Cost-Effective Hydrogen Oxidation Reaction Catalysts for Hydroxide Exchange Membrane Fuel Cells
Yanrong Xue , Xingdong Wang , Xiangqian Zhang , Jinjie Fang ,2, Zhiyuan Xu ,2, Yufeng Zhang ,Xuerui Liu ,2, Mengyuan Liu , Wei Zhu , Zhongbin Zhuang ,2,3,*
1 State Key Lab of Organic-Inorganic Composites, Beijing University of Chemical Technology, Beijing 100029, China.
2 Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China.
3 Beijing Key Laboratory of Energy Environmental Catalysis, Beijing University of Chemical Technology, Beijing 100029, China.
Abstract: Fuel cells are clean, efficient energy conversion devices that produce electricity from chemical energy stored within fuels. The development of fuel cells has significantly progressed over the past decades. Specifically, polymer electrolyte fuel cells, which are representative of proton exchange membrane fuel cells (PEMFCs),exhibit high efficiency, high power density, and quick start-up times.However, the high cost of PEMFCs, partially from the Pt-based catalysts they employ, hinders their diverse applicability. Hydroxide exchange membrane fuel cells (HEMFCs), which are also known as alkaline polymer electrolyte fuel cells (APEFCs), alkaline anionexchange membrane fuel cells (AAEMFCs), anion exchange membrane fuel cells (AEMFCs), or alkaline membrane fuel cells (AMFCs), have attracted much attention because of their capability to use non-Pt electrocatalysts and inexpensive bipolar plates. The HEMFCs are structurally similar to PEMFCs but they use a polymer electrolyte that conducts hydroxide ions, thus providing an alkaline environment. However, the relatively sluggish kinetics of the hydrogen oxidation reaction(HOR) inhibit the practical application of HEMFCs. The anode catalyst loading needed for HEMFCs to achieve high cell performance is larger than that required for other fuel cells, which substantially increases the cost of HEMFCs. Therefore,low-cost, highly active, and stable HOR catalysts in the alkaline condition are greatly desired. Here, we review the recent achievements in developing such HOR catalysts. First, plausible HOR mechanisms are explored and HOR activity descriptors are summarized. The HOR processes are mainly controlled by the binding energy between hydrogen and the catalysts, but they may also be influenced by OH adsorption, interfacial water adsorption, and the potential of zero (free)charge. Next, experimental methods used to elevate HOR activities are introduced, followed by HOR catalysts reported in the literature, including Pt-, Ir-, Pd-, Ru-, and Ni-based catalysts, among others. HEMFC performances when employing various anode catalysts are then summarized, where HOR catalysts with platinum-group metals exhibited the highest HEMFC performance. Although the Ni-based HOR catalyst activity was higher than those of other non-precious metalbased catalysts, they showed unsatisfactory performance in HEMFCs. We further analyzed HEMFC performances while considering anode catalyst cost, where we found that this cost can be reduced by using recently developed, non-Pt HOR catalysts, especially Ru-based catalysts. In fact, an HEMFC using a Ru-based HOR catalyst showed an anode catalyst cost-based performance similar to that of PEMFCs, making the HEMFC promising for use in practical applications. Finally,we proposed routes for developing future HOR catalysts for HEMFCs.
Key Words: Hydroxide exchange membrane fuel cell; Hydrogen oxidation reaction; Electrocatalyst; Platinumgroup metal; Cost
1 Introduction
The ever-increasing energy demands and serious environmental issues promote the development of efficient and clean renewable energy technologies over the past decades1-3.Among various new energy, hydrogen is considered as one of the most promising energy due to its abundant sources, high energy density, and zero pollution4-6. In the hydrogen economy, the excess renewable energy (solar, wind, and tidal energy, etc.) is stored in the form of hydrogen7,8. At the application terminal,the chemical energy stored in hydrogen can be directly converted into electricity by using fuel cells, which has the advantages of cleanliness and energy conversion efficiency9. The modern fuel cells employ solid electrolyte, which allows the fuel cells to compact and working under high current density, thus giving high power density. Great progress has been made for the proton exchange membrane fuel cells (PEMFCs), which has high efficiency, quick start-up time, low-temperature operation conditions and zero-emissions10,11. A high power density of more than 1 W·cm-2can be delivered by PEMFCs, making this type of solid electrolyte fuel cell highly promising12-14.However, the high cost for the PEMFCs, partially coming from the precious metal Pt catalysts, hindered their wide applications in the future hydrogen economy14-16. The alternative fuel cells with similar high performance, but with a lower cost are highly desired.
Hydroxide exchange membrane fuel cells (HEMFCs), which are also known as alkaline polymer electrolyte fuel cells(APEFCs), alkaline anion-exchange membrane fuel cells(AAEMFCs), anion exchange membrane fuel cells (AEMFCs)or alkaline membrane fuel cells (AMFCs), have the potential of using non-Pt electrocatalysts to reduce the cost, by switching from acid to alkaline environment17. The HEMFC has a similar structure to that of PEMFCs, except using the polymer electrolyte conducting hydroxide ions (Fig. 1). Although the intrinsic mobility of hydroxide ions is lower than that of protons,some highly conductive hydroxide exchange membranes(HEMs) have been developed. The stability of the HEMs also significantly improved over the recent decade18,19. HEMs contain polymer backbone and cationic functional groups, which are combined with appropriate ionomers to form the exchange channel for hydroxide in HEMFC20. According to the rational design of the unit structures, some reported HEMs shown high performance. Zhuang et al.21reported the polysulfone-based HEMs with double quaternary ammonium groups (DQAPS),which has advantages of high ion-exchange capacity, high chemical stability and low swelling degree. Bae et al.22successfully synthesized high-molecular-weight, solventprocessable HEMs based on poly(biphenyl alkylene)s (BPN1).The membranes showed high hydroxide ion conductivity (120 mS·cm-1) and alkaline stability at 80 °C. Varcoe et al.23used an organic-solvent-free method to synthesize poly(ethylene-cotetrafluoroethylene) (ETFE)-based radiation-grafted anionexchange membrane (RG-AEM), and it exhibited strong mechanical properties and high ion-exchange capacity (2.1 mmol·g-1). Yan et al.17reported HEMs and hydroxide exchange ionomers based on poly(aryl piperidinium) (PAP) consisting of a high-molecular-weight ether-bond-free, rigid and hydrophobic aryl backbone incorporating alkaline-stable piperidinium cations, which show sufficient ionic conductivity, mechanical robustness and chemical stability. And the HEMFCs employing the PAP HEMs can work at a temperature as high as 95 °C.
Owing to the development of the HEMs, the performances of the HEMFCs have been remarkably improved in these years.The well-optimized HEMFC shows an ultra-high peak power density (PPD) of 3.4 W·cm-2, making it competitive to the PEMFCs24. However, the current high performance HEMFCs still using the Pt or Pt alloy as the catalysts, facing the same cost issue as the PEMFCs25. Developing the Pt-free or low Pt HEMFCs is the key approach to promoting the HEMFCs.
The HEMFCs are composed of two half-cell reactions:hydrogen oxidation reaction (HOR) at anode and oxygen reduction reaction (ORR) at cathode3. Catalysts are required for both electrodes. Pt and its alloy are still the best catalysts for both HOR and ORR in base26. For the cathode, in which ORR occurs,some platinum-group-metal (PGM) free catalysts show the potential to replace the Pt, such as Ag, spinel and Fe-N-C type carbon-based materials. Especially, HEMFCs with a PPD higher than 1 W·cm-2have been reported using Ag and spinels as the PGM-free cathode catalysts. Varcoe et al.27reported the Ag/C based HEMFCs using their synthesized LDPE-based RG-AEM with a PPD of 0.85 W·cm-2at 80 °C. Recently, they developed a better AEM of high-density polyethylene-(HDPE)-based RGAEM, and the H2/O2HEMFC using Ag/C was elevated to 1.72 W·cm-2of PPD28. Zhuang et al.29reported the HEMFC employing the Mn-Co spinel cathode with a PPD of 1.1 W·cm-2at 60 °C. For the carbon-based ORR catalysts, they show high intrinsic activities in the RDE test. However, they face transport problems in the HEMFCs, because of the thick catalyst layer coming from the low density of the carbon based materials. Big progress has also been made for the HEMFCs using carbon based catalysts in these years. For example, Joo et al.30reported the HEMFCs using Fe-S-Phen/CNT as cathode catalyst exhibited a current density of 977 mA·cm-2at 0.6 V. Zhuang et al.31thought that the electronic conductivity of the Fe/N/C catalyst is a key factor for the cell performance, and the PPD of HEMFC with high conductivity Fe/N/C cathode (2 mg·cm-2) is over 450 mW·cm-2. These PGM-free ORR catalysts demonstrate the great potential to replace the Pt-based catalysts, thus reducing the cost of the cathode of the HEMFCs.
However, on the anode side, the situation is unlike. To achieve a high performance of the HEMFCs, it always requires a relatively high loading of Pt (a typical Pt loading of 0.4 mg·cm-2)on the anode, compared with the typical loading of 0.05 mg·cm-2used in the PEMFCs20,32,33. The reason is that the HOR activity of Pt drops approximately 100-fold when changing the electrolyte from acid to base34. The relatively sluggish HOR kinetics on Pt requires larger anode catalyst loading on HEMFCs to meet the performance of PEMFCs25. Thus, for the high performance HEMFCs using Pt as the catalysts, about half of the Pt is used in anode. Therefore, the development of high-activity and low-cost anode catalysts is of great significance to reduce the overall price of the HEMFCs.
In this review, we mainly summarize the progress of anode catalysts for HEMFCs. Firstly, we illustrated the plausible HOR catalytic mechanism proposed by researchers over the past decades, and discuss the factors that influent the HOR process in base. Then, we summarized the research progress of the HOR catalyst development on the basis of the elements. In the end, we summarized the HEMFC performance by using the various reported anode catalyst, and we also analyzed the HEMFC performances while considering anode catalyst cost. It was found that the anode cost for the HEMFC can be lowered to a similar level to that of PEMFCs by using the recently developed Pt-free HOR catalysts, especially the Ru-based catalysts,shedding light on the low cost HEMFCs.
2 Mechanism of the catalytic HOR process in base
The HOR process is a typical inner sphere catalytic process.The hydrogen molecules were adsorbed on the surface of the catalyst, transferred electrons, and then desorbed into the electrolyte. In a basic environment, the HOR reaction process is composed of two out of the three elementary steps(Tafel/Volmer or Heyrovsky/Volmer) described as follows35:
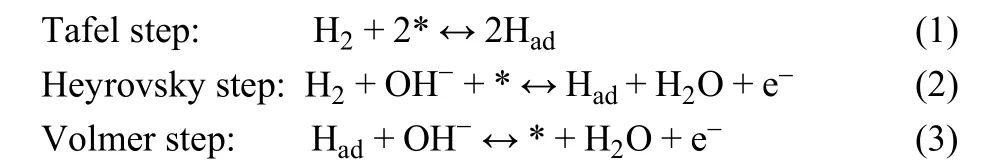
where Hadis adsorbed hydrogen, * is the active site of catalyst.
The HOR activity is significantly influent by the catalysts.The intermediate, Had, is found critical for the HOR. The hydrogen binding energy (HBE) is generally considered as the descriptor of the HOR activity on monometallic catalysts35-37.According to the Sabatier principle, the ideal catalysts should have HBE neither too strong nor too weak1. Trasatti first linked the exchange current for HOR/HER (HER is short for hydrogen evolution reaction, the reverse reaction for HOR) to the M―H bond strength, and a volcano relationship was obtained38. A similar volcano plot was also obtained by Nørskov et al.39, who obtained the HBE of various metal from the density functional theory (DFT) calculations. The volcano relationship to the DFT calculated HBE is also applied to the HOR/HER activity measured in base. Yan et al.40studied the HOR/HER activity of a series of metal in base, and a volcano relationship is showed versus the DFT calculated HBE.
Different from the HOR in acid, the OH-is a reactant for HOR in base. Several groups proposed that the adsorption of OH-also controls the kinetics of HOR in base. Markovic et al.41proposed that the HOR kinetics is limited by the Volmer step in base. The OH-in solution can absorb on the surface of the catalysts to generate the absorbed OH (OHad), and the OHadcan promote the desorption of Had. Thus the HOR in base is co-determined by the binding of the Had, as well as of the OHad. It explained the enhanced HOR activity of Pt by decorated with Ni(OH)2 adislands, which provides oxophilic sites for OHad. Koper et al.4drew a three-dimensional volcano curve to demonstrate this bifunctional mechanism, in which the activity for HOR/HER in base depends not only on the HBE, but also on the binding of OHad(the optimum binding of hydroxyl does not yet have a theoretical prediction). However, some groups found that the OH adsorption does not influent the kinetics of HOR in base significantly. Zhuang et al.42found that the enhanced HOR activity of PtRu to Pt is mainly due to the weakened HBE of PtRu. Zhuang et al.36found that PtNi and acid-washed PtNi(acid-PtNi) have much different OH adsorption ability, but similar HOR activity in base.
It was found that the HOR activity of Pt in acid is about two orders magnified than that in base35. This phenomenon is also applied to the other PGMs, such as Ir Pd and Rh43. Several groups tried to understand the reason for the lower HOR activity in base. One argument is the HOR in base is still controlled by the HBE, but the HBE of the metals is adjusted by the electrolyte.However, the influence of the electrolyte is generally neglected in the DFT calculation. Yan et al.44connected the HBE to the H under-potential deposition (Hupd) peak position (Eq. (4)), which can be obtained by the cyclic voltammogram in Ar-saturated electrolyte.
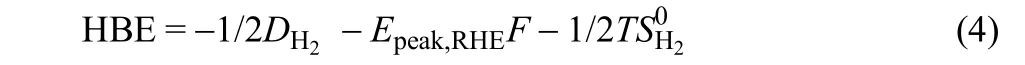
where DH2is the dissociation energy of hydrogen molecule(DH2= 436 kJ·mol-1), Epeak,RHEis the peak potential obtained from cyclic voltammograms, F is the Faraday’s constant, T is the temperature in Kelvin,is the entropy of H2in the gas phase at standard conditions (= 130.684 J·mol-1·K-1).
The measured HBE represents the metal-hydrogen interactions include the influence of the electrolyte. Yan et al.45found that the measured HBE of Pt increase with the increase of the pH of the electrolyte. In base, the HBE of Pt deviated from the ideal case, leading to lower HOR activity. The tuning of the HBE by pH was also found on various carbon supported PGMs,such as Pt/C, Ir/C, Pd/C, and Rh/C.
To further understand the origin of the tuning HBE by pH,Yan et al.46further proposed the apparent hydrogen binding energy (HBEapp). The HOR is an electrochemical process, which occurs in the presence of the electrolyte. The catalysts and the ions are covered by the solvent molecules, i.e. in the solvation forms. For the Volmer step, which is the desorption of Had, thus solvation of the catalysts and the protons also occurs simultaneously. Thus, for the HBEapp, it consists of two terms,which are the intrinsic HBE and water adsorption energy (Eq.(5)).whereandare the Gibbs free energy of hydrogen and water adsorption,andare the entropy of H2and H2O at standard conditions which are constant, T is the temperature in Kelvin. Yan et al.46proposed that theis an intrinsic property of metal and it is independent of pH.However, the water adsorbs on the metal surface weaklier in base than in acid, leading to a decrease of HBEappwith the rising of pH.

The stronger water adsorption in acid than in base is attested by Goddard et al.47from a theoretical calculation using a full solvent in situ quantum mechanics molecular dynamics(QMMD). The H2O/Pt(100) interface is closer in the acid,demonstrating the stronger H2O/Pt(100) interaction. The simulated HBEappincrease 0.13 eV from pH 0.2 to 12.8 with a slope of 10 meV·pH-1, which is close to the experimental result of 8 to 12 meV·pH-1, indicating the changes of water adsorption energy are the major factor for the pH-dependency of the HBEapp. Recently, Shao et al.48observed pH-dependent hydrogen and water binding on Pt surfaces by using in situ attenuated total reflection surface-enhanced infrared absorption spectroscopy. A larger Stark tuning effect for the peaks associated with water adsorption is observed in acid, proved the stronger Pt-H2O interaction. But surprisingly, the Pt-H interaction was also stronger in acid than in base. The overall HBEappis still weaker in acid, because of the dominated influence of water adsorption.
Koper et al.49also recognized the important role of the interfacial water on the kinetics of HER. However, they consider the impact of water by the reorganization in kinetics. The reorganization of interfacial water accommodates the charge transfer through the electric double layer, and the energetics of which are controlled by how strongly water interacts with the interfacial field. The strength of the interfacial electric field is determined by the distance of the potential of zero (free) charge(pzfc) from the actual electrode potential. In alkaline media,where the potential of H-UPD and HER occurs far from the pzfc,the interfacial water network interacts strongly with the strong interfacial electric field and is therefore more rigid and more difficult to reorganize during the charge transfer through the electrical double layer49. This effect makes slow kinetics of both hydrogen adsorption and hydrogen evolution. e.g. the Tafel plots for HER on Pt(111) increases from 37 mV·dec-1to 116 mV·dec-1from acidic (pH = 1) to alkaline electrolyte (pH = 13).
3 Measurement of the HOR activity
The intrinsic HOR/HER activity of the electrocatalyst can be obtained using rotating disk electrode (RDE) measurements in a standard three-electrode system. Well-defined and also enhanced mass transport characteristics can be obtained by the RDE method. KOH or NaOH are generally used to mimic the basic conditions provided by the HEMs. The polarization curves of catalysts were obtained in H2-saturated electrolyte after applying overpotential. The measured overall current (i) is composed of the diffusional current (id) and the kinetic current (ik), which can be described by the Koutecky-Levich equation (Eq. (6))35:

Assuming infinitely fast reaction kinetics for ideal reversibility of the HOR/HER, the idis obtained by Nernstian diffusional overpotential (ηd) (Eq. (7)):

where R is the universal gas constant, T is the temperature in Kelvin, F is Faraday’s constant. il is the HOR limiting current which is determined by the Levich equation (Eq. (8)):
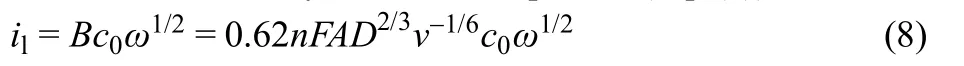
where n is the exchange electron numbers (n = 2 for the oxidation/evolution of one H2molecule); F is the Faraday’s constant; A is the available electrode area; D, c0and v are the hydrogen diffusion coefficient, hydrogen concentration and kinematic viscosity in the electrolyte, respectively.
The HOR/HER exchange current (i0) can be obtained by fitting the HOR/ HER kinetic current (ik) with the Butler-Volmer equation (Eq. (9)):

where η is the overpotential, R is the universal gas constant, F is the Faraday’s constant, T is the temperature in Kelvin. αaand αcare the transfer coefficients for HOR and HER, respectively.When the HOR/HER reaction pathway follows Tafel-Volmer mechanism where Volmer is the rate-determining step (RDS),there is only one electron transfer for a single pass making the αa + αc = 1. Besides, when the HOR/HER reaction pathway follows Heyrovsky-Volmer mechanism with Volmer remaining the RDS, the αawas replaced to 1 + αabecause of the multistep processes kinetics50,51.
The i0can also be obtained from the micro-polarization region(i.e., from -5 to 5 mV, the idcan be negligible and i can represent ik.). The Butler-Volmer equation can be simplified through expanded by Taylor’s formula (Eq. (10)).

by using this method, the i0can be obtained without knowing the id, which is preferred when the limiting current density is difficult to be obtained.
More attention is required for a special case discussed below.When the HOR catalysts have ultra-high HOR activity, which is commonly appeared for testing the PGMs in acidic media, the polarization curves approach to the id, making the i0 was generally underestimated. To get reliable data, a lower reaction rate is required, which can be realized by reducing the catalyst loadings. However, the catalysts can not uniformly be dispersed on the electrode when using ultra-low loadings, and catalyst agglomerates forms. Zhuang et al.7proposed an approach to measure the i0 of the catalyst accurately, which is to dilute the catalyst with a large amount of pristine carbon powder. The catalysts were uniformly dispersed in excessive pristine carbon particles, giving much enhanced mass transport conditions.
To evaluate the activity of the catalysts for HOR, specific activities or mass activities are generally applied. The HOR/HER i0 or the ik at a certain potential (e.g. 50 mV) is usually investigated. The specific activities are the current normalized by the electrochemical surface area (ECSA) of the catalysts and the mass activities are obtained by dividing the current by the mass of the catalysts on the electrode.
A more direct way to evaluate the performance of the catalysts under working conditions is testing it by assembling to HEMFCs. Although the HEMFCs performances account for the factor of anode catalysts, cathode catalysts, membranes and ionomers, they represent how much could be achieved by the catalysts in the real working condition. The current density at 0.6-0.8 V of cell voltage (typical operating potential of fuel cell for automotive applications) and the PPD of the HEMFCs are used to evaluate the catalyst and the mass transport capacity of the membrane electrode assembly (MEA), respectively52,53. The stability is another essential property for catalysts, which operated at a constant current density for HEMFCs.
4 The reported HOR catalysts
4.1 Pt-based catalysts
Although the HOR activity of Pt is two orders of magnitude lower in base than that in acid, Pt is still the best monometallic HOR catalyst in base50. The HOR activity of Pt can be further tuned by the size, shape, and exposed crystallographic plane of the Pt nanoparticle. Making alloy can also improve the HOR activity of Pt, and lower the Pt content.
Satsuma et al54. modified the Pt nanoparticles with fifteen metals (Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zr, Nb, Mo, Ru, Rh, Pd,and Ag), and they found most of the Pt alloys have the HOR activity higher than the pristine Pt. They attributed the enhanced HOR activity to the surface oxygen species of the modifying metals. Wong et al.55synthesized ultrathin Pt nanowires (NWs)which gave a better HOR performance compared with commercial Pt nanoparticles. They further alloyed the Pt NWs with other metals (i.e. Fe, Co, Ru, Cu, Au), and the activities of PtM alloy NWs were further improved. Furthermore, The order of HOR activity in alkaline solution increased in the sequence(111) ≈ (100) ≪ (110) on Pt(hkl) surfaces56. The activity differences arose mainly from the structure sensitivity of HUPDand OHadwith respect to the Pt crystal plane. Zhuang et al.36reported that the exchange current density of PtNi alloy (1.61 mA·cm-2) ca. 3 times higher than that of Pt/C (0.49 mA·cm-2) in base. The additional Ni tuned the HBE of Pt thus enhanced the HOR activity. Liu et al.57reported a PtRh nanoalloy aerogel(NAA) with unique lamellar architecture, hierarchical pores and abundant low-coordinated sites. The PtRh NAA exhibited a high HOR specific activity of 1.25 mA·cm-2at 50 mV, which was 5.5 times higher than that of the Pt/C (0.225 mA·cm-2) in 0.1 mol·L-1KOH electrolyte.
Other than the Pt alloys, core-shell structure with a Pt shell can reduce the amount of Pt and sometimes have enhanced activity. Yan et al.58reported the Pt-coated Cu nanowires (Pt/Cu NWs) with 100 nm of diameter and 25-40 μm of length, which are synthesized by the partial Galvanic displacement of Cu NWs.The mass exchange current density of Pt/Cu NWs is 1.9 times higher than that of Pt/C, and the improved HOR performance was attributed to the electronic tuning effect by the Cu substrate and Cu impurities in the Pt shell. Wang et al.59reported the high HOR activity of the Pt skin on structurally ordered intermetallic PdFe/C nanoparticles (O-PdFe@Pt/C), and the weakened hydrogen binding on the Pt shell can be ascribed to the compressively strained atomic layer of Pt induced by the ordered PdFe core.
Among the various Pt-based bimetal or multi-metal HOR catalysts, PtRu is the most popular one used for HEMFCs,because of its high HOR activity and stability43,60. Zhuang et al.42firstly reported the HEMFCs using PtRu as the anode, and a PPD of more than 1 W·cm-2was obtained at 60 °C, which broke the record at that time. By using a better HEM, Yan et al.61reported the HEMFCs with PtRu/C catalyst in anode and Pt/C catalyst in cathode achieved a PPD of 1.89 W·cm-2in H2/O2and 1.31 W·cm-2in H2/air (CO2-free) at 95 °C.
The reason for the high activity of PtRu has been discussed.Ru is considered to regulate the electronic structure of Pt to weaken the Pt-H interaction or to adsorb hydroxyl species to promote the oxidation of Had. Zhuang et al.42considered the weakened HBE on PtRu is the main factor. The DFT calculations show that the adsorption energies of H on Pt(111) and Pt3Ru(111) are -0.33 and -0.19 eV, respectively. However, Jia et al.62provided the experimental evidence for the presence of OHadon the surface Ru sites in the HOR potential region by using in situ XAS technique, and they concluded that the OHadpromoted the oxidation of Had for both PtRu/C and Ru/C. But recently, Yan et al.51proposed that surface Ru is not necessary for the HOR activity of PtRu, and the improved PtRu activity comes from the electronic interaction of sub-surface Ru with Pt,which can change the pathway from Tafel-Volmer to Heyrovsky-Volmer, and reduce the activation barrier of the Volmer step. The detailed mechanism for the HOR on PtRu is still not clear.
To maximize the HOR performance of the PtRu catalyst in HEMFCs, it requires that the ionomer has a minimum poisoning effect on the catalysts. The phenyl groups that existed in the ionomers may adsorb on electrocatalysts and inhibits the HOR performance. Bae and Kim et al.20designed an alkylammonium functionalized poly(fluorene) ionomer, which has non-rotatable phenyl group and leads to less adsorption on the catalyst. Higher HEMFCs performance was obtained by employing this rational designed ionomer.
The Pt-based catalysts are still the most used anode catalysts for HEMFCs. Coordinate with additional metal, the HOR activity can be enhanced. Higher HEMFC performance can be obtained by using these composite catalysts. However, a recent report shows that the Pt/C anode can be as good as the PtRu/C anode for HEMFCs operated at elevated temperatures (Fig. 2)52.Such an observation attribute to the apparent activation energy(Ea) of the HOR on Pt (35.2 kJ·mol-1) is greater than that on PtRu(14.6 kJ·mol-1). Thus, the development of Pt-free HOR catalyst is more urgent.

Fig. 2 The H2/O2 HEMFC performances by using Pt/C or Pt-Ru/C as anode catalyst (0.4 mg·cm-2) and Pt/C as cathode catalyst (0.4 mg·cm-2).The cell temperature is 80 °C, and the backpressure is 200 kPa 52.
4.2 Ir-based catalysts
Ir also has high HOR activity in base. Markovic et al.41reported that Ir has the highest HOR in monometallic catalyst,which is even higher than that of Pt, because Ir is more oxophilic than Pt and Pd. However, Gasteriger et al.63argued the HOR activity of Ir is only one-third and one-fifth to that for Pt in 0.1 mol·L-1NaOH and PEMFC, respectively. Although it is lower than that of Pt, Ir is still highly active compared with other metals. Chialvo et al.64reported the exchange current density of Ir is 0.28 mA·cm-2in 0.1 mol·L-1NaOH, and the HOR reaction takes place mainly through the Tafel-Volmer pathway with a small contribution of the Heyrovsky-Volmer pathway.Compared to acidic conditions, they thought OH-is more difficult than H+to achieve the appropriate spatial configuration in alkaline solution to enable the electron transference in the constrains of the superficial water network. The increased activation energies of the Heyrovsky and Volmer steps explained the lower HOR activity observed in alkaline solutions to that in acid solutions.
The HOR activity of Ir is influent by the particle sizes and the surface structures. Yan et al.44reported that the particle size(from 3 to 12 nm) effect of HOR/HER on Ir/C. The specific HOR/HER activities decrease as particle size decreases, because the portion of the sites with the lowest HBE increases with the decrease of the total electrochemical active surface area (i.e., the increase of the particle size). Luo et al.65successfully synthesized ultrafine worm-like Ir-oriented nanocrystalline assemblies (ONAs). Due to the long 1D nanostructure of Ir ONAs with low-index crystalline planes is beneficial for fast mass/charge transfer rate, the mass activity and specific activity of Ir ONAs is 0.080 mA·mg-1and 0.518 mA·cm-2in H2-saturated 0.1 mol·L-1KOH, which are higher than those of Ir NPs (0.066 mA·mg-1and 0.399 mA·cm-2), respectively.
The Ir can alloy with the other PGMs to achieve higher HOR activity. For example, the IrPd alloy showed higher HOR activity than that of Pt. Brett et al.66reported that the PdIr/C catalyst with 10 nm average size has an increase in exchange current density of over 80% compared with the commercial Pt/C catalyst. Song et al.67reported that the mass specific exchange current density of Pd0.33Ir0.67/N-C is 1.4 times that of commercial Pt/C, and the HEMFC using Pd0.33Ir0.67/N-C as the anode approached a PPD of 514 mW·cm-2, which is 1.3 times that of commercial Pt/C (Fig. 3). The IrRu alloy is also promising.Satsuma et al.68synthesized the carbon supported Ru-Ir catalyst and the HOR specific activity of Ru-Ir alloy was ca. 4 times higher than that of Pt/C. The HBE of the catalysts is experimentally evaluated from the Hupd on the metal surface, and the results showed that the HBE of Ru-Ir alloy is lower than that of Ir and close to that of PtRu. Shao et al.69reported that the HOR mass and specific activities at 50 mV of IrRu ultrathin NWs are 4.2 and 3.8 times that of commercial Pt/C, respectively.After 2000 cycles of accelerated durability tests under Ar conditions, the ECSA of IrRu NWs catalyst dropped 2.27%,which is less than the big decrease of 53.89% for commercial Pt/C. Furthermore, the HEMFC using IrRu NWs as the anode showed a PPD of more than 485 mW·cm-2, which is ca. 1.7 times than the PPD of the HEMFC using commercial Pt/C as the anode catalyst (292 mW·cm-2). Abruña et al.70synthesized the Ir3Pd1Ru6/C that has superior HOR activity and long-term stability than Pt/C and Ir/C in base, which is promising anode catalysts for HEMFCs. Furthermore, Zitoun et al.71reported that the excellent H2/O2HEMFCs using RuPdIr/C as anode and Agbased materials as cathode attained a PPD close to 1 W·cm-2.

Fig. 3 Left: the H2/O2 HEMFC performances by usingPd0.33Ir0.67/N-C or commercial Pt/C as anode catalyst (0.2 mg·cm-2) and Pt/C as cathode catalyst (0.3 mg·cm-2). The cell temperatures are 65 or 79 °C as marked. Right: the comparison of the PPD of the HEMFCs by using different anode catalysts 67.
Modification of Ir by non-precious metals can also improve its HOR performance. Shao et al.72prepared IrNi@PdIr/C catalyst via a Galvanic replacement reaction. The HOR mass and specific activities at 50 mV of IrNi@PdIr/C are 2.1 and 2.2 times that of commercial Pt/C in 0.1 mol·L-1KOH, respectively, and the PPD of HEMFCs with IrNi@PdIr/C as anode catalyst is 311 mW·cm-2. The remarkable HOR activity is attributed to the weakened HBE of the PdIr overlayers induced by the IrNi core.Zhuang et al.73synthesized IrNi@Ir core-shell HOR catalyst by a simple one-pot approach. Due to the fine-tuned nanostructure of the catalyst, the HOR mass activity of IrNi@Ir/C is 1.12 mA·mg-1at 0.05 V in 0.1 mol·L-1KOH, which is 2.4 times that of Ir/C. The metal oxides were found beneficial for the HOR activity of Ir. The oxygen vacancy on the surface of the CeO2can adsorb hydroxyl species to enhance HOR catalytic performance in base, and exchange current density, mass activity and specific activity of 10% Ir/CeO2-C are 2.4, 2.8 and 1.8 times that of 10%Ir/C, respectively74.
4.3 Pd-based catalysts
Pd has very similar electronic properties to that of Pt.However, the HOR activity of Pd is only about 1/20 to that of Pt63.One possible reason is that the absorbed hydrogen goes into the bulk lattice of massive Pd75. The hydride phase transition of Pd was observed with the change of the overpotentials (η) for HOR.The α-Pd/H (a solid solution with a low hydrogen concentration)is stable when η > 0.1 V, and β-Pd/H (a hydride form with a high hydrogen concentration) is found at η < 0.04 V. The decrease of the coverage of Hadfrom the β to α phase lead to the α-Pd/H have lower HOR activity than that of the β-Pd/H. Thus decreased HOR activity is observed at high overpotentials76,77.
Gasteiger et al.63proposed that the Volmer step is the ratedetermining step on Pd electrodes. Yan et al.78investigated the particle size effect of HOR/HER on Pd catalysts with different particle sizes ranging from 3 to 42 nm in both acid and base. The HOR/HER specific exchange current density increased as Pd particle size increases from 3 to 19 nm, and then reached a plateau with activity similar to that of bulk Pd. The enhanced HOR activities with arising particle sizes of Pd was ascribed to the increased ratio of the sites with weaker HBE, which is revealed in cyclic voltammograms (CVs). It also suggested the Volmer step is the rate-determining step.
Weakening the binding of H to Pd may enhance the HOR activity. The weakened HBE can be achieved by deposition of Pd on different substrates. Kolb et al.79calculated the ΔGHadof pseudomorphic Pd overlayers on the close-packed surfaces of Au, Pt, PtRu, Rh, Ir, Ru, and Re transition metal substrates with their exchange current density. They found volcano relationship,and Pd/PtRu resided near the peak of the curve with ΔGHad≈ 0.Yan et al.80synthesized Pd nanotubes (NTs) by Galvanic displacement of Cu NWs, and the HOR specific activity of Pd NTs was 20 times greater than that of Pd nanoparticles and about 80% higher than that of polycrystalline Pd. And the mass exchange current density of Pd coated Cu NWs is 7 times greater than that of the Pd nanoparticles.
Alloys were also found to have promoted HOR activity.Bimetallic Pd/Ni HOR catalysts have been studies to outperform pristine Pd in base, due to the presence of OHadspecies on the surface of the oxophilic Ni component together with the hydrogen binding Pd sites81-83. Alesker et al.84tested the HEMFCs based on Pd/Ni nanocomposite as anode catalyst showed a PPD of 400 mW·cm-2, more than twice that obtained using a Pd monometallic catalyst.
The HOR activity of Pd can be improved by the oxides,especially CeO2. CeO2is one of the most oxygen-deficient compounds, which could rapidly saturation with OH-in alkaline media and spillover of OH-to the supported metal nanoparticles85.Dekel et al.86employ a mixed carbon-CeO2supported Pd as HEMFC anode catalyst that showed a PPD of more than 500 mW·cm-2for H2/Air cell. Mustain et al.87reported that the HEMFCs (Pd-CeO2/C as anode and PdCu/C alloy as cathode)reach a PPD of 1 W·cm-2, which could operate continuously for more than 100 h at a constant current of 0.5 A·cm-2, and the voltage degradation rate is only 2.5 mV·h-1. Recently, Vizza et al.88synthesized a Pd-CeO2/C catalyst with engineered Pd-to-CeO2interfacial contact. The high HOR activity of Pd-CeO2/C catalyst lea to a 1.4 W·cm-2of PPD in H2/O2HEMFC test (Fig.4). DFT calculations suggested the optimized Pd-CeO2interfacial contact was a benefit to weaken the HBE through the interaction of Pd atoms with the oxygen atoms of CeO2.Furthermore, other materials (i.e. PdP2, WC/Pd and Pd-CNx)also exhibited high HOR activity89-91.

Fig. 4 The H2/O2 HEMFC performances by using Pd-CeO2/C as anode catalyst (0.25 mg·cm-2) and Pt/C cathode (0.4 mg·cm-2). The cell temperature is 80 °C 88. The black and red curves represent the anode Pd-CeO2/C catalysts synthesized by different methods.
4.4 Ru-based catalysts
Ru is generally used as the additive to promote the HOR activities of the PGMs, such as PtRu and IrRu. For example,Papandrew et al.92reported the high-surface-area Ru-based RuxMy(M = Pt or Pd) alloy catalysts. The HOR exchange current density of Ru0.20Pt0.80and Ru0.20Pd0.80catalysts were 1.42 and 0.148 mA·cm-2, respectively, which were nearly 3 times that of the Pt (0.49 mA·cm-2) or Pd (0.05 mA·cm-2).
Recently, Ru itself showed promising HOR activity and gained more attention due to the advantages of Ru in terms of price and element abundances. The HOR kinetics is sensitive to the structure of Ru. Adzic et al.93reported that the exchange current densities are 0.13 and 0.16 mA·cm-2for Ru(0001) and Ru(10¯ 0) in 0.05 mol·L-1H2SO4at 40 °C, respectively.Furthermore, the apparent electrochemical activation energy for the surface of Ru(0001) is about 120 kJ·mol-1, and that for the Ru(10¯0) surface is 80 kJ·mol-1. On Ru(0001), Hadcompeted with OHadfor adsorption Ru sites. In the positive-going scan, the more stable OHadwill tend to displace Hadat E > 0.1 V in 0.1 mol·L-1HClO4. However, a sufficiently high H2concentration would raise the chemical potential that the gained adsorption free energy would be sufficient to thermodynamically displace OHadby Hadat 0.1 V< E < 0.2 V. Thus, the HOR current for Ru increased with positive-going sweeps up to around 0.2 V and then decreased94. Chialvo et al.95calculated the corresponding set of kinetic parameters from the proposed Ru HOR process and kinetic model, and verified the reversible electroadsorption of OHadon active sites. They thought the mechanism of monometallic Ru electrodes via a Heyrovsky-Volmer pathway is more important than Tafel-Volmer in 0.5 mol·L-1H2SO4. The facet dependency towards HER was also investigated by Liu et al.96The found the HER activity of Ru follows the order of hcp(100) > hcp (002) > hcp (101) > fcc (111).
The size-dependent HOR activity of Ru nanoparticles showed volcano shaped with maximum activity at approximately 3 nm.The Ru NPs structure changed from amorphous-like structure below 3 nm to metal nanocrystallite with roughened surface at ca. 3 nm, and then displays well-defined facets above 3 nm97.Furthermore, 3 nm Ru NPs as anode represented higher HEMFC PPD of 250 mW·cm-2than the Pt/C, Rh/C, Pd/C, and Ag/C catalyst98. Zhuang et al.99designed Ru support carbon (Meso C) with mesoporous ravine-like channel as anode achieves superior HEMFCs performance with a PPD of 1.02 W·cm-2. The higher performance of Ru/Meso C than Ru/C is attributed to the mesoporous microstructure in Ru/Meso C which enables the formation of an inner hydrophobic surface and prevents the oxidation of the metallic Ru NPs, facilitating the oxidation of hydrogen.
The Ru alloy shows enhanced HOR activity than the pristine Ru. Recently, Zhuang et al.100reported a highly active Ru7Ni3/C catalyst. The HOR mass activity and specific activity of Ru7Ni3/C is ca. 21 and 25 times that of Pt/C, and 3 and 5 times that of PtRu/C, respectively. The HEMFC using Ru7Ni3/C as anode catalyst can deliver a high PPD of 2.03 W·cm-2in H2/O2and 1.23 W·cm-2in H2/air (CO2-free) at 95 °C, surpassing that using PtRu/C anode catalyst, and good durability with only about 4.4% voltage loss over 100 h of operation at a constant current density of 0.5 A·cm-2under H2/air (CO2-free) (Fig. 5).The electrochemical in situ ATR-SEIRAS results suggested that the high HOR activity of Ru7Ni3/C from the alloying effect of the additional Ni in the interior of the catalyst nanoparticles, and the enhanced water adsorption by the presence of surface Ni oxides.

Fig. 5 The H2/O2 and H2/air (CO2-free) HEMFC performances by using Ru7Ni3/C or PtRu/C as anode catalyst (0.2 mg·cm-2) and Pt/C as cathode catalyst (0.4 mg·cm-2). The cell temperature is 95 °C 100.
4.5 Nickel-based catalysts
Ni-based materials are the most promising non-PGM HOR electrocatalysts in base. Leger et al.101. investigated the HOR electrocatalytic behavior of low-index nickel single-crystal electrodes that is related to the crystallographic structure of the electrode surface and the pH of the electrolyte. The Ni(100) and Ni(110) are 5 to 6 times active than polycrystalline nickel, which is close to that of Ni(111). For the Ni(100) and Ni(110) planes,the current densities increase as the pH and the maximum current densities appear at pH 13. Although the Ea of Ni in acid is lower than that in base (i.e. the Eais 50 kJ·mol-1at pH 9, while it is 40.2 kJ·mol-1at pH 4.8), the dissolution of Ni causes its deactivation under acidic condition. Yan et al.40calculated the HBE of (111) plane for fcc Ni is -0.51 eV, and the measured exchange current density is ca. 0.008 mA·cm-2in 0.1 mol·L-1KOH.
Raney Ni has been widely used in hydrogenation reactions.The Raney Ni-based catalysts show promising HOR activity and have been used as the anode catalyst in the alkaline fuel cells(AFC)43. Modification on the Raney Ni can further improve its HOR activity. The Raney Ni electrodes with carbon black showed higher HOR performance than bare Raney Ni electrode.The improved HOR activity of Raney Ni electrode was caused by the increase of electrode conductivity and the enhancement of gas and electrolyte diffusivity by the addition of the highly porous carbon black102. Wendt et al.103fabricated the electrode using Raney Ni with copper oxide for enhancing electronic conductivity and also introducing PTFE as a hydrophobic binder.It showed long durability of approximately 5000 h with 100 mA·cm-2at 50 °C. Alloying can promote the HOR activity of Raney Ni. Nissinen et al.104investigated the effect of additional transition metals on the HOR performance of Raney nickel, and the effect of the doped metals follows the sequence of Cr > La >Ti > Cu > Fe. Surface analysis of the electrodes after electrochemical tests have shown that the enrichment and aggregation of the transition metals (especially for the Cr and Ti)on the Raney Ni surface affect its activity and stability. However,Raney Ni is commonly used in AFC under high alkalinity (6 mol·L-1KOH), which can not work well for HEMFC (the alkalinity can be mimicked as 0.1-1 mol·L-1KOH).Furthermore, the thick gas diffusion electrodes (GDEs) of Raney Ni based catalysts are inapplicable for HEMFCs with a delicate MEA structure43.
Supported Ni based nanoparticles, which have higher surface areas, have been also reported for the HOR catalyst in base. Yan et al.105reported the HEMFC with a Ni/C anode and the Ag cathode, showed 1.06 V open-circuit voltage and 76 mW·cm-2PPD (at 0.68 V) (at 70 °C and under 250 kPa backpressure).Doping Ni nanoparticles with transition metals can also enhance their HOR activity, because of the tuning effect on the HBE of Ni. In order to predict the feasibility of the Ni-alloy for HOR,Dekel et al.106calculate the HBE and hydroxide binding energy values for bare Ni and binary Ni3TM1, and they found that Ni3Fe1is the most promising HOR catalyst in base. Highly dispersed supported Ni-based catalysts as anode for HEMFCs have been reported over the past decade. Zhuang et al.107employed W-doped Ni and Co-polypyrrole as the anode and cathode catalyst,and the H2/O2and H2/air HEMFCs are shown a decent performance with PPD being 40 and 27.5 mW·cm-2at 60 °C,respectively. Serov et al.108reported that the HBE of Ni modified by Mo can reduce ca. 0.14 eV, and the H2/O2 HEMFC using NiMo/KB as anode and Pd/C as cathode can output high PPD of 120 mW·cm-2at 0.5 V, and the MEA could hold a constant potential at 0.7 V for 115 h. Atanassov et al.109reported that HEMFCs using the nickel-rich Ni95Cu5-alloy as anode and using Pd/C as cathode, demonstrated the PPD of 350 mW·cm-2at 80 °C, which set a technical record for the PGM-free anode in HEMFC (Fig. 6).

Fig. 6 The H2/O2 HEMFC performance by using NiCu/KB as anode catalyst (4 mg·cm-2) and Pd/C as cathode catalyst (0.2 mg·cm-2).The cell temperatures are 60, 70, 80 °C, respectively 109.
Modifying the Ni based catalysts by non-metallic elements,especially N, also shows the benefits. The modification can be done either on the support or Ni itself. Yan et al.110using nitrogen-doped carbon nanotubes (N-CNT) as the support and the mass activity and exchange current density of Ni/N-CNT increases by a factor of 33 and 21, respectively. The DFT calculations indicate that N-CNT stabilizes Ni nanoparticle and locally activated for the HOR because of nitrogen located at the edge of the nanoparticle tunes local adsorption sites by affecting the d-orbitals of Ni. Yang et al.111found that the HOR activity of Ni nanoparticles supported on N-doped carbon nanosheets exhibits a volcano dependence on the N-doping level, and the 8.7% (atomic fraction) N content of catalyst exhibits an optimal HOR activity. Directly doping N into Ni nanoparticles can also enhance its HOR activity. Sun et al.112reported a unique Ni3N/Ni electrocatalyst that exhibits exceptional HER/HOR activities in base, and the active sites are located at the interface between Ni3N and Ni. Hu et al.113using the ultraviolet photoemission spectroscopy (UPS) technique analyzed the downshift of the Ni d-band and interfacial charge transfer from Ni3N to the carbon which lead to the weak HBE in Ni3N/C. The HBE has the order of Ni > Ni3N > Ni3N/C, so the Ni3N/C has the highest HOR activity. In addition, the weak oxygen species adsorption energy of Ni3N/C is not conducive to the oxidation of Ni and therefore has a higher break-down potential.Furthermore, the doping with other non-metal elements than N has also been reported as efficient ways to improve the HOR activities of Ni. For example, Luo et al.114reported the HOR activity of Ni which supported on heteroatom (S, N, or B) doped carbon, and the trend following the sequence of Ni/S-C > Ni/NC > Ni/B-C.
Although the HOR activities of Ni-based catalysts are still lower than that of PGMs, the ultralow cost of Ni makes it competitive for the price-sensitive applications26,107. However,stability is one of the most important issues that limited the application of Ni-based HOR catalysts115. The passivation of the Ni surface by the formation of Ni(OH)2significantly poison the Ni-based HOR catalysts. When a positive potential (> 0 vs. RHE)in H2-saturated electrolyte is applied, the surface of Ni is gradually oxidized to α-Ni(OH)2. When the applied potential beyond 0.3 V, α-Ni(OH)2would irreversible converted to β-Ni(OH)2101,116. For the Ni alloys, they also have stability issues.Cherevko et al.115investigated the stability of Ni3M/C HOR electrocatalysts (M = Co, Fe, Cu, Mo) in alkaline medium. It was found that Mo is unstable due to its intense dissolution in the short HOR region (0-0.3 V), and Cu is stable below 0.4 V due to its onset dissolution potential is at ca. 0.45 V. The irreversible oxidation of Ni to Ni(OH)2can occur at HOR potential region,and the incorporation of Co and Fe into the Ni(OH)2lattice lead to no significant dissolution was found for Ni, Fe, and Co when the potential up to 0.7 V.
Several methods have been reported to enhance the stability of Ni. The most popular way is the growth of a protective layer on the Ni-based catalyst. Bao et al.117reported a novel Ni@h-BN core-shell nanocatalysts consisting of Ni nanoparticles encapsulated in a few-layer of h-BN shells. The h-BN shells help to maintain the active metallic Ni phase in both air and the electrolyte, and it also weakens the interactions of the O, H, and OH species with the Ni surface. Zhuang et al.118synthesized the Ni@C catalysts by a vacuum pyrolysis method, and the antioxidation capability of Ni has been improved by the carbon shells. The graphitization degree of the carbon shells is the key factor affecting Ni utilization and its HOR activity. The HEMFC achieves a PPD of 160 mW·cm-2by using Ni@C-500 as the anode, and it could stably discharge for 120 h at 0.7 V.
The completed oxidized Ni does not have HOR activity.However, it was found that partially oxidized Ni may helpful for the activity of Ni. Savinova et al.119found that the presence of NiO species formed by Ni contact with air at ambient conditions can enhance the HOR/HER activity of monometallic Ni to ca.10 times. By combining the experimental observations with microkinetic modeling, the enhancement of the HOR activity of Ni in the presence of surface NiO can be related to a decrease of the HBE of Ni, along with an increase of the rate constant of the Volmer step. Zhuang et al.120plotted a volcano curve between the HOR activity and the surface molar ratio of Ni(OH)2 /Ni, and the ratio of 1.1 : 1 exhibit exchange current ca. 6.8 times than that of Ni/C. Sun et al.121synthesized a low-cost Ni/NiO/C catalyst with abundant Ni/NiO interfacial sites, which exhibits one order of magnitude HOR activity higher than Ni/C in alkaline media due to both optimal hydrogen and hydroxide binding energies at the Ni/NiO interface. Moreover, Ni/NiO/C also shows CO tolerance and better stability than Pt/C. The function of Ni oxides can be replaced by the other metal oxides.Zhuang et al.26using Cr-decorated Ni catalyst as anode and Ag as cathode for HEMFC which shows a PPD of 50 mW·cm-2at 60 °C and almost no degradation over a test period of 100 h.Wang et al.122prepared that Ni/MoO2 nanosheet by using a facile hydrothermal-reduction method. The Ni/MoO2has higher HOR activity than that of Ni, which is attributed to the positively charged MoO2accelerates the dissociation of water molecules and promotes the OH adsorption at the Volmer step, while the nearby Ni act as the hydrogen adsorption sites and MoO2 optimizes the Ni-H bond energy.
4.6 Other metal based catalysts
Among various PGM catalysts for HOR, Rh has also attracted attention because of its ΔGHadis close to Pt39. Satsuma et al.98reported that the HEMFCs with Rh/C anode obtained the PPD is ca. 50 mW·cm-2. Abruña et al.123investigated the HOR activity of carbon-supported Rh and Rh alloy nanoparticles. The experimental results show that HOR activity significantly increased on Rh nanoparticles, relative to bulk Rh. Furthermore,the HOR activity of PtRh and IrRh alloy catalysts outperformed that of Ir/C and Pt/C due to the synergistic effect. Recently, Luo et al.124synthesized the monodisperse Rh2P nanoparticles with high HOR/HER activity. DFT results reveal that the introduction of P into Rh can weaken the ΔGHadof Rh2P to nearly zero. The HOR specific and mass activities of Rh2P nanoparticles are 0.65 mA·cm-2and 0.52 mA·μg-1, which is 2.4 and 1.4 times higher than those of Rh/C (0.27 mA·cm-2and 0.37 mA·μg-1), and even surpass the activity of Pt/C in alkaline media125. However,considering the element abundance and price of Rh, it is not a good choice as a HOR catalyst than other inexpensive materials.
For non-PGM catalysts except for Ni, transition metal carbides (TMC) display electronic and catalytic properties that are similar to Pt-group metals126. The stability of the TMCs is related to the oxygen binding energy of the parent metal, so W,Ta, Ti, and Zr carbide show stability under acidic conditions; Ti,Ta, Zr, V, and Nb carbide show stability under basic conditions.Generally, all of the TMCs are stable in acidic and alkaline media under the operating conditions for HOR/HER127. Due to the low intrinsic activities of TMC, the low loading of PGMs was deposited on TMC substrates128. Chen et al.129plotted the volcano relationship between HER activity and HBE for all metal, TMC, and monolayer (ML)-metal-TMC thin films, and the ML Pd-WC and ML Pd-Mo2C thin films showed the most promise as HER catalysts. Chen et al.130was synthesized six low-loadings Pt transition metal carbide (Pt/TMC) powder catalysts, and 1% (w) Pt on VC achieved comparable HOR/HER activity than that of the 46% (w) Pt/C catalyst in alkaline electrolytes. However, the slow research progress of TMC leads to it has a long dance to replace traditional HOR catalysts in HEMFCs.
5 Summary of the HEMFC performance by using different HOR catalysts
We summarize the HEMFC performance by using the reported HOR catalysts as the anode. For the HEMFC, either pure O2or CO2-free air can be feed on the cathode side.Generally, feeding pure O2gives higher performance, because of the high O2concentration. Fig. 7 summarizes the H2/O2and H2/air (CO2-free) HEMFC performances respectively. The data are also listed in Table S1.

Fig. 7 The summary of the reported (a) H2/O2 and (b) H2/air (CO2-free) HEMFC performances using different anode catalysts in literature 24,26,52,53,67,69,71,72,84,86-88,98-100,105,107-109,131,132.

Fig. 8 (a) The prices of the precious metals during the years of 2015-2020. The numbers at the top, middle and bottom of the histogram are the highest, average and lowest price from 2015-2020, respectively 133. (b) Element abundances of the precious metals in the lithosphere 134.
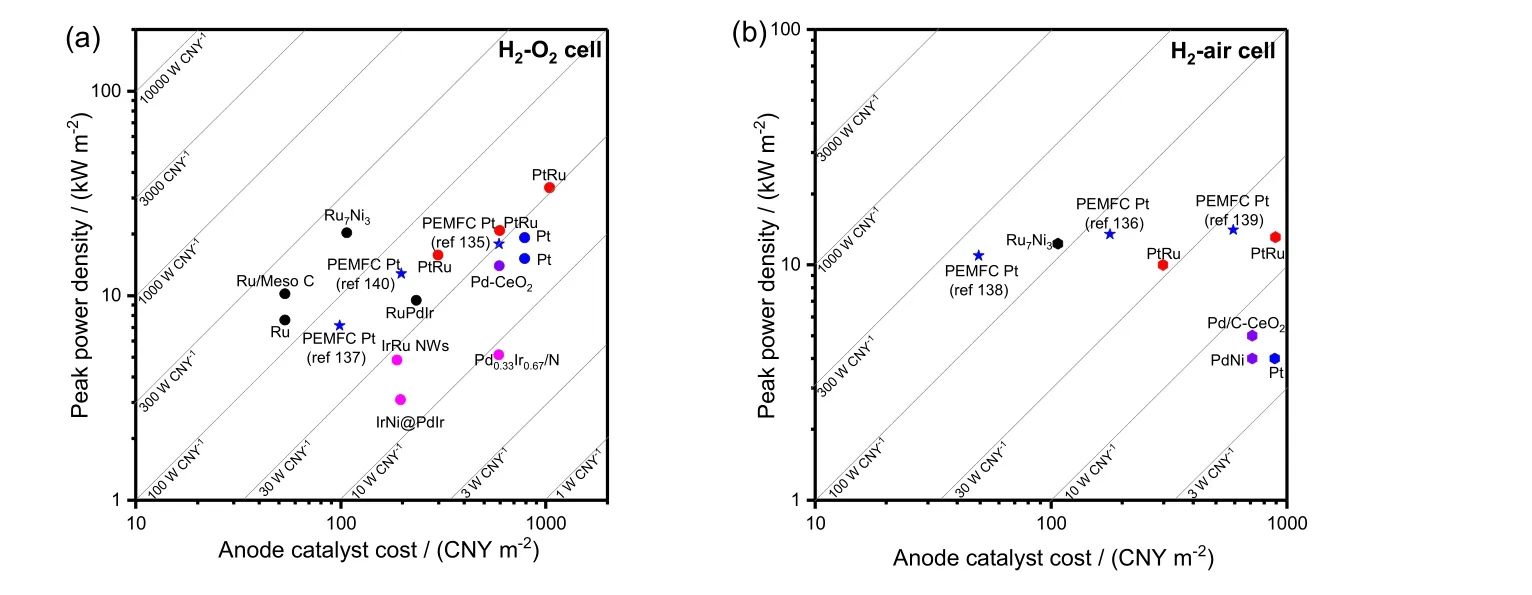
Fig. 9 The PPD of HEMFC versus the anode catalyst cost. (a) H2-O2 cell. (b) H2-Air cell. Diagonal lines denote the values of anode cost specific PPD. As a reference, the blue star is the corresponding value of PEMFCs using Pt/C as the anode 135-140.
For the H2/O2HEMFC, PtRu is the most used anode catalysts,and the PPD is in the range of 1-3.4 W·cm-2dependent on the cell temperature, back pressure and the used HEMs. Employing the PtRu catalysts, high HEMFC performance can be obtained,making the HEMFCs competitive to the PEMFCs. However,higher anode catalyst loading is required for HEMFCs. By using the pristine Pt, high PPD of near 2 W·cm-2also can be obtained at high cell temperature of 80 °C, close to the HEMFC using PtRu anode at similar conditions. Apart from Pt, the other PGMs,such as Pd, Ir and Ru based materials, have also been reported as the anode for HEMFC, and generally a PPD more than 0.5 W·cm-2can be obtained. Cooperating with the other metals, the HEMFC performance using the PGM anode catalysts can be elevated. For example, the CeO2modified Pd can support the HEMFCs with a PPD of ~1.3 W·cm-2. In particular, the recent reports show that the HEMFC using Ru based catalysts can deliver high PPDs. For example, the HEMFC using RuNi as anode can deliver a PPD of ~2 W·cm-2, which is slightly higher than the HEMFC using PtRu anode working at the same condition. For non-precious metal catalysts, only Ni-based materials were assembled on HEMFCs in the previous reports.The performance of Ni-based HEMFCs can deliver a PPD in range of several tens of mW·cm-2to ~350 mW·cm-2. Thus there is still large room for the development of non-precious metal based anode HEMFCs.
For the H2/air (CO2-free) HEMFCs, it has a similar situation.High HEMFC performance of more than 1 W·cm-2of PPD can be obtained by using PtRu or Ru based anode catalysts.
6 Cost on the anode catalysts for HEMFCs
One of the most important goals of the development of HEMFCs is to reduce the cost of the fuel cells. For the PEMFCs,the anode catalyst loading (~0.05 mg·cm-2) is much lower than that for the cathode. Thus, the cost of the anode catalyst for the PEMFCs is not a big problem. However, for the HEMFCs, the anode catalyst cost is a big problem, coming from the lower HOR catalytic activity of the Pt in base. Here, we summarize the anode catalyst cost for the reported HEMFCs. Because of the unsatisfied HEMFC performance of the non-precious metal based HEMFCs, we do not analyze the PGM-based HEMFCs.The prices of the PGMs fluctuates. Fig. 8a shows the highest,lowest and average price of the PGM in the past five years. Ag is the cheapest PGMs, but Ag has very low HOR activity. Ru is the second cheap PGMs, with the price in the range of 1/10-1/3 to that Pt. Owing to the good performance of the Ru-based HEMFCs, it is very promising to reduce the anode cost of HEMFC by using the Ru-based HOR catalysts. Moreover, the element abundances of PGM in the global lithospheres are in the order of Ag > Ru > Pt > Au ≈ Ir > Os > Pd > Rh (Fig. 8b). Ru also has a relatively high abundance on the earth.
We calculated the anode catalyst cost through the PGM prices and the anode catalyst loadings in the HEMFCs, and they are illustrated with the PPD of the HEMFCs in Fig. 9a, b. The anode cost specific PPD can be obtained by diving the PPD by the anode cost, and they are denoted by the diagonal lines. The anode catalyst cost for the typical PEMFCs is also added for comparison. PtRu shows relatively high anode cost specific PPD, because of the high PPD obtained by using PtRu. However,due to the relatively high PtRu catalyst loading on the anode side for HEMFCs, the anode cost specific PPD of PtRu based HEMFC is lower than that of the typical Pt based PEMFCs.Excitingly, the Ru based catalysts show the high anode cost specific PPD, beneficial from the high performance obtained by the Ru-based HEMFCs and also the relatively low cost of Ru.And the anode cost specific PPD of Ru-based HEMFC is similar to that of the Pt based PEMFCs, making the anode catalyst cost no longer a drawback of HEMFC compared with the PEMFCs.
7 Conclusions
In this review, we discussed the HOR mechanism in base,summarized the reported HOR catalysts and analyzed the HEMFC cost from the anode catalyst. Significantly progresses have been made for the anode catalyst of the HEMFCs.Previously, HEMFCs have an order lower of anode cost specific PPD than that of the PEMFCs. In recent years, the HEMFC performance improved remarkably. Beneficial from the understanding of the HOR mechanism and the development in the materials synthesis, the HOR activity in base has been improved and lower Pt loading was required on the anode side.More important, some highly active non-Pt catalysts have been developed, especially the Ru based catalysts. High PPD can be obtained by using the Ru-based HOR catalyst, and the anode cost specific PPD of Ru-based HEMFC is now similar to that of the Pt-based PEMFCs. Thus, the anode catalyst cost is no longer a drawback of HEMFC compared with the PEMFCs. Coupled with the PGM-free cathode catalysts, the HEMFC has become very promising to achieve the reduced catalyst cost for fuel cells,which is fruitful to realize the hydrogen economy.
There are still several challenging tasks for the further development of the HOR electrocatalysts in base.
(i) Further lower the loading of the PGM catalyst. Although the anode catalyst loadings and cost can be reduced by the recently developed Ru-based HOR catalysts, its cost is still higher than the potential PGM-free cathode catalyst and the anode catalysts become the major catalyst cost for HEMFCs.Thus, to further lower the cost of the HEMFCs, the anode catalyst cost could be further reduced. It requires to further lower the catalyst loading on the anode side, by further increasing the activity of the catalyst. The catalysts with advanced structures,such as the core-shell nanoparticles with PGM-free cores, may also reduce the PGM amount in the catalysts. At an ultralow loading of the catalyst, the mass transportation may also huge influence, thus the structure of the catalyst layers should be also considered.
(ii) Development of highly active and stable PGM-free catalyst. Using the PGM-free catalyst to replacing the cost PGM is attractive. However, up to now, only Ni-based catalyst shows moderate HOR activity in base. There still a large room to improve the HOR activity of the Ni-based catalysts. More important, the Ni is likely to be oxidized and lose its HOR activity, making it have low stability. How to stabilize the Nibased HOR catalyst is the priority. Cooperation with other metals may stabilize the Ni. Developing PGM-free HOR catalysts without using Ni is particularly concerned, but still challenging.
(iii) Understanding the HOR mechanism in base. The detailed mechanism of the HOR in base is still unclear. There is still no generally accepted explanation for the low HOR activities of the PGMs in base. The role of OH-is still debatable. It suggested that water adsorption may plays important role in catalytic processes. However, the depth understanding of how the electrolyte influent the water adsorption and direct observation of the water catalyst interactions are still lacking. Furthermore,there are many debates on the mechanism of the HOR process on the catalyst with two or more composites. The in situ spectroscopic techniques, such as infrared spectroscopy (IR),Raman spectroscopy, and extended X-ray absorption fine structure spectroscopy (EXAFS) are helpful to direct observation of the intermediates for the catalytic processes, thus helping to understand the HOR processes.
(iv) Understanding the catalyst-solid electrolyte interface. In the working condition of the HEMFC, the reaction occurs on the interface of the catalysts and the solid electrolyte. The double layer structures have been proposed for the liquid electrolyte.However, there is still lack of a model to describe the “double layer structure” for the solid electrolyte. The charge distribution of the solid electrode at the near catalyst region is still unclear.The advanced model method, such as DFT or molecular dynamics, may be used for understanding the catalyst-solid electrolyte interface.
(v) The HOR catalysts under harsh conditions. It is possible to deviate from the normal anode working condition for HEMFCs when in pratical application. For example, at the start-up and shut-down of the HEMFCs, the anode may expose to high potential and a reverse current may generate. Thus, it requires the anode catalyst to have higher stability. And low ORR activity is also desired for reducing the reverse current. It is also desired to feed the HEMFC directly by the H2from reforming gas, which contains a certain amount of CO. Thus the HOR catalysts with good CO-tolerance are expected. If the HOR can work under these harsh conditions, the HEMFC system would be simplified,and the cost of the HEMFC system would be reduced, making us closer to the realization of the hydrogen economy.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- 有序金属间化合物电催化剂在燃料电池中的应用进展
- Recent Progress in Proton-Exchange Membrane Fuel Cells Based on Metal-Nitrogen-Carbon Catalysts
- 提升燃料电池铂基催化剂稳定性的原理、策略与方法
- 高温聚合物电解质膜燃料电池膜电极中磷酸分布及调控策略研究进展
- Formic Acid Electro-Oxidation Catalyzed by PdNi/Graphene Aerogel
- Enhanced Performance and Durability of High-Temperature Polymer Electrolyte Membrane Fuel Cell by Incorporating Covalent Organic Framework into Catalyst Layer

