Evidence based tools to improve efficiency of currently administered oncotherapies for tumors of the hepatopancreatobiliary system
Zoltan Herold,A Marcell Szasz,Magdolna Dank
Zoltan Herold,A Marcell Szasz,Magdolna Dank,Division of Oncology,Department of Internal Medicine and Oncology,Semmelweis University,Budapest H-1083,Hungary
Abstract Hepatopancreatobiliary tumors are challenging to treat,and the advanced or metastatic forms have a very low 5-year survival rate.Several drug combinations have been tested,and new therapeutic approaches have been introduced in the last decades,including radiofrequency and heat based methods.Hyperthermia is the artificial heating of tumors by various biophysical methods that may possess immunostimulant,tumoricidal,and chemoradiotherapy sensitizer effects.Both whole-body and regional hyperthermia studies have been conducted since the 1980s after the introduction of deep-seated tumor hyperthermia techniques.Results of the effects of hyperthermia in hepatocellular and pancreatic cancer are known from several studies.Hyperthermia in biliary cancers is a less investigated area.High local and overall responses to treatment,increased progression-free and overall survival,and improved laboratory and quality-of-life results are associated with hyperthermia in all three tumor types.With the evolution of chemotherapeutic agents and the introduction of newer techniques,the combination of adjuvant hyperthermia with those therapies is advantageous and has not been associated with an increase in alarming adverse effects.However,despite the many positive effects of hyperthermia,its use is still only known at the experimental level,and its concomitant utilization in routine cancer treatment is not certain because of the lack of thorough clinical studies.
Key Words:Hyperthermia induced;Carcinoma hepatocellular;Cholangiocarcinoma;Gallbladder neoplasms;Pancreatic neoplasms
INTRODUCTION
Hepatopancreatobiliary cancers are fatal diseases that can be characterized by very low 5-year survival rates[1-3].In 2018,over 1.5 million new cases and approximately 1.4 million deaths from hepatocellular(HCC),biliary(BC)including gallbladder and cholangiocellular(CCC),and pancreatic cancers(PC)were reported[4].Diagnosis at a more advanced stage is characteristic to all four tumor types[1-3],which is usually accompanied by other comorbidities like a cirrhotic state of the liver,which further worsens patient life expectancy[1].In the last decades,several new techniques and possible multimodal therapies have emerged that support conventional surgical resection and facilitate chemoradiotherapy,including but not limited to various thermal ablative methods[5]including radiofrequency[6]and microwave ablation[7],laser-induced thermotherapy[8],hyperthermic intraperitoneal chemotherapy[9],percutaneous ethanol injection[10],transcatheter arterial chemoembolization[11],highintensity focused ultrasound[12],and various types of hyperthermia[13].
The available literature on the clinical applications of hyperthermia in hepatopancreatobiliary cancers is discussed in this review,focusing on survival and safety data that might be an interest to the practicing oncologists.The presentation of in-depth technical details of hyperthermia and its effects on cells and biochemical processes is not the aim of the current review,focused publications on those subjects are available[13-18].
METHODLOGY
A literature search was conducted in PubMed/MEDLINE using the strings “cholangiocarcinoma hyperthermia”,“gallbladder cancer hyperthermia”,“hepatocellular hyperthermia”,and “pancreatic cancer hyperthermia”,for articles published from January 1,1964 to January 31,2021.After removing duplicates,a total of 780 potential articles were found,from which 39 full-text articles were selected(Figure 1).A secondary search in ClinicalTrials.gov was conducted,and an additional three finished and four currently running clinical trials were identified.Five additional studies were included from another search in meta-analysis and review citations,raising the total number of 47 publications included in this review(Table 1).
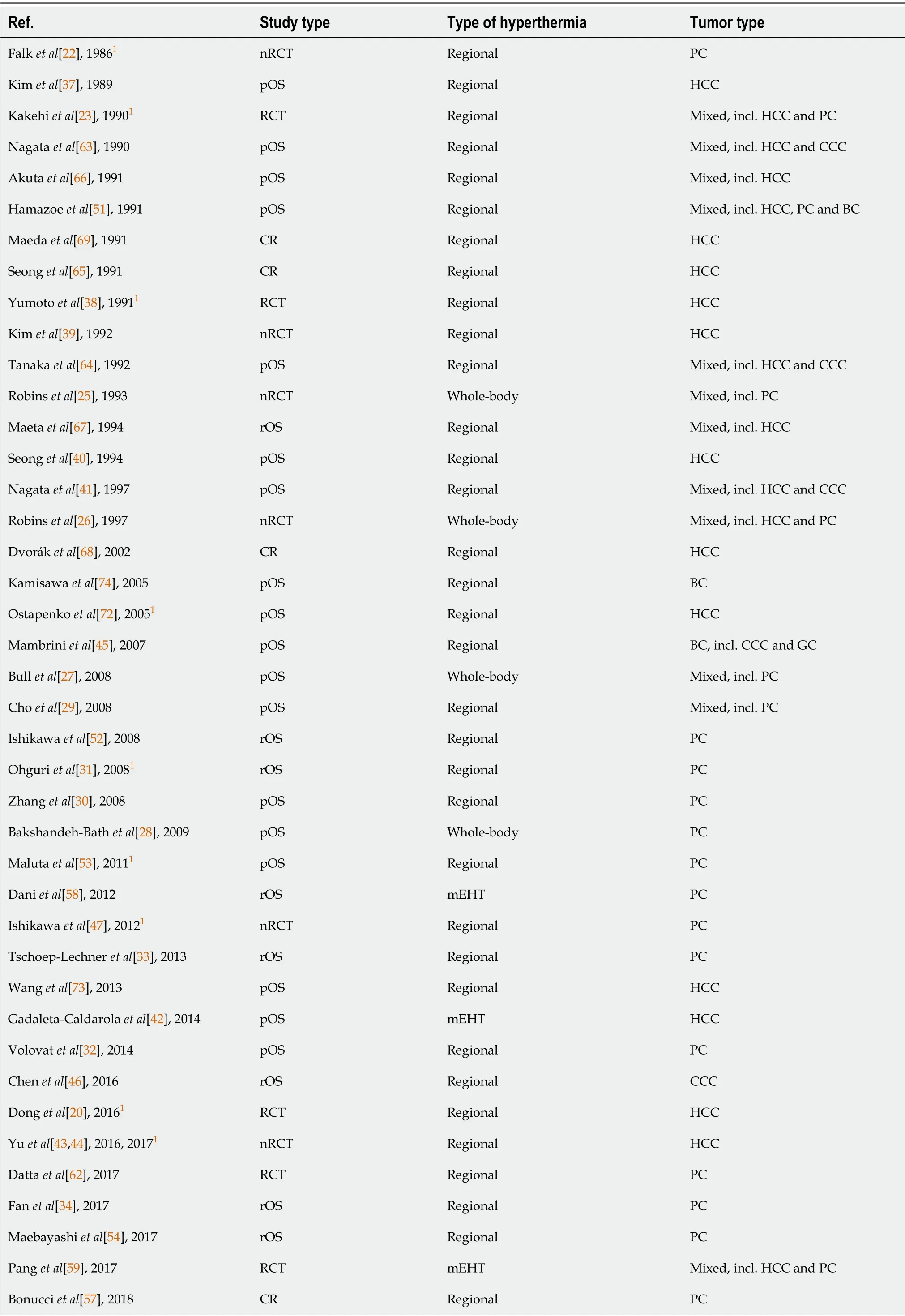
Table 1 Details of the articles retrieved from the searches
Most subjects included in the studies were patients with advanced or metastatic malignancies with no probability of cure with conventional treatments(Stage III and IV).1Study including some Stage I and II patients.BC:Biliary cancer;CCC:Cholangiocellular cancer;CR:Case report;GC:Gallbladder cancer;HCC:Hepatocellular cancer;mEHT:Modulated electrohyperthermia;nRCT:Non-randomized clinical trial;PC:Pancreatic cancer;pOS:Prospective observational study;RCT:Randomized clinical trial;rOS:Retrospective observational study.
Figures 2 and 3 were drawn with R version 4.0.4(R Foundation for Statistical Computing,Vienna,Austria,2021)and the R package forestplot(version 1.10.1,Max Gordon and Thomas Lumley,2020).Data were obtained from eligible articles,and a simple difference in overall survival and disease control rate(the sum of complete or partial response to treatment and stable disease)was calculated from the results of the cohorts with and without hyperthermia.
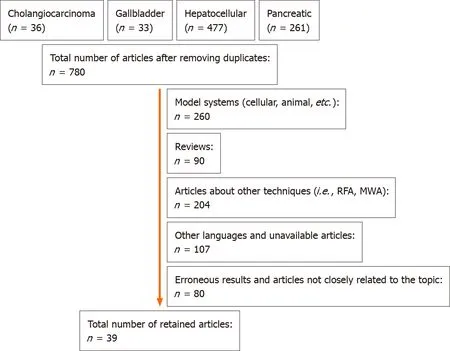
Figure 1 Flow diagram of the selection of PubMed/MEDLINE articles.MWA:Microwave ablation;RFA:Radiofrequency ablation.
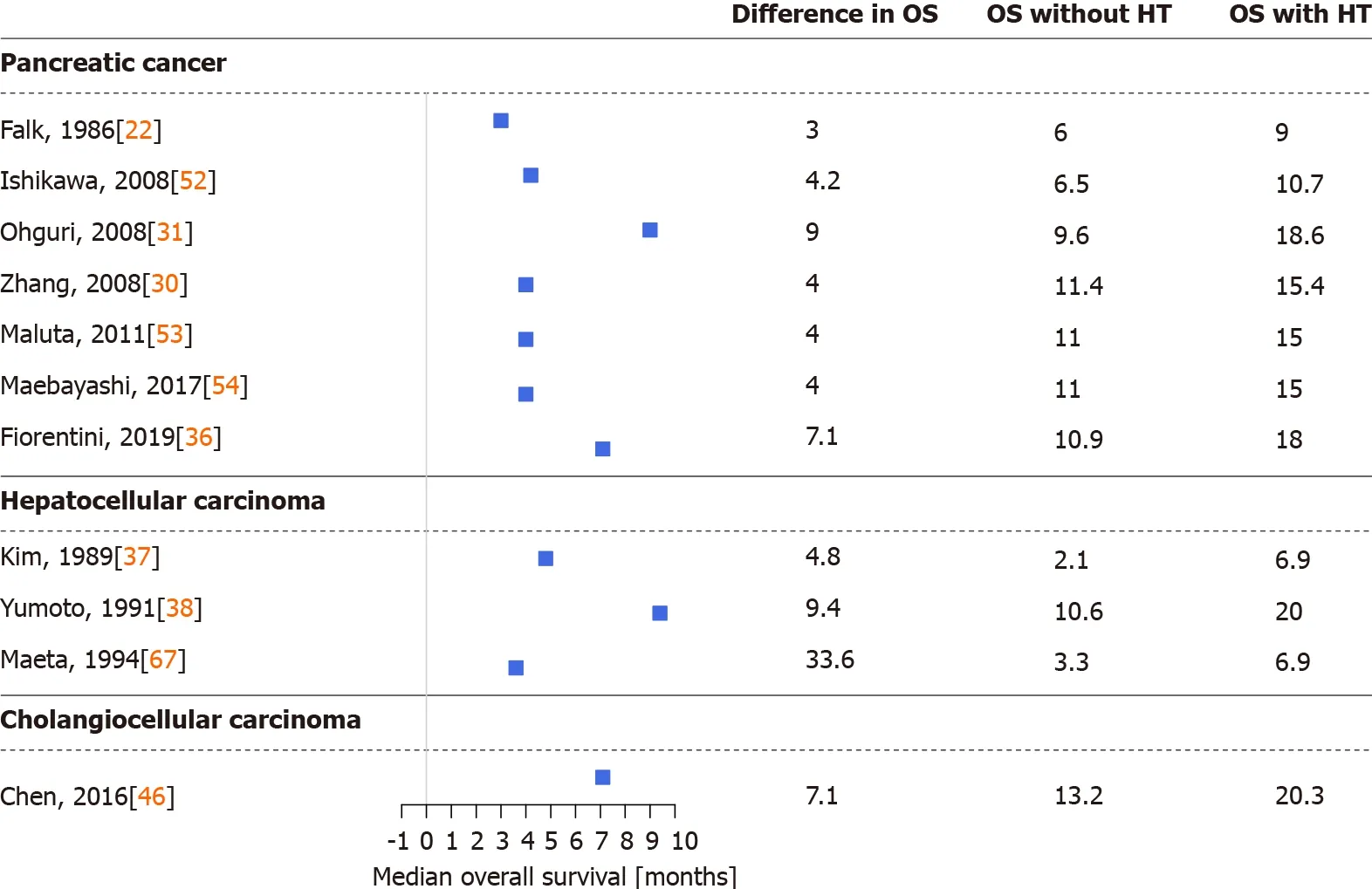
Figure 2 Differences in median overall survival between cohorts treated with and without hyperthermia.HT:Hyperthermia;OS:Overall survival.
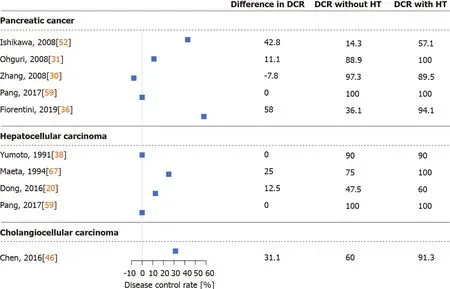
Figure 3 Differences in the disease control rate between cohorts treated with and without hyperthermia.DCR:Disease control rate;HT:Hyperthermia.
HYPERTHERMIA IN CANCER TREATMENT
Hyperthermia in oncology is the artificial raising of the temperature of the tumor to approximately 39-45 ℃viafull body or local heating methods[18].In brief,heating isachieved by various biophysical techniques,namely capacitive and radiative methods,which have tumoricidal effects on cancer cells through direct metabolic effects or heating or an indirect immunomodulatory impact.Radiotherapy-resistant,hypoxic,and S-phase cells can be killed.Enhancement of the radiation effectviainhibition of DNA damage repair has been observed following combined administration of adjuvant hyperthermia with radiotherapy[18-20].Hyperthermia also has a sensitizer effect on chemotherapyviaincreased cytotoxicity[13,15,18].
The earliest studies on hyperthermia,published in the late 1970s investigated only superficial cancers[21];trials on deep-seated tumors have been conducted since the 1980s[22,23].Two major hyperthermia types can be distinguished.Whole-body hyperthermia involves the patient’s entire body surface,while in local/regional hyperthermia techniques only the tumor and its surroundings(i.e.the pelvic or abdominal region)are exposed to the electric field[18].Early hyperthermia studies of hepatopancreatobiliary cancers included both whole-body and regional hyperthermia,but most of the latest studies evaluated only regional hyperthermia and modulated electrohyperthermia(mEHT),the latest advancement in regional hyperthermia[24].The easier application of regional hyperthermia and a few additional adverse effects of whole-body hyperthermia,detailed below,may among the factors behind why wholebody hyperthermia has recently been used less frequently.
After whole-body hyperthermia,a significant increase in beta-endorphin,adrenocorticotropic hormone,and cortisol levels have been observed.Furthermore,the longer the hyperthermia treatment lasted,the higher the hormone levels rose,and normalization of hormone levels required at least a day following treatment[17].Side effects of whole-body hyperthermia can be vomiting,nausea,low-grade fever ≤ 24 h after treatment,as well as a small increase in liver functions,and increasedHuman alphaherpesvirus 1and urinary tract infections[25-27].It should be also emphasized that during the procedure,patients have to be in a light conscious sedation.Moreover,whole-body hyperthermia is a longer procedure.Usually,1-2 h are necessary to raise the body temperature,followed by maintaining the temperature reached,and a cooling phase is also necessary,resulting in a total procedure time of at least 4-6 h[25-28].
Regional hyperthermia,in comparison,does not require sedation and only takes 30-60 min.Common adverse events related to regional hyperthermia and mEHT during the procedure are thermal stress,local discomfort,hot sensation,abdominal and back pain,bolus pressure,position-related discomfort or pain,tachycardia,and grade I and II burns at the heated region[29-46].Increased liver enzymes,skin rashes at the heated region,and occasional mild subcutaneous fat necrosis,nausea,dyspnea,and urinary urgency can also occur after the procedure[29,39-46].Several studies have reported a higher incidence of hematotoxicity in patients treated with hyperthermia than in those without hyperthermia[30,31,47],possibly associated with the increased cytotoxicity of chemotherapeutic agents in hyperthermia[13,15,18].Various combination of therapy options can also increase or decrease hyperthermia-related tolerability.Most of the studies reported basically 100% tolerability,but Nagataet al[41]reported that hyperthermia had to be discontinued in almost every fifth patient.
HYPERTHERMIA IN PC
Current treatment options for PC are surgical resection with postoperative chemoradiotherapy,and systemic chemotherapy for borderline resectable and locally advanced or metastatic PCs[3].Historically 5-fluorouracil was the most used chemotherapy agent in advanced and metastatic PCs,but was replaced by gemcitabine and FOLFIRINOX(folinic acid + 5-fluorouracil + irinotecan + oxaliplatin)starting in the mid-1990s[48,49].Hyperthermia has been introduced as an auxiliary treatment for advanced and metastatic PCs.
Whole-body hyperthermia was shown to improve the effect of melphalan on blood cell counts in refractory cancers including PC[26],but no such effect was observed if carboplatin was administered alone[25].A treatment regimen of monthly cisplatin +gemcitabine with whole-body hyperthermia combined with continuous low-dose interferon-α had somewhat improved survival compared with standard chemoradiotherapy in metastatic PC,and a higher partial response rate to thermo-chemotherapy[27].In another study,advanced PC patients,who had achieved partial remission,had longer median survival than those who had not responded to the treatment(11.4 movs15.8 mo)[28].A clinical study(NCT04467593)is currently investigating the effects of whole-body hyperthermia on current chemotherapy with gemcitabine and FOLFIRINOX[50].
Several studies demonstrated that thermo-chemotherapy of locally advanced or metastatic PCsviaregional hyperthermia had a positive effect on patient survival and therapeutic efficacy.During the last three decades,multiple chemo,radio,and other therapies and procedures were combined with regional hyperthermia in PC[22,23,29-36,47,51-59].In detail,thermo-chemotherapy with selective immune stimulation by copovithane or PZ-73C resulted in longer patient survival[22].In other studies[23,51],patients treated with chemoradiotherapy and complementary hyperthermia had better disease control rates than those without hyperthermia,doubling the observed responses to treatment.Furthermore,the partial response rate increased along with increased maximum output power of the hyperthermia device[51].Three-dimensional conformal γ-knife radiotherapy with thermo-chemotherapy has also shown improved survival and response rates when combined with hyperthermia[30](Figures 2 and 3).
After the introduction of routine clinical use of gemcitabine and FOLFIRINOX,several studies investigated the effect of regional hyperthermia on those treatments[29,31-35,47,52-57].Gemcitabine alone had worse overall survival and treatment response than when used with complementary hyperthermia[29,47,52],and better progressionfree and overall survival have also been reported with the concomitant use of hyperthermia in combination with radiotherapy[31,53,54].The previous observation that increasing the power output of the hyperthermia device increases treatment response[51]was not confirmed in the case of gemcitabine[31].Supplementation of gemcitabine thermo-chemotherapy with other chemotherapeutic agents such as cisplatin[33,34]or oxaliplatin[32]had similar results.Patients in the hyperthermia arm had better partial response rates and better survival than those without hyperthermia(Figure 2).Combined hyperthermia with metabolically supported chemotherapy(i.e.gemcitabine or FOLFIRINOX during artificial mild hypoglycemia,serum glucose <4.0 mmol/L caused by low-dose bolus insulin),hyperbaric oxygen,and a ketogenic diet[55];and hyperthermia with a modified FOLFIRINOX protocol(i.e.a reduced dose of irinotecan and no 5-fluorouracil)[35]have highlighted the importance of the impact of hyperthermia on overall and progression-free survival.In addition,the currently running HEAT(NCT01077427)[60]and HEATPAC(NCT02439593)[61,62]clinical trials will further broaden our knowledge of the efficacy and safety of hyperthermia combined with gemcitabine in PC.
A few case reports have been published recently where chemoradiotherapy and hyperthermia were combined with herbal remedies.One German[56]and two Italian[57]reports described treating metastatic PC patients with chemoradiotherapy and hyperthermia supplemented with subcutaneous,fever-inducing mistletoe(Viscum album)extract and other immunomodulating supplements including curcumin or shiitake(Lentinula edodes)derivatives.All three patients had survived more than 30 mo with unrestricted quality of life;no deaths were reported at the time of publication[56,57].
As with conventional regional hyperthermia,positive effects of mEHT on progression-free and overall survival,and on improved disease control rate have been observed[36,58].Metastatic tumors,including PC patients with ascites,have shown better response(absorption of ascites)and quality of life when mEHT with traditional Chinese herbal remedy therapy was administered,compared with patients on chemotherapy and regular drainage[59].A possible correlation between the time from diagnosis to the first mEHT treatment and the survival time from first mEHT treatment was proposed[36].
HYPERTHERMIA IN HCC
Early-stage HCCs are treated with surgical resection or liver transplantation,radiofrequency or microwave ablation,or embolization methods with or without chemoradiotherapy.Intermediate and advanced-stage HCCs are generally treated with systemic and combination therapies[1].Early studies of hyperthermia in HCC investigated the combination of hyperthermia with chemoradiotherapy,transarterial embolization,or transcatheter arterial chemoembolization[23,37-41,63-69].Significantly longer survival,lower serum alpha-fetoprotein levels,and better response to treatment,even in tumors>7 cm[23],were observed in those reports[23,37-41,63-69].According to the results of one study[64],the best results were achieved if the intratumor temperature reached >42 °C,while in another study[39]tumors located in the left lobe of the liver were more responsive to combined treatment with hyperthermia.The latter observation may have resulted from a technical aspect of the treatment,as noted by the authors[39].In addition to the above,investigations of which treatment option benefits the most from hyperthermia in HCC have found that the best survival and response data have been observed in cases of immunotherapy with hyperthermia[41](Figures 2 and 3).
Combining transcatheter arterial chemoembolization,radiotherapy and hyperthermia[43,44],conformal radiotherapy with hyperthermia[20],and mEHT with sorafenib[42]or traditional Chinese herbal remedy therapy[59]were shown to improve the normalization of laboratory results,disease control rate,progression-free and overall survival,and 1-year recurrence and mortality rates.Results of the CERT[43,44]study supplement the above with the following:(1)radiotherapy related gastroduodenal toxicity(e.g.,ulcers,gastroduodenitis,and others)were significantly lower in the hyperthermia cohort;and(2)patients with better tolerance for higher power hyperthermia had the same treatment response rate and survival,suggesting that an increased power output level did not adds to treatment efficacy.
It is known from model systems[70-72]that heating to fever-range temperatures improves the immune response against tumors,while tumoricidal temperatures(>42°C)inhibit host competence.Because of the latter observation,whole body hyperthermia at tumoricidal temperatures is considered to have an unfavorable effect on the immune system,while regional hyperthermia does not have this effect,as the tumorsurrounding tissue only heats to fever-like temperatures[70-72].Investigation of the CD4+and CD8+T,and natural killer(NK)cell autoimmunity after the first treatment and after a whole hyperthermia treatment course in HCC[72]revealed that antitumoral changes occur in those cells and last up to at least 2 mo:NK cells are the first to respond to hyperthermia.Increased NK cell activity has been observed in patients with below normal or normal levels of pretreatment NK activation.Patients with increased pretreatment NK activity had a slight decrease after treatment,but the difference was not statistically significant.CD4+T cell activation was slightly decreased and CD8+increased,both after the first and after the complete hyperthermia regimen[72].Furthermore,in addition to a decrease of serum alpha-fetoprotein levels and abdominal circumference,intraperitoneal perfusion of cytokine-induced killer cells in combination with regional hyperthermia increased the post-treatment activation of CD4+,CD3+CD8+,and CD3+CD56+T-cell populations in advanced HCC patients[73].
HYPERTHERMIA IN BILIARY TRACT CANCERS
Of the hepatopancreatobiliary cancers,hyperthermia is the least studied in BC.Conventional treatment options of BC are similar to those of PC and HCC;surgical resection for resectable cases and chemoradiotherapy and systematic combination therapies for advanced and metastatic cases[2].Until the mid-2000s no studies had been specifically designed to investigate hyperthermia in BC.The results of studies investigating the effect of hyperthermia on mixed tumor types are available[26,29,41,51,63,64],in which a few biliary cases were also presented.Positive effect of hyperthermia on tumor response rate,tumor markers,and survival have been reported[26,29,41,51,63,64];and the combination of hyperthermia and transcatheter arterial embolization,chemo- or radiotherapy have been considered as equally good combinations of possible therapies in advanced tumors of the biliary tract[41].
Adjuvant hyperthermia with chemotherapy has increased the treatment response rate and overall survival of BC patients[74,75].Improvements in quality of life(i.e.fewer tumor related symptoms)and laboratory results were reported in a detailed case report of a patient with unresectable hilar CCC[75].Similarly,extending hepatic arterial infusion chemotherapy with hyperthermia in patients with advanced BC have resulted in longer progression-free and overall survival and in better disease control rate[45,46](Figures 2 and 3).The positive effects of hyperthermia were observed after the first few treatments[46],complete responses have been reported[46],and no increased toxicity after chemotherapy occurred[45].
CONCLUSION
One of the major challenges in advanced and metastatic hepatopancreatobiliary cancers is to find a treatment regimen that increases therapy efficacy but has no additional adverse effects.Adjuvant hyperthermia fulfills those expectations.Patients treated with additional hyperthermia have been reported to have significantly higher local and overall response to treatment,longer progression-free and overall survival,and improved laboratory results.Several studies and case reports suggest that complementary hyperthermia also improved quality of life.Furthermore,there have been a few cases in which patient returned to his or her previous active life after treatment.As with every therapeutic method,hyperthermia also has some adverse effects,which are associated with the local heating process;all have been considered to be grade II complications at the highest.It has to be mentioned however,that regional hyperthermia and mEHT is more advantageous and more accepted among patients than whole-body hyperthermia as no conscious sedation is needed,and the former do not require long treatment times.
The application of hyperthermia has not found its exact clinical indication in the setting of disease stage.For most patients,it is a palliative treatment when no other possibility is available in the traditional oncological armamentarium.Thus,a refined or subdivided stage 4 category would be most beneficial to stratify the patients according to tumor load and involved organs,supplemented by serum tumor marker levels.Tumor markers like lactic dehydrogenase as used in stage 4 melanoma might be an option[76].Certain organs that are vital or less crucial could also be taken into account when containing metastases.To our knowledge,no such classification exists for the stratification of patients for hyperthermia treatment.Higher Eastern Cooperative Oncology Group performance status or increased amount of body fluids(ascites,hydrothorax,other edema)may be limitations in the administration of hyperthermia.In future analyses,it is encouraged to address the question of tumor load and the capabilities of the patient to overcome the neoplastic disease.
Summarizing all of the above,hyperthermia in advanced hepatopancreatobiliary cancers is an effective complementary treatment option with several promising positive results extending current protocols.To date,its usage in these three cancer types is mainly limited to research only.However,based on the clinical results and observations provided in this review,its routine use in advanced liver-PC care,especially with further immunomodulation,should be considered.
ACKNOWLEDGEMENTS
We are grateful to Viktor Madar-Dank for English proofreading.
 World Journal of Gastrointestinal Oncology2021年9期
World Journal of Gastrointestinal Oncology2021年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Use of liquid biopsies in gastrointestinal cancers
- Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer:A metaanalysis
- Prognostic value of modified Lauren classification in gastric cancer
- Scoparone inhibits pancreatic cancer through PI3K/Akt signaling pathway
- Effect of oncometabolic surgery on gastric cancer:The remission of hypertension,type 2 diabetes mellitus,and beyond
- Characterization of metabolic landscape in hepatocellular carcinoma
