Immune aspects of hepatocellular carcinoma:From immune markers for early detection to immunotherapy
Ângelo Z Mattos,Jose D Debes,Andre Boonstra,Arndt Vogel,Angelo A Mattos
Ângelo Z Mattos,Angelo A Mattos,Graduate Program in Medicine:Hepatology,Federal University of Health Sciences of Porto Alegre,Porto Alegre 90050-170,Brazil
Ângelo Z Mattos,Angelo A Mattos,Gastroenterology and Hepatology Unit,Irmandade Santa Casa de Misericórdia de Porto Alegre,Porto Alegre 90020-090,Brazil
Jose D Debes,Department of Medicine,Division of Gastroenterology and Infectious Diseases,University of Minnesota,Minneapolis,MN 55812,United States
Jose D Debes,Andre Boonstra,Department of Gastroenterology and Hepatology,Erasmus Medical Center,Rotterdam NL-3015,The Netherlands
Arndt Vogel,Department of Gastroenterology,Hepatology and Endocrinology,Hannover Medical School,Hannover 30625,Germany
Abstract Hepatocellular carcinoma(HCC)is one of the most prevalent cancers and one of the main causes of cancer-related deaths worldwide.Most HCCs develop in an inflammatory microenvironment,and mounting evidence emphasizes the importance of immune aspects in hepatocarcinogenesis.In normal physiology,both innate and adaptive immune responses are responsible for eliminating malignantly transformed cells,thus preventing the development of liver cancer.However,in the setting of impaired natural killer cells and exhaustion of T cells,HCC can develop.The immunogenic features of HCC have relevant clinical implications.There is a large number of immune markers currently being studied for the early detection of liver cancer,which would be critical in order to improve surveillance programs.Moreover,novel immunotherapies have recently been proven to be effective,and the combination of atezolizumab and bevacizumab is currently the most effective treatment for advanced HCC.It is expected that in the near future different subgroups of patients will benefit from specific immunotherapy.The better we understand the immune aspects of HCC,the greater the benefit to patients through surveillance aiming for early detection of liver cancer,which allows for curative treatments,and,in cases of advanced disease,through the selection of the best possible therapy for each individual.
Key Words:Hepatocellular carcinoma;Immunology;Hepatocarcinogenesis;Surveillance;Biomarker;Immunotherapy
INTRODUCTION
Liver cancer has a global incidence of 11.6/100000 individuals(905677 new cases in 2020)and a mortality rate of 10.7/100000 individuals(830180 deaths in 2020),which places it sixth among all malignant neoplasms regarding incidence and second concerning mortality worldwide[1].Moreover,liver cancer accounted for 12.5 million disability-adjusted life years across the globe in 2019[2].Also,it is estimated that the age-standardized incidence rate of liver cancer will increase between 2017 and 2030 from 11.80 to 14.08 per 100000 individuals[3].Hepatocellular carcinoma(HCC)is responsible for the vast majority of primary liver cancers[4,5].HCC develops most frequently in patients with cirrhosis,and,while the most important causes of liver disease in patients with HCC are still chronic hepatitis B and C,non-alcoholic fatty liver disease is continuously growing in importance[6].
Current guidelines recommend surveillance for HCC with semiannual ultrasound in high-risk individuals,particularly in patients with cirrhosis[7-10].The American Association for the Study of Liver Diseases makes it optional to add alpha-fetoprotein(AFP)to the surveillance program and it also recommends surveying high-risk individuals with hepatitis B who do not have cirrhosis(patients with African ancestry,Asian males over 40 years of age or females over 50 years of age,and patients with a family history of HCC)[7,8].Regarding surveillance for patients without cirrhosis,the European Association for the Study of the Liver(EASL)also targets people with advanced liver fibrosis(Metavir fibrosis stage ≥ F3)and individuals with hepatitis B and a PAGE-B score ≥ 10[9].Patients with cirrhosis presenting with liver nodules ≥1 cm on ultrasound should be referred for diagnostic evaluation of HCC with either a multiphasic computed tomography scan or magnetic resonance imaging[7-9].Unfortunately,different studies demonstrate that only half of cases with HCC are diagnosed while patients are under surveillance,despite the association between diagnosis in a surveillance program and better prognosis[11-13].HCC management depends on the stage of the disease.Table 1 shows the most commonly recommended therapeutic options according to the Barcelona Clinic Liver Cancer staging system at the time of publication of the current guidelines,in 2018[7-9].

Table 1 Recommended treatments for hepatocellular carcinoma according to the Barcelona Clinic Liver Cancer staging system
Since then,many advances in the field of HCC have occurred.Several of these advances are directly related to the immune aspects of this disease,as it must be highlighted that HCC usually develops in an inflammatory and immunogenic background,such as that of viral hepatitis.The aim of this article is to review the immune characteristics of hepatocarcinogenesis,as well as the impact of the immune features of HCC on its early detection and on the novel systemic therapies against it.
IMMUNE ASPECTS OF HEPATOCARCINOGENESIS
Similar to many other tumors,HCC is infiltrated by a wide variety of immune cells that shape the microenvironment of the tumor.Tumor-associated macrophages(TAM)are considered major players in the inflammation process and in dampening the antitumor response.These macrophages are of the so-called M2-type,meaning that they express immunomodulatory cytokines such as interleukin(IL)-10 and transforming growth factor-β(TGF-β)capable of inhibiting anti-tumor immunity[14,15].In addition,TAM express low levels of pro-inflammatory cytokines,and are poor antigen presenters.Consequently,the presence of M2 macrophages has been associated with poor clinical outcomes in patients with HCC[16].In recent years,more data has become available on the importance of TAM in promoting tumor cell proliferation,angiogenesis,and metastasis[17].Viathe release of chemokines,regulatory T cells are attracted towards the tumor,which contributes to impairment of the T cell response at the tumor site.Recent developments in transcriptomic profiling of the HCC material by single cell RNA sequencing clearly revealed the presence of multiple macrophage subsets in the tumor at distinct states,which were also found to be associated with disease progression[18,19].
Besides TAM,other cells of the innate immune system,such as natural killer(NK)cells,and cells of the adaptive immune system,such as CD8+ T cells,are also detected in the tumor tissue and are able to kill the transformed cells by the release of cytolytic enzymes,such as perforins and granzymes.Perforin acts by making pores in the cellular membrane,while granzymes are serine proteases which,among other activities,cleave and activate caspase-dependent apoptotic pathways thereby killing the cells.Recognition of the tumor by NK cells occursviagerm-line encoded receptors that are triggered when their stimulatory and inhibitory signals are out of balance due to their encounter with virus-infected or tumor cells[20].CD8+ T cells,on the other hand,recognize the tumorviathe interaction of specific receptors on T cells with a major histocompatibility complex class I(MHC-I)molecule present on tumor cells that harbors tumor-associated antigens(TAA).A number of classical tumor antigens have been described for HCC,including cancer-testis antigens(such as melanomaassociated gene-A1 — MAGE-A1 and New York-esophageal squamous cell carcinoma-1 — NY-ESO-1 proteins),and oncofetal antigens(such as AFP and glypican-3 — GPC-3)[21,22].Antigen-specific CD8+ T cell responses directed against all four of these TAA have been readily observed in the blood of more than 50% of HCC patients[23],and found to be expressed exclusively or at much higher levels in HCC tumor tissue than in tumor-free liver tissue[22].
HCC is an immunogenic tumor,and this has been convincingly demonstrated by the fact that extensive immune cell infiltration is seen in HCC tissue,predominantly consisting of T cells.It has been reported that increased T cell infiltrates in the tumor are associated with improved overall survival(OS)in HCC and with lower tumor recurrence following resection[23,24].Also,higher numbers of infiltrating NK cells in the neoplastic tissue have been shown to be associated with better survival of patients.In the same study,patients with advanced-stage HCC exhibited not only lower numbers of NK cells,but these cells were also functionally impaired,with lower production of interferon-γ and tumor necrosis factor(TNF)[25].The impairment of NK cell function is,at least in part,ascribed to downregulation or enhanced shedding of activating receptors or their ligands,as has been reported for the interaction between the receptor NK group 2 member D(NKG2D)on NK cells and its ligands MHC-Irelated chain A(MICA)and UL16-binding protein-1(ULBP1)on tumor cells[26,27].In addition,increased expression of the inhibitory ligand NK group 2 member A(NKG2A)on NK cells has been reported in HCC and has been shown to induce IL-10,which may further impair NK cell activity[28].
Despite the importance of NK cells in immune surveillance and progression of HCC,most studies on the immunology of HCC have focused on T cell responses,and especially on CD8+ T cell responses.Due to the wide availability of reagents to detect TAA-specific CD8+ T cells using MHC-I multimers,studies on CD8+ T cells have outnumbered those on TAA-specific CD4+ T cells.As mentioned above,the tumorimmune microenvironment of HCC is characterized by an abundance of tumor-infiltrating T cells and,although TAA have been identified during recognition of the tumor,the responses are too weak to eliminate the tumor and the effects are dampened by diverse immunosuppressive mechanisms in the tumor environment.It is crucially important to gain in-depth knowledge on these suppressive mechanisms,as shifting the balance by improving and restoring the quality of the immune response in the tumor environment may strongly benefit treatment outcomes for HCC patients[19].However,the immunosuppressive mechanisms affecting the function of tumorspecific T cells are complex and diverse.Indeed,numerous suppressive immune populations able to inhibit T cell responses have been described to be present at higher numbers in the tumor environment of the liver as compared to the tumor-free liver.These cells include classical forkhead box P3(FoxP3)positive regulatory T cells,IL-10 producing regulatory cells,myeloid derived suppressor cells and TAM[29-31].Besides increased numbers,enhanced expression of inhibitory molecules and release of suppressive cytokines,such as IL-10 and TGF-β,have also been reported for these cells[32].
In addition to these suppressive immune cells,it has long been recognized that continuous tumor antigen exposure for prolonged periods of time leads to a differentiation program in T cells which gradually switches off specific activities of the affected cell,such as the capacity to perform cytotoxicity,to produce cytokines or to proliferate.These so-called exhausted T cells are characterized by overexpression of inhibitory receptors,including programmed cell death protein 1(PD-1),cytotoxic T-lymphocyteassociated protein 4(CTLA-4),lymphocyte-activation gene 3(LAG-3)and T cell immunoglobulin and mucin domain-containing protein 3(Tim-3)[33].Importantly,higher numbers of exhausted CD8+ T cells have been reported to be associated with poor prognosis in HCC.The inhibitory intracellular signals are delivered to the T cell upon encountering its ligands on the tumor cell.This interaction is complex and diverse due to the high number of different inhibitory receptors known and the different levels of expression depending on the individual patient,but also on the activation state of the T cell and its antigen-specificity.However,mouse studies andin vitrostudies have convincingly shown that blockade of inhibitory receptors can restore T cell function and lead to a reduction in tumor size[33,34].
In HCC patients,enhanced fractions of CD8+ T cells expressing PD-1,CTLA-4,LAG-3 and Tim-3 were observed in the tumor as compared to tumor-free tissue and blood of the same patient,with higher expression found on TAA-specific as compared to non-TAA-specific CD8+ T cells[35].In vitroblockade of PD-1,CTLA-4 or LAG-3 using monoclonal antibodies increased the proliferation and cytokine production of CD8+ T cells isolated from the tumor upon polyclonal stimulation,and combinations of PD-1 blockade with any of the other antibodies further enhanced the CD8+ T cell activities[35].Soluble PD-1 and PD ligand-1(PD-L1)can be detected in serum,but no significant associations of soluble PD-1 or soluble PD-L1 with either intra-tumoral PDL1 expression or the numbers of CD8+ T cells in the tumor have been determined[36].Importantly,it has been suggested that,within the PD-1 expressing CD8+ T cell population,subgroups can be identified in which HCC with a discrete subgroup of cells that express high levels of PD-1 are more aggressive than HCC without such a population of cells,and that the levels of PD-1 play a role in the response to anti-PD-1 blockade[37].The immune mechanisms involved in hepatocarcinogenesis are depicted in Figure 1.
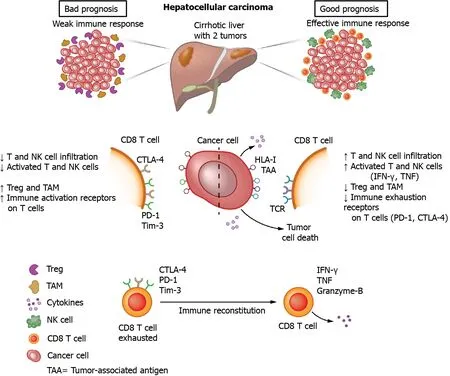
Figure 1 Immune mechanisms of hepatocarcinogenesis.NK:Natural killer;Treg:Regulatory T cells;TAM:Tumor-associated macrophages;CTLA-4:Cytotoxic T-lymphocyte-associated protein 4;PD-1:Programmed cell death protein 1;Tim-3:T cell immunoglobulin and mucin domain-containing protein 3;HLA:Human leucocyte antigen;TAA:Tumor-associated antigens;TCR:T cell receptor;IFN:Interferon;TNF:Tumor necrosis factor.
PERSPECTIVES ON IMMUNE MARKERS FOR EARLY DETECTION OF HCC
Regardless of the underlying liver disease,chronic inflammation is a common denominator present in more than 90% of patients with HCC[38].Indeed,local activation of intrahepatic cell populations can trigger coordinated processes followed by immune cell infiltration.It is with these concepts in mind that a broad array of inflammatory molecules,including cytokines and chemokines,have been investigated as potential biomarkers to predict early development of HCC,and to understand the mechanisms involved in HCC formation[39-41].
Peripheral immune markers represent an attractive option as they are easily measured in plasma or serum with relatively low interface technology.There are,however,several concerning issues when using immune markers for the detection of HCC.The first one is the poor reproducibility in measuring immune analytes by different platforms.This is mainly based on the variable expression level of these analytes at any given time in the organ system(serum or plasma),as well as collection and storage variability of samples.The second issue is that of the underlying liver disease.Immune markers that show promise for early detection of HCC in individuals with hepatitis B might not be reliable in individuals with HCC secondary to alcoholic liver disease due the differently activated immune pathways.Finally,a major issue lies in immunosurveillance,the recognition of tumor cells by leucocytes,which has been well-described for a variety of tumors and plays an important role during oncogenesis[42].In this regard,it is difficult to distinguish the modulation of immune markers secondary to the underlying liver disease from that associated with a response to HCC formation.Nonetheless,some data suggest that,in the setting of chronic viral hepatitis,a hyperimmune environment due to the continuous presence of a virus in the liver over the years could pose a hyperreactive immune response with larger modulations of immune markers measurable in blood during the transition from a liver nodule to HCC[43,44].
A multitude of individual immune markers has been studied with the goal of early recognition of HCC.Among the best studied immune markers are:Osteopontin(OPN),growth differentiation factor 15(GDF15),vascular endothelial growth factor(VEGF)and TGF-β.Other immune markers,not described here,have been studied for recognition of HCC at different stages,such as IL-6,IL-10,monocyte chemoattractant protein 1,and fibroblast growth factor 2,among others.
OPN has been examined as an early HCC marker by several research groups.OPN mediates a large array of different biological functions in the immune system and has been extensively studied in a variety of cancers[45,46].Increased serum and plasma levels of OPN in individuals with HCC compared to those with liver cirrhosis or controls have been reported by multiple studies[47-50].Most of the studies showing a high area under the receiver operating characteristic curve(AUROC)for OPN have been performed in Asian cohorts.However,more recent studies with West-African and European cohorts,including the EPIC study,have also shown promising findings[51].
GDF15 has been shown to modulate intrinsic pathways in inflammation,ischemia and several different cancers[52].A Chinese study looking at GDF15 in predominantly viral hepatitis-related HCC showed elevated levels of this immune marker in those with early HCC compared to controls,with an AUROC of 0.84[53].
VEGF is an angiogenic factor related to vascular endothelial formation.A small retrospective Japanese study showed increased serum VEGF levels in hepatitis Crelated HCC.The diagnostic performance of VEGF in this study was better than that of AFP[54].Nonetheless,a later study performed in Egypt also in hepatitis C-related HCC did not detect serum VEGF differences between the HCC and the control groups[55].Both studies were relatively small,and larger cohorts to further clarify these ambivalent results are needed.A more recent longitudinal study from our group identified serum VEGF as one of 12 immune mediators able to predict HCC development in individuals with hepatitis C[43].However,this evaluation was also performed in a small cohort and in co-measurement with other immune analytes.
TGF-β regulates a variety of inflammatory processes and has been implicated in modulation of cell proliferation,differentiation and survival[56].Previous studies have shown that serum levels of TGF-β are associated with HCC development,as well as early detection[49,57].Nevertheless,most of these studies are small,mainly in individuals with hepatitis B or C,and several of them have shown improvement in the detection of HCC only when in conjunction with another analyte[58].
Most of the studied immune biomarkers show some degree of limitation in their role to detect early HCC.Recently,further attention has been placed on multiple immune-analyte detection rather than on individual measurements.Our group found a series of co-measured immune analytes to be associated with the future development of HCC in a cohort of individuals with hepatitis C,even when the cancer occurred up to two years later.The C-statistic for correct prediction of HCC was >0.90 for four of these markers(monokine-induced gamma interferon,IL-2,TNF-related apoptosisinducing ligand and A proliferation-inducing ligand),and >0.80 for the rest.However,this study was performed in a small and specific cohort,and larger studies will be needed to evaluate the use of these and other immune markers in HCC detection[43].
IMMUNOTHERAPY FOR HCC
Considering the clear role of the immune system in the genesis and progression of HCC,attempting to treat HCC with immunotherapy has been a reasonable approach evaluated by many groups.However,the complex interactions among the immune system,the underlying liver disease and the tumor make it challenging to develop effective therapies.This helps explain why only now immunotherapy is becoming part of the therapeutic armamentarium for HCC.
Until recently,sorafenib was the only drug approved for advanced HCC.It was the first medication with proven efficacy for this stage of the disease and it was the standard of care for over 10 years[59].After many phase III failures,additional multitargeted tyrosine kinase inhibitors such as lenvatinib,regorafenib and cabozantinib and the anti-VEGF receptor antibody ramucirumab have been approved for both first- and second-line treatment in the last four years[60].
During the same time,the first promising results for immunotherapy in HCC were published and specifically the data for nivolumab in the phase II CheckMate-040 study provided compelling evidence that at least a subgroup of HCC patients is sensitive to immune-oncology(IO)based therapies[61].A high disease control rate and overall response rate(ORR)were observed across all investigated subgroups,including“poor-prognosis patients” with impaired liver function(Child-Pugh B),extrahepatic tumor burden,or in patients after treatment with sorafenib.Based on this study,nivolumab was approved in the United States before the publication of the pivotal CheckMate-459 trial.The latter study investigated nivolumab in comparison to the standard of care(sorafenib)in the first-line setting,but failed to show a statistically significant improvement in OS,despite an OS of 16.4 mo,which had not been achieved in a phase III trial by that time.The response rate of just 15% confirmed the observation from the phase II study that immunotherapy alone has clinically meaningful efficacy only in a subset of patients[62].
Similar to nivolumab,promising data were initially reported for pembrolizumab in second-line HCC therapy in the phase II KEYNOTE-224 study,which led to its approval in the United States[63].However,the subsequent KEYNOTE-240 pivotal trial also failed to reach the significance level for the predefined primary endpoints[an improvement in OS and/or progression-free survival(PFS)compared to placebo]due to the dual endpoints and several predefined interim analyses[64].The response rate in both studies was 17% and 18.4% respectively,with a remarkable OS of 12.9 and 13.9 mo,which also stands out in comparison to non-IO phase III studies in the second-line setting[63,64].Overall,despite good phase-II data,neither nivolumab nor pembrolizumab met their primary endpoints in the pivotal studies,and,accordingly,there is no approval for both drugs in Europe for advanced HCC.
To date,there is not a definitive correlation between clinical efficacy of IO and specific biomarkers.Initial evidence suggests that higher PD-L1 expression might correlate with improved survival under treatment with nivolumab[65].On the other hand,despite initial suggestion that the wingless-related integration site(Wnt)/βcatenin pathway might convey resistance to IO-based therapies,these findings have not yet been confirmed in any of the prospective clinical trials[66].
Due to the lack of clinically meaningful biomarkers at the time,there were early efforts to develop IO-based combination therapies.The combination of nivolumab plus ipilimumab has already shown a superior OS in patients with different cancer types,including advanced melanoma and advanced non-small-cell lung cancer,compared to nivolumab monotherapy.In the CheckMate-040 study,three combinations were evaluated and revealed a consistently high ORR,as well as a promising OS in the second-line setting[67].Based on these results,the United States Food and Drug Administration(FDA)approved nivolumab plus ipilimumab as second-line treatment for patients with advanced HCC previously treated with sorafenib.Currently,the CheckMate-9DW study is evaluating the efficacy of nivolumab plus ipilimumab compared to sorafenib or lenvatinib in first-line therapy.
Overexpression of VEGF has been implicated in the development and progression of liver cancer,and previous phase II studies have shown modest anti-tumor efficacy of bevacizumab as monotherapy in advanced HCC[68].Additionally,there is increasing evidence that anti-VEGF therapies can enhance anti-PD-1 and anti-PD-L1 efficacy by reversing VEGF-mediated immunosuppression and promoting T-cell infiltration in tumors.Based on these observations,there was a rationale to evaluate the combination of the PD-L1 inhibitor atezolizumab with the VEGF-inhibitor bevacizumab in advanced HCC.In a relatively large phase Ib study for various tumor entities,bevacizumab in combination with atezolizumab achieved an impressive response rate of 36%(37/104 patients)in the HCC subgroup.The disease control rate was 71%,with a PFS of 7.3 mo and an OS of 17.1 mo[69].The results were subsequently confirmed in the phase III IMbrave 150 trial in the first-line setting[70].Of note,the OS at 12 mo was 67.2% among patients receiving bevacizumab plus atezolizumab and 54.6% among those receiving sorafenib,with a hazard ratio for death of 0.58(P<0.001).Moreover,the confirmed objective response was higher with the combination therapy than with sorafenib[27.3%vs11.9% by Response Evaluation Criteria in Solid Tumors(RECIST)1.1 and 33.2%vs13.3% by modified RECIST(mRECIST),P<0.0001].In addition to the significantly higher clinical activity,the side-effect profile and the quality-of-life evaluation were also considered favorable[70];thus,the combination of atezolizumab and bevacizumab was approved by the FDA and the European Medicines Agency for the first-line therapy of HCC and it will become the next standard of care in first-line therapy for advanced HCC.
Currently,several ongoing phase III trials are evaluating the efficacy of IO/IO combinations and combinations of checkpoint inhibitors with tyrosine kinase inhibitors based on promising phase I and II studies.In agreement with findings for other solid tumors[71],the combination of lenvatinib and pembrolizumab,for instance,achieved a very high disease control rate of 88%,with an ORR of 46% by mRECIST and 36% by RECIST 1.1 as first-line therapy against unresectable HCC in the phase Ib KEYNOTE-524 study.The high ORR translated into an encouraging PFS of 9.3 mo,which is the longest PFS reported so far in any IO-based study in HCC,and an OS of 22 mo[72].Based on these findings,an FDA breakthrough therapy designation was granted to the combination in 2019.However,due to the approval of atezolizumab and bevacizumab,the FDA denied approval of pembrolizumab and lenvatinib as frontline therapy in 2020.Now,the efficacy of the combination is being evaluated in comparison to lenvatinib in the phase III LEAP-002 trial(NCT03713593).
CONCLUSION
The progressive knowledge on the immune aspects of HCC is leading to important clinically applicable breakthroughs.Novel immunotherapies against advanced HCC are now available,resulting in increased survival,and many more are under development.Research on immune markers must be intensified,in order to identify tools for early detection of HCC,allowing for curative treatments to be offered to a larger proportion of patients,as well as to recognize which subgroups of individuals with advanced HCC will benefit the most from each systemic therapy.
 World Journal of Gastrointestinal Oncology2021年9期
World Journal of Gastrointestinal Oncology2021年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Use of liquid biopsies in gastrointestinal cancers
- Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer:A metaanalysis
- Prognostic value of modified Lauren classification in gastric cancer
- Scoparone inhibits pancreatic cancer through PI3K/Akt signaling pathway
- Effect of oncometabolic surgery on gastric cancer:The remission of hypertension,type 2 diabetes mellitus,and beyond
- Characterization of metabolic landscape in hepatocellular carcinoma
