Histone methyltransferase SDG8 in dehydration stress
Sun Xingwang, Chen Lan, Su Yanhua, Ding Yong, Zheng Han
School of Life Sciences, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230027, China
Abstract: The covalent modifications of histone in plants have changed dynamically during the development and adaption to dehydration stress. However, the histone modification enzymes involved in dehydration stress are mostly unknown. Here, we show that the SDG8, responsible for di- and tri-methylation of H3 lysine36, is involved in dehydration stress in Arabidopsis. The expression analysis shows that mutations in SDG8 result in altering a cluster of gene transcripts, including genes in salt, cold, and dehydration stress. Loss-of-function of SDG8 displays faster transpiration, larger stomatal apertures, less sensitivity to the ABA treatment, and decreased tolerance to dehydration stress. Together, our study suggests that SDG8 might be a novel factor involved in the dehydration stress process.
Keywords: dehydration stress; epigenetic regulation; histone methylation; SDG8
1 Introduction
As sessile organisms, plants need to adapt to environmental changes rapidly. Therefore, plants have developed a sophisticated and complicated system in responding to environmental stimuli. In addition to the transcriptional factor and proteins kinase, chromatin modifications, such as histone methylation, were involved instress[1-4]as well.
Histone lysine methylation occurs predominantly at lysine4, lysine9, lysine27, and lysine36 of H3. In general, Lys4 and Lys36 methylation of H3 are associated with the gene activation, whereas Lys9 and Lys27 methylation are associated with transcriptional gene silence. The dynamic changes in genome-wide histone H3 lysine 4 tri-methylation (H3K4me3) patterns in response to dehydration stress in Arabidopsis was observed[5].
ARABIDOPSIS TRITHORAX-RELATED 1 (ATX1) is responsible for tri-methylation of H3 lysine 4, and involved in different dehydration stress signaling stress response pathways, including ABA-dependent and ABA-independent pathways. Theatx1mutants under mannitol conditions exhibited reduced germination, larger stomatal aperture, and dehydration stress sensitivity. ATX1 binds toNCED3and increased Pol-II occupancy atNCED3to elevate H3K4me3 level at this locus. ATX1 modulates the gene expression in ABA-dependent and ABA-independent pathways, implicating ATX1 is involved in multiple dehydration stress response mechanisms inArabidopsisthaliana[6]. In addition to the histone methylation, H3K4 demethylase JMJ17 also functions in dehydration stress[7].
Histone methylations are crucial for plant develop-ment and abiotic stress response. Although H3K4me3 in dehydration stress was characterized, the response of tri-methylation of H3 lysine36 (H3K36me3) in dehydration stress is mostly unknown. SDG8 is originally identified as the enzyme responsible for di- and tri-methylation of H3 lysine 36. Loss of SDG8 function resulted in early flowering and reduced expression ofFLC[8]. SDG8 is also engaged in other biological processes, including ovule and anther development[9], seed development[10], carotenoid biosynthesis[11], branching[12], biotic stress response[13], as well as nitrate signal response[14].
Here, we report thatSDG8is involved in dehydra-tion stress. Based on the microarray data, we found that mutations inSDG8resulted in altering a cluster of gene transcripts, including genes in salt, cold, and dehydration stress. Physiological results showed thatsdg8mutants displayed faster transpiration, larger stomatal apertures, less sensitivity to ABA treatment, and decreased tolerance to dehydration stress. Our study suggests that SDG8 might be a novel factor involved in the dehydration stress process.
2 Materials and methods
2.1 Plant material and growing environment
TheArabidopsisthalianaecotype Col-0 plants were grown at 22°C under a long-day photoperiod (16-h-light/8-h-dark cycles)or a short-day photoperiod(8-h-light/16-h-dark cycles). The mutant strains obtained from the SALK collection were as follows:sdg8-2, SALK_026442;sdg8-4, SALK_036941.
2.2 Microarray data
The gene expression microarray of GSE109424 was downloaded from the Gene Expression Omnibus (GEO(1)https://www. ncbi. nlm. nih. gov/geo/.) of the National Center for Biotechnology Information (NCBI). Threesdg8samples and three Col-0 samples were obtained from GSE109424. RNA was isolated using TRIzol Reagent (Invitrogen) from 6-day-old seedlings cultivated at 22°C on 1/2 MS medium under the long-day photoperiod. The platform of this expression microarray was GPL198:[ATH1-121501] Affymetrix Arabidopsis ATH1 Genome Array. The differential expression genes(DEGs) insdg8compared with Col-0 were determined by using the limma package. Details are as follows:a series of matrix files were input and normalization was conducted by using the limma package (v3.4.4) in the R environment. The probes were converted into matched gene symbols according to annotation information. If multiple probes corresponding to a single gene, the value of gene expression was designated as the probes’mean. The adjustedp<0.05 and|log2fold change|≥0.585 were considered the cutoff values for DEGs screening.
2.3 GO enrichment analysis
A gene ontology analysis was performed using the enrichGO function of clusterProfiler packages[15].p. value<0.05 was considered to be statistically significant.
2.4 Detached leaf air dry assay
Col-0 andsdg8mutant seedlings from the same container under a short-day for 4 weeks. The fifth to seventh rosette leaves of Arabidopsis were selected and placed on weighed plates with a total weight of fresh leaves greater than 0.1 g and approximately the same number of leaves. The weight of water loss was recorded for different times (0 min, 15 min, 30 min, 60 min, 90 min, 120 min, 150 min, 180 min, and 210 min). Statistical data to calculate the rate of water loss at different times were plotted.
2.5 Mannitol treatment assay
The 5-day-old seedlings were transferred on 1/2 MS medium with or without mannitol. The seedlings were grown at 22℃ for 14 d, and the phenotype was then recorded.
2.6 Soil water deficits experiment
The Col-0 and thesdg8mutant were planted side by side in the same container under a short-day photoperiod. Plants (14-day-old) were grown with (Water) or without water (No water) for 10 d, followed by a 3-day watering recovery period (Rewater).
2.7 Measurement of stomatal apertures
The fifth to seventh rosette leaves of 4-week-old plants were selected. The leaves were placed in a stomatal opening buffer(20 mmol·L-1KCl, 1 mmol·L-1CaCl2, 2.5 mmol·L-1Mes-KOH) with or without the different concentration of ABA. After 2 hours of treatment with light, the epidermal cells were peeled, and stomatal aperture sized was examined with a microscope. The number of stomata and the size of stomatal closure were measured and counted. Fifty or more mature stomata of the fixed epidermal strips were examined in each experiment.
2.8 Total RNA extraction and real-time PCR
Total RNA was isolated from the leaf of 3-week-old seedlings with or without air dry treatment, and reverse transcribed with oligo(dT) primers. The amounts of individual genes were measured with gene-specific primers. Real-time PCR analysis was performed with the CFX real-time PCR instrument (Bio-Rad) and SYBR Green mixture (Vezyme). The relative expression of the genes was quantitated with the 2-ΔΔCTCt calculation, usingUBIQUITINas the reference housekeeping gene for the expression analyses.
3 Results
3.1 SDG8 modulates the transcription levels of stress-responsive genes
We first compared the transcriptome ofsdg8and the wild type from the GSE109424 dataset of the Gene Expression Omnibus (GEO) database. 474 differentially expressed genes (170 up-regulated and 304 down-regulated genes insdg8relative to Col-0) were identified insdg8(Figure 1(a), Table S1). These genes are shown in a hierarchical cluster (Figure 1(b)). Gene Ontology (GO) annotation and GO enrichment were performed by using the clusterProfiler package. The results revealed that the enrichment was overwhelmingly associated with responses to stimuli in biological process categories, including that response to oxidative stress (GO:0006979), defense response to fungus (GO:0009620), response to drug (GO:0042493), response to water deprivation (GO:0009415), response to cold (GO:0009409). Of the 474 differentially expressed genes, 20 were associated with cold stimulation, 20 with oxidative stress, and 7 with response to water deprivation (Table 1, Table S2). These results suggest that SDG8 might be particularly involved in abiotic stress-related genes.

Figure 1. (a) A volcano plot of significant genetic differences between Col-0 and sdg8. The x-axis represents the fold change, and the y-axis represents the p. value. The adjusted p. value<0.05 were considered to be statistically significant, and |log2FC|>0.585 was set as the threshold for identifying DEGs. Each point represents one gene. (b) Heatmaps of the DEGs between Col-0 and sdg8 in GSE109424.

Table 1. The significantly enriched analysis of differentially expressed genes.
We then analyzed the SDG8 interaction proteins using two public protein databases (BioGRID(2)https://thebiogrid. org/.and STRING(3)https://string-db. org/.). Eight candidate proteins were found (Figure S1(a)). Among these, ATX1(AT2G31650), DEK3(AT4G26630), SUMO1(AT4G26840), and SUMO2 (AT5G55160), were associated with abiotic stresses, such as salt, heat, and water deprivation (Figure S1(b)) (TAIR(4)https://www. arabidopsis. org/.).
3. 2 Mutations in SDG8 displayed super-sensitivity to the dehydration stress
The transcriptomic and protein interaction data suggested that SDG8 might regulate stress responses in Arabidopsis. The two T-DNA insertions were obtained, namely,sdg8-2andsdg8-4. The genotypic analysis revealed a T-DNA insertion in exon 2 and intron 5, respectively (Figure S2(a)). No full-length SDG8 transcripts were detected in thesdg8-2orsdg8-4mutants, indicating that both mutants are null alleles. Under the LD photoperiod conditions,sdg8showed an early-flowering phenomenon(Figure S2(b,c)). Then, we performed soil water deficit experiments. WT andsdg8grew well under adequate water conditions.sdg8, but not wild type, severely wilted, after 10 d without water treatment. The plants were re-watered for 3 d. Wild type survived over 70% compared with around 20% forsdg8, suggesting that SDG8 is sensitive to dehydration stress (Figure 2(a-c)). Subsequently, we performed a water loss assay with detached leaves. The wild type weighed 62% of fresh weight, whereassdg8leaves weighed around 50% of fresh weight with 210-minute treatment (Figure 2(d)).

Figure 2. (a)14-day-old seedings were grown for 10 d with or without water, followed by a 3-day watering recovery. (b,c)The survive percentage of Col-0 or sdg8 for 10 d under dehydration and re-watered for 3 d. Significant t-test differences are marked as **p<0.01, *p<0.05. (d) Water loss using detached leaves in air drying. The water loss ratio was calculated with a percentage change of real-time leaves weight with fresh leaves (FW). Representative experiments are shown and were performed at least three times.
We also use mannitol to mimic osmotic stress. 5-day seedlings were transferred to mannitol-containing plates and treated for 2 weeks. The wild type andsdg8plants grow well on 1/2 MS medium, and the growth of wild type andsdg8were retarded in 250 mmol·L-1mannitol plates. At this time, the leaves ofsdg8plants turned white and yellow relative to the wild type (Figure S3(a)). These results suggest thatsdg8is sensitive to osmotic stress. In general,sdg8exhibited sensitivity to osmotic stress, and this sensitivity can be explained, at least in part, by the faster transpiration ofsdg8leaves. Precise regulation of stomatal aperture in response to endogenous and environmental stimuli, such as hormones, and dehydration, is essential for plant growth and survival. The rapid transpiration indicates a possible malfunction in the regulation of stomata closure.

Figure 3. (a) The representative stomatal images of wild type and sdg8(scales:100 μm). (b) The stomata numbers of wild type and sdg8(unit field of view area is 0.15 mm2). (c) The average stomatal area of wild type and sdg8 (unit field of view area is 0.15 mm2). (d) The average width of stomatal openings of the wild type or sdg8 leaves at different concentrations of ABA(unit field of view area is 0.15 mm2). A representative experiment is shown and was performed at least three times (means were derived from 50 stomatal measurements). Significant t-test differences are marked as **p<0.01, *p<0.05.
3.3 Stomatal closure of sdg8 is insensitive to ABA
To further investigate SDG8 in dehydration, we performed a stomatal observations assay. Leaves in the same parts of wild-type andsdg8were obtained, and the stomata were observed (Figure 3(a)). The number of stomata of wild-type was 40, whereas the number of stomata ofsdg8-2was 31 and the number of stomata ofsdg8-4was 30 in the unit field of view area, suggesting that the stomatal density per unit area in the mutantsdg8was significantly reduced relative to the wild type(Figure 3(b)). However, the opening width and opening area insdg8are larger than those of WT (Figure 3(c,d)). The rapid closure of stomata was observed with ABA treatment in the wild type, but not insdg8, suggesting that SDG8 is involved in stomatal closure via ABA pathway (Figure 3(d), Figure S3(b)).
3.4 Loss of SDG8 function resulted in transcriptional level changes of response genes
To validate the transcriptional level changes insdg8mutants, we selected the six genes related to water deprivation stress (GO: 0009414) in analysis and examined their transcription levels at the dehydration stage (Table 1). Transcript levels of four genes, includingNCED3,GOLS2,COR413IM, andAAP4, were reduced in the water-well and dehydration stage, which are in consistent with microarray analysis. However, the other two genes, includingGRP7andWRKY70, were increased in both stages (Figure 4). Together, SDG8 might regulate dehydration stress response by modulating the transcriptional levels of genes related to the water deprivation stress response.
4 Discussion
In this study, we found that SDG8 is involved in dehydration stress. The sensitivity ofsdg8is partly due to the rapid water loss, larger stomata opening, and less ABA sensitivity. Our study suggests that SDG8 is involved in dehydration stress via stomata size and ABA pathway. The microarray analysis and RT-PCR showed that transcripts level ofNCED3,GOLS2,COR413IM,AAP4were down-regulated in thesdg8plant. These results are consistent with the observation in dehydration stress.
In addition, some water-deprivation genes associated with SDG8 were down-regulated, such asNCED3[16],GOLS2[17],COR413IM[18],AAP4[19]are considered to be key positive components of the plant tolerance to dehydration stress. RT-PCR results showed that the transcription of these genes was reduced to a normal and dehydration level .GRP7promotes stomatal opening and reduces tolerance under salt and dehydration stress conditions[20].WRKY70negatively regulates drought stress response[21]. RT-PCR results showed increased transcription levels ofGRP7andWRKY70genes(Figure 4). This is consistent with the drought-sensitive phenotype ofsdg8. These results suggest that SDG8 might regulate the dehydration stress response by regulating the transcriptional levels of genes related to the water deprivation stress response.
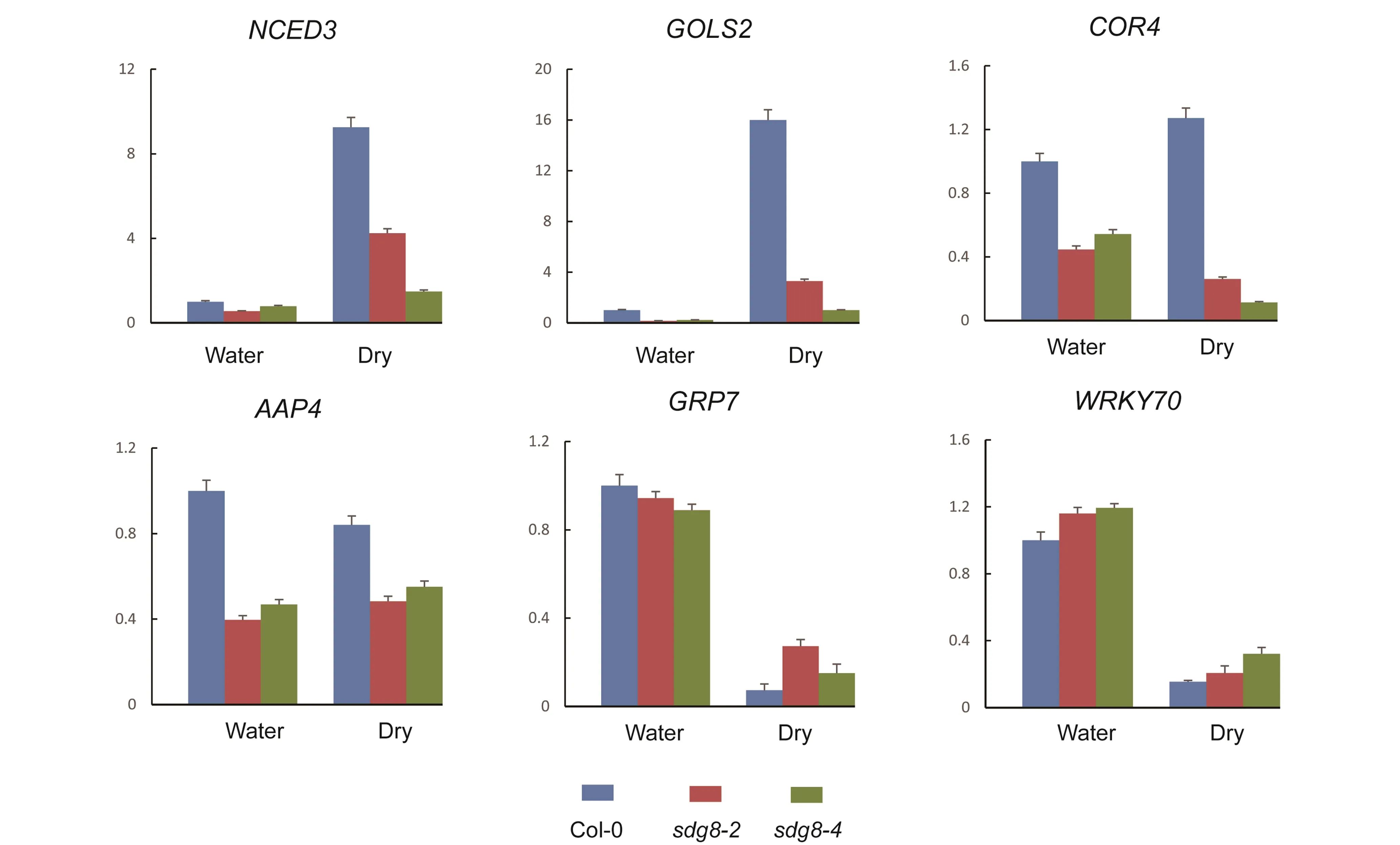
Figure 4. Transcript levels of genes are related to the water deprivation stress. Transcript levels were measured in non-stressed leaves (Water), air-dried for 1h (Dry) in Col-0 (blue), sdg8-2(red) or sdg8-4 (green) genotypes.
Although SDG8 is responsible for H3K36me3 modification, the relationship between down-regulated genes and the histone modification needs further study. Together, our study suggests that SDG8 might be a novel factor in the dehydration stress process.
Supplementary data
Supplementary data are available at J.Univ.Sci.Tech.China online.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: SDG8 (DQ340869.1).
Acknowledgement
This work was supported by the National Natural Science Foundation of China(32000241, 32000242), and the Fundamental Research Funds for the Central Universities (WK2070000178, WK2070000179).
Conflictofinterest
The authors declare no conflict of interest.
Authorinformation
SunXingwangis a MD student under the tutelage of Prof. Ding Yong at University of Science and Technology of China. His research interests are focused on the mechanisms of epigenetic regulation of drought stress in plants.
DingYong(corresponding author) is a professor and a PhD supervisor at University of Science and Technology of China. His research interests are in histone modifications to regulate plant growth and development.
ZhengHan(corresponding author) is currently a postdoctoral fellow at University of Science and Technology of China. He received his PhD degree under the tutelage of Prof. Ding Yong in 2019 from USTC. His research interests are in the epigenetic regulation of plant flowering.
- 中国科学技术大学学报的其它文章
- Three-dimensional array materials for electrocatalytic water splitting
- Synthesis of protected amines from N-hydroxyphthalimide esters via Curtius rearrangement
- LncRNA GIMA promotes hepatocarcinoma cell survival via inhibiting ATF4 under metabolic stress
- Effects of alanine-serine-cysteine transporter 2 on proliferation and invasion of hepatocellular carcinoma
- The asymptotic properties of least square estimators in the linear errors-in-variables regression model with φ-mixing errors
- Electrodialysis to concentrate high-salinity solutions: The matching relation between cation- and anion-exchange membranes

