Linking Cellular Metabolism to Epigenetics in Melanoma
Wei-Nan Guo and Chun-Ying Li∗
Department of Dermatology,Xijing Hospital,The Fourth Military Medical University,Xi’an,Shaanxi710032,China.
Abstract
Keywords:melanoma,metabolism,epigenetics,autophagy,histone acetylation,miRNAs,lipid metabolism,targeted therapy
Introduction
Melanoma originates from epidermal melanocytes and is the most malignant form of skin cancer,with an increasing incidence worldwide.Although recent advances in targeted therapy and immunotherapy have brought revolutionary progress in the prognosis of patients with advanced melanoma,the survival remains suboptimal,1indicating the complexity of melanoma etiology and pathogenesis that requires more in-depth investigation.Apart from the known genetic mutations(such as V-raf murine sarcoma viral oncogene homolog B[BRAF],neuroblastoma RAS viral oncogene homolog[NRAS],and phosphatase and tensin homolog[PTEN]mutations)closely associated with ultraviolet radiation or other environmental risk factors that contribute to melanoma carcinogenesis,the remodeling of epigenetic modification has been well documented to orchestrate the malignant behaviors of tumor cells and anti-tumor immunity,emerging as an irreversible tumorigenic event that is more preventable and treatable with medications.2-3Epigenetic modification refers the heritable changes in gene expression without an alteration in genome sequence.The main paradigms of epigenetic modification including DNA methylation,histone modification and non-coding RNAs.To be specific,DNA methylation is the process of the addition of a methyl group to the5position of cytosine by DNA methyltransferase to form5methylcytosine(5-mc).In addition,histone modification is defined as a series of modifications occuring on the N-terminal“tails”of hitones,including histone acetylation,methylation and ubiquitination.What’s more,non-coding RNAs are alternative type epigenetic regulation involving lncRNAs,microRNAs and circRNAs.The dysregulation of epigenetics in cancer has a broad-spectrum impact on biological activities,4and recent studies by other groups and our laboratory have reported that disorders of cellular metabolism(including autophagy,mitochondrial function,and the oncometabolite acetyl-CoA)that are related to epigenetic malfunction play a pivotal role in melanoma progression.5-6Therefore,targeted metabolism-associated epigenetic modulators may be a potential novel approach for melanoma therapy.
This review was conducted by searching PubMed and Web of Science for original research artilces in English.By taking epigenetics(espeically DNA methylation,histone modifiation and non-coding RNA),metabolism(especially autophagy,mitochondiral function and lipid metabolism)and melanoma as key words,we mainly emphasized on the advances of this field from2016.1.1to2020.12.30.Data were independently reviewed by two researchers and underwent thematic analysis by the research team.
The paradoxical role of autophagy in melanoma and its epigenetic regulators
Autophagy is a dynamic metabolic process that delivers intracellular proteins and organelles into autolysosomes for digesting and recycling.The pathologic role of autophagy in melanoma remains unclarified,and the paradoxicality is probably attributable to tumor stage and genetic background.Liu et al.first detected the autophagy marker ATG5and found that its level is lowered in earlystage melanomas compared with nevi;this downregulation of ATG5and the autophagy level prevents oncogeneinduced senescence in melanocytes to facilitate malignant transformation.7-8However,in an established geneticallyengineered mouse model of melanoma driven by oncogenic BRAF and PTEN tumor suppressor deficiency,Autophagy-related protein7(ATG7)gene deletion reportedly restrains tumor growth and extends survival by amplifying oxidative stress and inducing cell senescence in tumors.9To further investigate these contrary reports,our laboratory systemically examined the autophagy markers LC3and p62in melanoma tissues at different clinical stages,and revealed that the autophagy level is downregulated in primary melanomas and upregulated in metastatic lesions.5Moreover,our data demonstrated the bimodal role played by autophagy that suppresses melanoma growth at the early stage,but acts as an oncogenic factor at the advanced stage both in vitro and in vivo.5The potentiated autophagy level endows melanoma cells with a high capacity for invasion and migration,which enables the formation of metastases in lung and liver by reshaping the cytoskeleton.10In addition,the latest research reported that autophagy affects tumor development in a manner dependent on the PTEN status.ATG7 deletion promotes the onset of melanomas in mice harboring only the BRAF mutation,but not in mice hemizygous for PTEN.11Further investigation is needed to determine whether the effect of autophagy is associated with alternative clinicopathological factors in addition to stage and genetic mutations.
Studies have shown that multiple epigenetic modifiers are responsible for the dysregulation of autophagy.The loss of ATG5protein at the early stage occurs due to hypermethylation of its promoter.7Our laboratory showed that the bimodal level of autophagy is paralleled with upstream Type I insulin-like growth factor receptor-Akt signaling that is silenced by histone deacetylase Sir2-related protein type6(SIRT6),and that SIRT6expression level also fluctuates during melanoma progression.5Targeting SIRT6also exerts a bimodal function on melanoma growth similar to that of autophagy intervention.5Furthermore,we performed genome-wide transcriptional analysis to reveal the mRNA expression profile of melanomas,and found that runt-related transcription factor2induces the downregulation of miR-23a,which contributes to autophagy activation in metastatic melanoma by relieving its suppression of Atg12.10Other studies also report that the dysregulation of miRNA is a crucial epigenetic event in trigging the disorder of melanoma cell autophagy.12–14Overall,the research suggests that DNA methylation enzymes,histone acetylation modifiers,and miRNAs are promising targets for autophagy intervention as melanoma therapy(Fig.1).
Oncogenic mitochondrial function potentiated by histone acetylation and non-coding RNA
The mitochondrion is a crucial organelle responsible for producing ATP and metabolic intermediates via oxidative phosphorylation.Mitochondrial function is generally repressed as a result of the Warburg effect in cancer.15However,Vazquez et al.16reported that a subset of melanomas defined by a relatively high endogenous PGC1α expression level show increased mitochondrial capacity and are more resistant to oxidative stress,and that PGC1α deficiency renders melanoma cells more susceptible to reactive oxygen species(ROS)-inducing drugs.Thus,the acquisition of the mitochondrial genome is capable of potentiating the respiratory function and tumorigenic potential.17There is a close relationship between mitochondrial function and currently available targeted therapy and immunotherapy.In particular,increased mitochondrial biogenesis orchestrated by transcription factor a,mitochondrial(TFAM)mediates the adaptive resistance to MAPK inhibitors in BRAF-mutant melanomas.18Furthermore,proteomics analysis of clinical samples reveals that higher oxidative phosphorylation and lipid metabolism in mitochondria dictates the response to immunotherapy by elevating antigen presentation and increasing melanoma cell immunogenicity.19Based on these findings,multiple agents targeting mitochondrial function and oxidative phosphorylation have proven to have high potential in restraining tumor progression and synergizing with targeted therapy.20–22
Transcriptional cascades govern the activation of mitochondrial regulators and downstream components of oxidative phosphorylation machinery in melanoma.23-24However,the epigenetic regulation mechanism remains poorly defined.Our results proved that the transcriptional cascade Microphthalmia-associated transcription factor(MITF)-Peroxisome proliferator-activated receptor gamma coactivator1alpha(PGC1α)is regulated by p300-mediated histone acetylation.Pharmacological inhibition of p300 suppresses the expression of the MITF-PGC1α axis and downstream mitochondrial function,paralleled with the downregulation of histone acetylation.6Chromatin immunoprecipitation assay reveals direct binding of acetylated histone to the promoter of MITF.6In addition,a recent report demonstrated that long non-coding RNA SAMMSON is amplified in melanoma under the control of the lineage-specific transcription factor SRY-box transcription factor10(SOX10).25Further study demonstrated that SAMMSON directly interacts with p32to increase its mitochondrial targeting and pro-oncogenic function.25The knockdown of SAMMSON disrupts vital mitochondrial functions in cancer cells,which is therefore expected to deliver highly effective and tissue-restricted anti-melanoma therapeutic responses.25Taken together,the targeting of mitochondrial function through histone acetylation or noncoding RNA has great therapeutic potential in the management of melanoma(Fig.2).
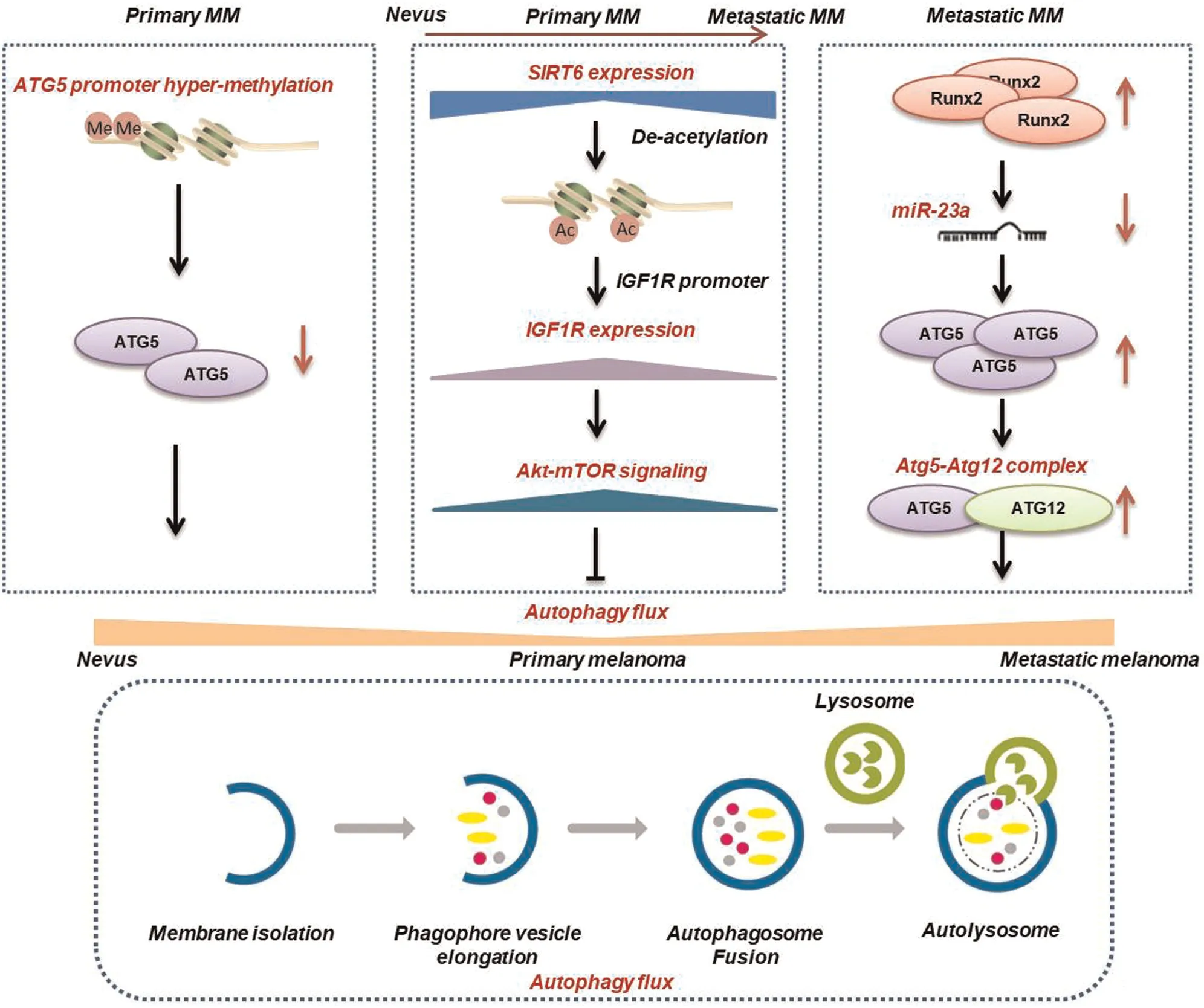
Figure1.Key epigenetic regulators of the fluctuating autophagy level in melanoma at different clinical stages.MM:melanoma;ATG5:autophagy-related protein5;SIRT6:Sir2-related protein type6;IGF1R:type I insulin-like growth factor receptor;mTOR:mammalian target of rapamycin;Runx2:runt-related transcription factor2.
Oncometabolite acetyl-coenzyme A connects cell metabolism to epigenetics
Autophagy and mitochondrial metabolism are two critical metabolic processes that occur in cytoplasm and mitochondria,respectively,both of which are sensitive to the alteration of the intracellular energy status.Acetylcoenzyme A(Ac-CoA)is a central metabolic intermediate,and its abundance in distinct subcellular compartments reflects the general energetic state of the cell.26In particular,disturbance of the intracellular Ac-CoA level is directly coupled to cellular autophagic flux and oxidative phosphorylation27–29as a critical nexus of multiple biological processes.More importantly,Ac-CoA is the sole donor of the acetyl group for acetylation.Nuclear Ac-CoA-mediated histone acetylation fluctuates during the cell cycle and promotes gene transcription via the relaxation of chromatin.26Therefore,Ac-CoA acts as the second messenger connecting cell metabolism to histone acetylation-mediated epigenetics.Recent studies have revealed that the intracellular Ac-CoA content and histone acetylation increase in carcinogenesis and tumor progression under the control of oncogenic factors such as Akt.30Multiple Ac-CoA-producing rate-limiting enzymes like ATP-citrate lyase(ACLY)and pyruvate kinase,muscle are reportedly hyperactivated in cancers.31-32Moreover,the nuclear translocation of pyruvate dehydrogenase complex from mitochondria also potentiates nuclear Ac-CoA generation and histone acetylation and is implicated in cancer pathogenesis.33These reports emphasize the role of Ac-CoA as an oncometabolite through its epigenetic modification function.

Figure2.Involvement of histone acetylation and lncRNA in the regulation of mitochondrial biogenesis and oxidative phosphorylation in melanoma,and the resultant effect on melanoma progression,targeted therapy,and immunotherapy.MITF:microphthalmia-associated transcription factor;PGC1α:PPAR-gamma co-activator-1α;SOX10:SRY-related HMG-box10protein.
We recently investigated the role of the Ac-CoAproducing enzyme ACLY,which is the node of glucose and lipid metabolism.34ACLY is prominently activated in melanoma and its expression is highly associated with patient survival.In particular,upregulated ACLY expression promotes Ac-CoA production to enhance histone acetylation in an acetyltransferase p300-dependent manner,which contributes to mitochondrial oxidative phosphorylation and tumor growth.6To the best of our knowledge,the above-mentioned investigation by our group was the first to reveal the pivotal role of Ac-CoA-mediated crosstalk between different metabolic processes in tumors and demonstrate that ACLY and p300are promising therapeutic targets associated with metabolism and epigenetics in melanoma therapy(Fig.3).
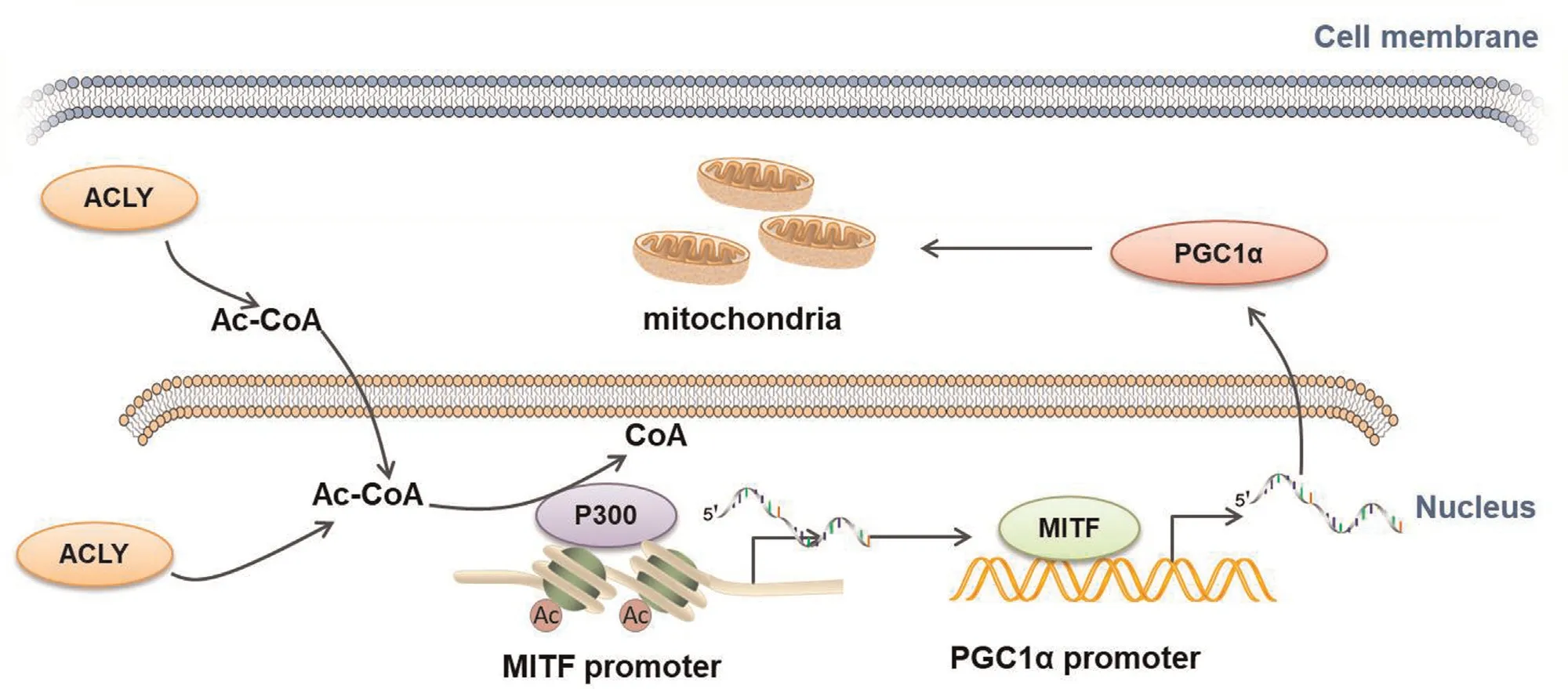
Figure3.Schematic presentation of the mechanism underlying the facilitative role of ACLY in mitochondrial function via acetyl-coenzyme A(Ac-CoA)in melanoma.ACLY expression is upregulated in melanoma,followed by an increase in Ac-CoA content,enhancement of P300activity,and increase in histone acetylation at the MITF promoter.This leads to the transcriptional activation of the MITF-PGC1α axis and subsequent mitochondrial biogenesis.ACLY:ATP-citrate lyase;PGC1α:peroxisome proliferator-activated receptor gamma coactivator1α;MITF:microphthalmia-associated transcription factor.
Future perspectives
Although the linkage between epigenetics and metabolism in melanoma pathogenesis has been preliminarily revealed by our group and some other investigations,there are several limitations and questions waiting to be solved or answered.To be specific,genome-wide transcriptional analysis or proteomics analysis should be employed to systemically testify the regulatory relationship between these two crucial hallmark characteristics.In addition,more paradigms of cell metabolism and epigenetic modifications like amino acid metabolism,nucleotide metabolism,histone methylation,and other forms of histone modifications should also be emphasized and taken into consideration in further studies.What’s more,more pre-clinical animal models need to be used to elucidate the translational potential of targeting the crosstalk between metabolism and epigenetics in melanoma therapy.
In summary,reports from other groups and our laboratory have provided evidence revealing the interplay between metabolism and epigenetic modification in the pathogenesis of melanoma.The above-mentioned epigenetic regulators of autophagy,mitochondrial function,and Ac-CoA production might be valuable as potential therapeutic targets for melanoma treatment.This therapeutic approach warrants future investigation.
Acknowledgments
The authors are grateful to Dr.Jian-Ru Chen for revising the manuscript.
Source of funding
The research leading to these results has received funding from the National Natural Science Foundation of China(No.81625020,No.81902791)and the Xijing Hospital(No.2019cyjhgwn).
- 国际皮肤性病学杂志的其它文章
- Capsaicin Regulates Mitochondrial Fission to Promote Melanoma Cell Apoptosis
- Oxyresveratrol-induced Activation of Nrf2/HO-1 Signaling Pathway Enhances Ability ofResveratrol to Inhibit UVB-induced Melanin
- mTOR-Dependent Autophagy Machinery Is Inhibited in Fibroblasts of Keloid
- Ablative Carbon Dioxide Laser Treatment for Papular Scars of Nose and Chin Due to Acne:A Case Series
- Scrofuloderma:A Rare Case Report of Sequelae of Intestinal Tuberculosis
- Treatment of Nevoid Basal Cell Carcinoma Syndrome by Surgery Combined With ALA-PDT:A Case Report

