subunit IκB-α and remains inactive in the cytoplasm.When IκB-α is phosphorylated and degraded by inflammatory stimuli such as LPS,NF-κB migrates to the nucleus and triggers the transcriptional activity of inflammation-inducing cytokines and catabolic enzymes including iNOS and COX-2[36,37].Therefore,the efficacy of the ethanol extract ofC.tenellus
on LPS-induced NF-κB activation was investigated because the inhibition of the activity of NF-κB could be effective against inflammation.The current results showed that the translocation of NF-κB from the cytoplasm into the nucleus and the degradation of IκB-α were increased in LPS-treated RAW 264.7 cells,but the ethanol extract ofC.tenellus
effectively blocked the nuclear translocation of NF-κB and the degradation of IκB-α,an essential step for NF-κB activation.Therefore,the inhibitory effect of the ethanol extract ofC.tenellus
on the increased expression of pro-inflammatory enzymes and cytokines following LPS stimulation can be ascribed to the suppression of the nuclear translocation of NF-κB.Accumulated evidence clearly shows that activation of NF-κB is involved in the activation of various intracellular upstream signaling molecules[4,38].Among them,activated kinases belonging to the MAPKs and PI3K/Akt signaling pathways phosphorylate IκB for degradation through the ubiquitin-proteasome system,eventually enhancing NF-κB release and translocation from the cytoplasm to the nucleus[36,37].Therefore,targeting the inactivation of the MAPKs and PI3K/Akt signaling pathways may be effective in treating inflammatory diseases.In this study,LPS-induced phosphorylation of p38 MAPK,ERK,and JNK belonging to MAPKs was remarkably down-regulated by pre-treatment of the ethanol extract ofC.tenellus
.We further proved that LPS-stimulated phosphorylation of Akt and its upstream kinase,PI3K,was strongly inhibited by exposure to the ethanol extract ofC.tenellus
.These results indicate that the blocking effect of NF-κB by the ethanol extract ofC.tenellus
was at least involved in the obstruction of the MAPKs and PI3K/Akt signaling pathways.In addition,these observations support the reason for the reduction in pro-inflammatory cytokines and catabolites in the supernatant of the ethanol extract ofC.tenellus
-treated cells,similar to the actions of other anti-inflammatory agents reported in many previous studies[39-43].However,our results suggest that the real targets of the ethanol extract ofC.tenellus
may be other upstream kinases other than MAPKs and PI3K/Akt,and questions about this await further research.As noted in many previous studies,excess ROS accumulation can cause oxidative damage to cellular macromolecules,including nucleic acids,proteins,and lipids,and organelles[1,10].There is well known that ROS result in the activation of macrophages,and are involved in initiating and promoting inflammatory response by proinflammatory mediators and cytokines[1,10,11].In this study,the ethanol extract ofC.tenellus
substantially suppressed LPS-induced ROS formation in RAW 264.7 macrophages.This result supported the application of the ethanol extract ofC.tenellus
as an antioxidant for the management of oxidative stress associated with inflammatory responses.In this study,the anti-inflammatory and antioxidant potential of the ethanol extract ofC.tenellus
was demonstrated in an LPStreated RAW 264.7 macrophage model.According to our results,the morphological changes and phagocytic activation of RAW 264.7 cells by LPS were significantly suppressed by the ethanol extract ofC.tenellus
.Furthermore,the LPS-induced overexpression of pro-inflammatory enzymes including iNOS and COX-2 and overproduction of inflammatory cytokines such as TNF-α,IL-6,and IL10 were significantly attenuated in the presence of the ethanol extract ofC.tenellus
.These results suggested that the ethanol extract ofC.tenellus
exhibits potential suppressive effect on LPSinduced activation and inflammation of macrophages.In addition,the ethanol extract ofC.tenellus
attenuated the accumulation of ROS and suppressed the activation of NF-κB,MAPKs,and PI3K/Akt signaling pathways,indicating that the anti-inflammatory and antioxidant effects of the ethanol extract ofC.tenellus
on LPS stimulation are related to the inactivation of these signaling pathways in RAW 264.7 macrophages.However,further studies are needed to explore the role of cellular signaling pathways that may be involved in the antioxidant activity of the ethanol extract ofC.tenellus
and to determine a direct relationship with its anti-inflammatory efficacy.It is also necessary to verify the efficacy of the ethanol extract ofC.tenellus
through animal experiments and to identify the active ingredients contained in the ethanol extract ofC.tenellus
.Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This research was a part of the project titled ‘Omics based on fishery disease control technology development and industrialization(20150242)’,funded by the Ministry of Oceans and Fisheries,Republic of Korea.
Authors’ contributions
Collecting data,drafting the manuscript and critical revision of the manuscript were done by DHK,CP,HL,SHH and YHC.YHC,CP and HJH designed the study.Data analysis and interpretation were done by GYK,HJC,HSK and HJH.In addition,YHC was responsible for supervision,as well as SK and HJH contributed to project administration and funding acquisition.
 Asian Pacific Journal of Tropical Biomedicine2021年10期
Asian Pacific Journal of Tropical Biomedicine2021年10期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for Authors Asian Pacific Journal of Tropical Biomedcine
- Antioxidant,cytotoxic,and anti-venom activity of Alstonia parvifolia Merr.Bark
- Gracilaria fisheri oligosaccharides ameliorate inflammation and colonic epithelial barrier dysfunction in mice with acetic acid-induced colitis
- Effects of Sirt1 on proliferation,migration,and apoptosis of endothelial progenitor cells in peripheral blood of SD rats with chronic obstructive pulmonary disease
- Potential immunomodulatory role of sesamin in combating immune dysregulation associated with COVID-19
