Neoadjuvant chemotherapy for colorectal liver metastases:A contemporary review of the literature
Marissa Guo,Ning Jin,Timothy Pawlik,Jordan M Cloyd
Marissa Guo,Department of Surgery,The Ohio State University Medical Center,Columbus,OH 43210,United States
Ning Jin,Department of Internal Medicine,Division of Medical Oncology,The Ohio State University Medical Center,Columbus,OH 43210,United States
Timothy Pawlik,Department of Surgery,The Ohio State University,Columbus,OH 43210,United States
Jordan M Cloyd,Department of Surgery,Division of Surgical Oncology,The Ohio State University Medical Center,Columbus,OH 43210,United States
Abstract Colorectal carcinoma(CRC)is one of the leading causes of cancer-related deaths worldwide,and up to 50% of patients with CRC develop colorectal liver metastases(CRLM).For these patients,surgical resection remains the only opportunity for cure and long-term survival.Over the past few decades,outcomes of patients with metastatic CRC have improved significantly due to advances in systemic therapy,as well as improvements in operative technique and perioperative care.Chemotherapy in the modern era of oxaliplatin- and irinotecancontaining regimens has been augmented by the introduction of targeted biologics and immunotherapeutic agents.The increasing efficacy of contemporary systemic therapies has led to an expansion in the proportion of patients eligible for curative-intent surgery.Consequently,the use of neoadjuvant strategies is becoming progressively more established.For patients with CRLM,the primary advantage of neoadjuvant chemotherapy(NCT)is the potential to down-stage metastatic disease in order to facilitate hepatic resection.On the other hand,the routine use of NCT for patients with resectable metastases remains controversial,especially given the potential risk of inducing chemotherapy-associated liver injury prior to hepatectomy.Current guidelines recommend upfront surgery in patients with initially resectable disease and low operative risk,reserving NCT for patients with borderline resectable or unresectable disease and high operative risk.Patients undergoing NCT require close monitoring for tumor response and conversion of CRLM to resectability.In light of the growing number of treatment options available to patients with metastatic CRC,it is generally agreed that these patients are best served at tertiary centers with an expert multidisciplinary team.
Key Words:Colorectal liver metastases;Neoadjuvant chemotherapy;Hepatic resection;Conversion therapy;Chemotherapy-associated liver injury;Disappearing liver metastases;Future liver remnant;Immunotherapy
INTRODUCTION
Colorectal carcinoma(CRC)is a leading cause of cancer-related death among Western populations and the second most common malignancy worldwide[1,2].In the United States,around 148000 new cases are diagnosed each year.Between 40%-50% of patients with CRC develop colorectal liver metastases(CRLM)during the course of their disease[3-5].Up to 70% of these patients have synchronous CRLM at their initial presentation[4-7].Historically,metastatic CRC has been associated with poor survival.However,outcomes of patients with CRLM have improved significantly over the past few decades due to advances in systemic therapy and locoregional treatment,each of which have contributed to the expansion of safe hepatic resection.As a result of these advances,the median five-year survival rate of patients with metastatic CRC has risen from <10% to 35%-40%,while median overall survival(OS)has increased from <12 mo to approximately 42 mo[7-9].
Surgical resection of CRLM is generally considered necessary for potential longterm survival and has been shown to improve OS[9-11].Fortunately,advances in operative technique,perioperative care,and effective chemotherapy have increased the proportion of patients eligible for curative-intent surgery[12].Indeed,the growing efficacy of systemic treatments has accentuated the role of surgery for CRLM by improving patient selection for liver-directed therapies and downstaging initially unresectable tumors.Nevertheless,the routine delivery of chemotherapy before surgery remains controversial given the lack of consistent evidence demonstrating an associated survival benefit,as well as the risk for chemotherapy-associated liver injury(CALI).In this review,we present the most recent literature on neoadjuvant chemotherapy(NCT)for CRLM and discuss the rationale,supporting evidence,technical considerations,and current indications for its use.
RATIONALE FOR NCT
Several empirical and theoretical benefits are associated with NCT,and in turn,it is increasingly utilized in the treatment of many solid-organ cancers[13].With the improved availability and effectiveness of modern systemic therapies,neoadjuvant strategies have become progressively more established in the management of advanced malignancies requiring a multimodal approach.For patients with CRLM,the primary advantage of NCT is the potential to render previously unresectable tumors resectable in what is often termed “conversion therapy.” NCT can down-stage metastatic disease to resectability in up to 35% of these patients[14-17].Given the importance of surgical resection to patient prognosis,current guidelines recommend the initiation of systemic therapy in those who have unresectable CRLM,coupled with close monitoring and continued re-evaluation for operative candidacy by an experienced multidisciplinary team[18].
Even among patients with initially resectable disease,downstaging with NCT could have certain advantages.Preoperative chemotherapy may facilitate a parenchymalsparing or minimally invasive surgical approach,as well as increase the likelihood of obtaining negative margins.In addition,the use of NCT could lead to better selection of surgical candidates by allowing time to identify those who have rapidly progressive metastatic disease and therefore would not benefit from a potentially morbid operation.In contrast,postoperative complications following major liver surgery can prohibit some patients from receiving adjuvant chemotherapy,and the administration of chemotherapy preoperatively can increase the probability of completing all intended treatment by those who would benefit the most from a multimodal approach.Moreover,it is thought that the delivery of early chemotherapy may reduce the risk of recurrence by prioritizing the treatment of micro-metastatic disease.Lastly,monitoring of the radiographic,biochemical,and histological response to NCT provides important prognostic information that may guide future management.
Despite these potential advantages,enthusiasm for NCT has been tempered by reports of hepatotoxicity associated with common chemotherapeutic agents used to treat CRC,especially given the concern that the resulting liver injury may preclude hepatic resection.5-Fluorouracil can cause steatosis,although this is generally considered clinically insignificant.Oxaliplatin can lead to sinusoidal injury,which,in the absence of severe injury resulting in portal hypertension,does not usually contribute to increased postoperative mortality[19].Irinotecan,however,has been linked with clinically significant steatohepatitis that,in retrospective studies,has been associated with increased rates of postoperative liver insufficiency and mortality[20-22].However,evidence is mixed on the association of NCT with increased risk for postoperative complications.Although higher rates of complication have been reported in patients who received NCTvsindividuals who underwent immediate hepatectomy for CRLM(25%vs16%,P=0.04)in the European Organization for Research and Treatment of Cancer(EORTC)intergroup trial 40983[23],a populationbased study using the American College of Surgeons National Surgical Quality Improvement Program found no significant difference in the rates of postoperative morbidity or mortality[24].
Other commonly cited disadvantages of NCT include failure to proceed with potentially curative resection due to local disease progression,toxicity of systemic therapy,challenges in identifying sites of metastases experiencing complete macroscopic response(disappearing liver metastases,DLM),and inconsistent data related to survival benefits associated with its routine use(Table 1).
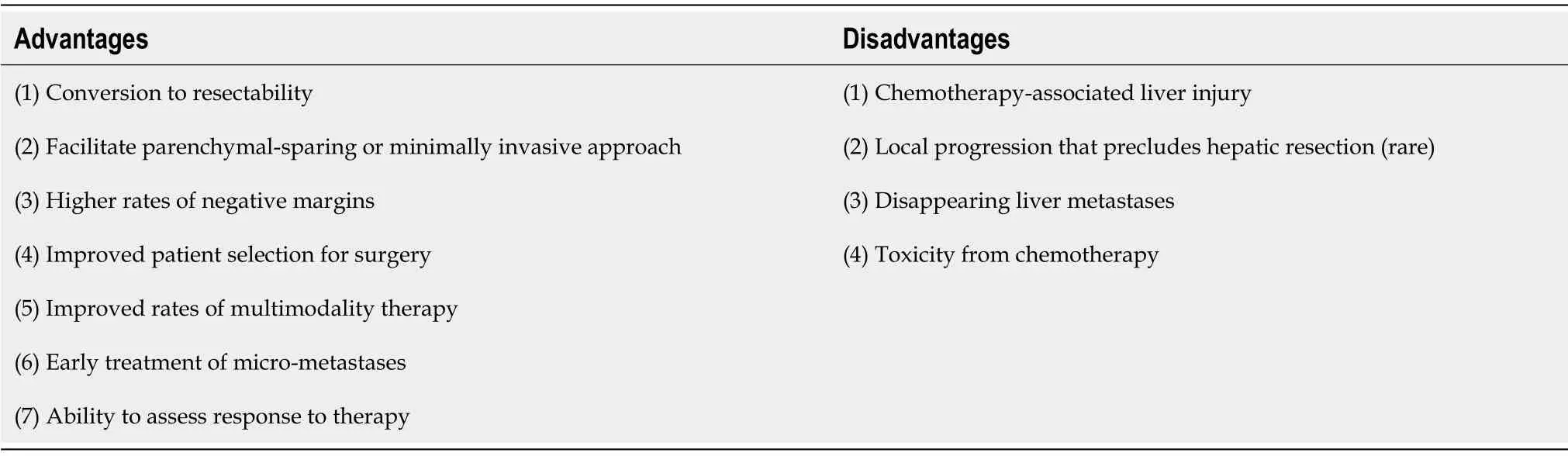
Table 1 Potential advantages and disadvantages of neoadjuvant chemotherapy for colorectal liver metastases
NCT
Chemotherapy regimens
First-line chemotherapy for metastatic CRC primarily consists of fluorouracil-based regimens containing oxaliplatin and/or irinotecan.Current NCCN guidelines list FOLFOX(fluorouracil,leucovorin,and oxaliplatin),FOLFIRI(fluorouracil,leucovorin,and irinotecan),XELOX(capecitabine and oxaliplatin),and FOLFOXIRI(fluorouracil,leucovorin,oxaliplatin,and irinotecan)as recommended courses for systemic therapy[18].The choice of regimen largely depends on the patient’s performance status and the presence ofRASorBRAFmutation[25].Among patients who can tolerate a more intensive regimen,triplet therapy with FOLFOXIRI is an option that may lead to higher response rates[17,26].
Biological agents
The recent development of targeted biologic agents has further increased the number of treatment options available to patients with metastatic disease and become the focus of newer studies investigating perioperative systemic therapy.The COIN trial was a randomized trial examining the effects of adding cetuximab,a monoclonal antibody that binds EGFR,to standard oxaliplatin-based chemotherapy regimens as first-line treatment for patients with advanced CRC.Although the addition of cetuximab did not affect overall or progression-free survival(PFS),higher rates of tumor response were reported in patients with wild-typeKRASgenotype[27].Based on these results,the New EPOC(eloxatin perioperative chemotherapy)randomized controlled trialwas conducted to evaluate the outcomes of adding cetuximab to oxaliplatin- or irinotecan-based perioperative chemotherapy in patients with potentially resectable CRLM.With an overall median follow-up of 66.7 mo,both PFS(15.5 movs22.2 mo,P=0.304)and median OS(55.4 movs81.0 mo,P=0.036)were shorter in the chemotherapy plus cetuximab group than in the chemotherapy alone group[28,29].The results of this study were unexpected,and it was theorized that molecular modifications in theKRASpathway rendered the tumors more resistant to treatment.Indeed,significantly higher rates of response,PFS,and OS had previously been observed in patients withKRASwild-type disease treated with cetuximab in addition to FOLFIRI compared with patients receiving FOLFIRI alone[30,31].Similarly,the addition of panitumumab,another anti-EGFR monoclonal antibody,to FOLFOX was shown to improve PFS in patients withKRASwild-type CRC[32].While the addition of EGFR inhibitors to chemotherapy regimens continues to be recommended for patients with advanced metastatic CRC,it should not be used in those with initially resectable CRLM.
Bevacizumab,a monoclonal antibody that binds VEGF,is a newer biologic agent that is theorized to increase response rates and median OS among patients with metastatic CRC when added to standard chemotherapy regimens[33].Currently,there are two ongoing clinical trials investigating its role as an adjunct to perioperative chemotherapy for CRLM.The goal of the PERIMAX trial will be to compare resection and adjuvant FOLFOX to perioperative FOLFOXIRI with bevacizumab in patients with resectable CRLM,while the CHARTA trial intends to evaluate the effect of adding bevacizumab to either FOLFOX or FOLFOXIRI in the treatment of patients with unresectable CRLM[34].Initially,there was concern that major complications associated with bevacizumab,such as thromboembolism,bowel perforation,bleeding,and impaired wound healing,could interfere with surgical resection.However,numerous studies have since demonstrated the safety of its use in the preoperative setting when administered 5 or more weeks prior to surgery[35-41].In the BECOME trial examining the use of bevacizumab in patients withRASmutant,unresectable CRLM,significantly improved outcomes were noted among individuals treated with chemotherapy plus bevacizumab compared to those treated with chemotherapy alone.With a median follow-up time of 37.0 mo,patients who received mFOLFOX6 plus bevacizumab had higher rates of R0 resection(22.3%vs5.8%,P<0.01),response rates(54.5%vs36.7%,P<0.01),median PFS(9.5vs5.6 mo,P<0.01),and median OS(25.7vs20.5 mo,P=0.03)vstheir counterparts who received mFOLFOX6 alone[42].In practice,bevacizumab is frequently omitted from the final cycle of NCT prior to liver resection in order to limit safety concerns at the time of surgery.
INDICATIONS FOR NCT
Patients with initially resectable disease
For patients with resectable CRLM at the time of presentation,the routine use of NCT over upfront surgery is controversial.For some malignancies,such as pancreatic adenocarcinoma,all patients have been found to benefit from adjuvant chemotherapy.Therefore,strategies involving the delivery of chemotherapy before surgery for these patients is rational as it ensures the receipt of all intended treatment[43].In contrast,it is not clear that all patients with resectable CRLM would benefit from the receipt of systemic therapy at all,whether in a neoadjuvant or adjuvant setting.
The use of fluorouracil-based chemotherapy as an adjuvant treatment strategy following resection of CRLM was investigated in two multicenter phase III trials,the Federation Francophone de Cancerologie trial 9002[44]and the EORTC trial 40923[45].Although preliminary results demonstrated a trend toward improved disease-free survival(DFS)and OS in patients who received chemotherapy after hepatic resection,both were closed prematurely due to slow accrual.In a pooled analysis of both trials(n=278),longer median PFS(27.9 movs18.8 mo,P=0.058)and OS(62 movs47 mo,P=0.095)were associated with adjuvant chemotherapyvssurgery alone[46].In a recent study conducted by the Japan Clinical Oncology Group,300 patients were randomized to either hepatectomy alone or hepatectomy with adjuvant mFOLFOX.While DFS was improved in the adjuvant chemotherapy group,OS was worse,potentially due to an increased number of deaths following disease recurrence[47].
Only one randomized controlled trial has been conducted on the use of chemotherapy prior to surgery in patients with resectable CRLM.In the EORTC intergroup trial 40983[48],364 patients were randomized to receive either perioperative chemotherapy(with six cycles of FOLFOX4 before and after surgery)or surgery alone.Resectable disease was defined as £4 Liver lesions,absence of extrahepatic metastases,and a primary tumor that had been or could be completely resected;the primary endpoint was PFS.The administration of perioperative chemotherapy resulted in a 9.2% increase in 3-year PFS from 33.2% to 42.4%(P=0.025),but no significant difference in OS.Although patients receiving perioperative chemotherapy experienced higher rates of hepatic failure(7%vs5%),biliary fistulas(8%vs4%),and intraabdominal infection(7%vs2%),operative mortality was <1% in both groups.Overall,these early results demonstrated a modest but statistically significant benefit for perioperative administration of FOLFOX without significant added morbidity or mortality.Long-term results of the EORTC 40983 trial were published five years later focusing on OS.After a median follow-up of 8.5 years,no significant difference in OS was noted between the two groups(52.4% of chemotherapy patientsvs48.3% of surgery only patients,P=0.34)[23].Median OS was 63.7 mo among individuals who received perioperative chemotherapy and 55.0 mo among those who underwent surgery alone.
Interestingly,the EORTC 40983 trial continues to be cited in support of both NCT and upfront surgery.Proponents of NCT cite the nearly 10% improvement in PFS,while those who support upfront surgery point to the lack of difference in OS.It is important to note that this trial was largely comprised of metachronous and oligometastatic CRLM,which typically represent patients with more favorable prognoses.Focusing solely on patients with synchronous liver metastases,a large retrospective study was published reporting no difference in OS between patients who received NCTvsthose underwent surgery alone.However,patients who received NCT had worse DFS in the setting of multi-centric metastatic disease[49].Of note,the same study reported that postoperative chemotherapy was associated with improved OS and DFS.In contrast,the use of NCT was associated with decreased OS and DFS in a meta-analysis including 17 cohort studies including the EORTC 40983 trial[50].However,these results appeared to be confounded by the fact that patients undergoing NCT typically presented with a larger disease burden.When only studies with patients at high risk for disease recurrence were included,NCT was associated with improved survival(pooled HR for 5-year OS=0.69).CHARISMA is a currently ongoing multicenter phase III clinical trial that aims to determine whether the addition of NCT to surgery will improve OS in a well-defined high-risk patient group with CRLM[51].
Patients with initially unresectable disease
For patients with unresectable disease at the time of diagnosis,the decision to proceed with chemotherapy is more straightforward.Multiple studies have demonstrated the ability to down-stage initially inoperable CRLM with preoperative chemotherapy to facilitate secondary hepatic resection.Between 12%-35% of patients with unresectable CRLM experience a sufficient response to chemotherapy and are subsequently able to undergo surgery with curative intent[14-17].Five- and ten-year survival following NCT and resection in these patients have been reported at 33% and 23%,respectively[14,52].Some of the variability in survival between different studies can be attributed to the use of different chemotherapeutic regimens.Indeed,one study reported that rates of R0 resection and OS were higher among patients who were treated with FOLFOXIRI compared with individuals receiving FOLFIRI before surgery[17].In addition,the definition of “unresectable” disease varied depending on study criteria,surgeon opinion,and quality of cross-sectional imaging.Nevertheless,it is clear that some patients with primarily unresectable CRLM can experience successful downstaging prior to hepatic resection which,when feasible,is associated with improved OS.
Current guidelines
At this time,NCT is not recommended for all patients with CRLM.Recommendations from multiple consensus statements generally advise upfront resection followed by adjuvant chemotherapy in patients with resectable disease and low operative risk(medically fit with four or fewer lesions),and reserve NCT for patients at higher operative risk with borderline resectable or unresectable disease(Table 2).Overall,guidelines on the delivery of perioperative chemotherapy,either pre- or posthepatectomy,in patients with resectable CRLM are not well established as more conclusive data on the optimal regimen and timing of chemotherapy is necessary.

Table 2 Consensus statements and guidelines for the use of neoadjuvant chemotherapy in patients with colorectal liver metastases
TECHNICAL CONSIDERATIONS
Timing and duration
The optimal timing and duration of chemotherapy in relation to surgical resection for CRLM is not definitively known.The duration of NCT has varied widely among studies,though in most studies examining perioperative chemotherapy for CRLM,patients have undergone between 6 cycles to 10 cycles preoperatively,usually followed by 6 cycles to 8 cycles postoperatively[14,15,17,48,52].In one report,administration of more than 12 wk of chemotherapy and/or an interval of less than four weeks between chemotherapy and surgery was associated with increased postoperative complications,rates of reoperation,and longer hospital stay[53].Another study found that NCT consisting of FOLFOX with or without bevacizumab was not associated with an increase in complete or major pathologic response after 9 cycles,though it was associated with higher incidences of liver insufficiency and sinusoidal injury[54].Patients receiving extensive chemotherapy are also at risk for experiencing hepatic atrophy,which is associated with higher rates of postoperative mortality related to liver failure[55].Thus,avoiding unnecessarily lengthy courses of systemic therapy should be a priority before planned hepatectomy.For patients with initially unresectable disease,radiographic assessment should be performed every 2-3 mo and patients should proceed to surgery as soon as they achieve resectability.For individuals with resectable disease,2-3 mo of chemotherapy(as conducted in the EORTC 40983 trial)before and after liver resection is often preferred.
No prospective randomized trials have been performed to compare neoadjuvant(or perioperative)to adjuvant chemotherapy.This topic was evaluated in a retrospective multi-institutional study comparing the outcomes of different chemotherapy strategies,including pre-hepatectomy alone,post-hepatectomy alone,perioperative,and no chemotherapy,used to treat synchronous CRLM.Multivariate analysis revealed that timing of chemotherapy relative to hepatic resection did not impact recurrence-free survival(RFS).However,post-hepatectomy chemotherapy was associated with increased OS.Median OS for patients treated with no chemotherapy,pre-hepatectomy chemotherapy,post-hepatectomy chemotherapy,and perioperative chemotherapy were 36,53,76,and 67 mo,respectively(P<0.001).When narrowing the patient population to individuals with low-risk disease,patients receiving posthepatectomy and perioperative chemotherapy experienced even higher rates of survival relative to other treatment groups:median OS among patients receiving no chemotherapy,pre-hepatectomy chemotherapy,post-hepatectomy chemotherapy,and perioperative chemotherapy were 39,56,99,and 97 mo,respectively(P<0.001)[56].
Clinical risk scoring
The Fong clinical risk score is arguably the most well-known algorithm to assess prognosis in patients with CRLM being considered for resection.It assigns a point for each of the following variables:positive margin,extrahepatic disease,node-positive primary,disease-free interval from primary to metastases <12 mo,number of hepatic tumors >1,largest hepatic tumor >5 cm,and carcinoembryonic antigen(CEA)level >200 ng/mL,as these factors were significant and independent predictors of poor longterm outcomes[57].A variety of other models for clinical risk stratification have since been developed.Of note,in a study evaluating the accuracy of eight recognized scoring systems,including the Fong clinical risk score,only the Rees postoperative index was a significant predictor of disease-free and disease-specific survival at 1,3,5,and 10 years[58].Overall,the clinical relevance of risk scores has been challenged.In particular,while these risk scores may function relatively well in predicting survivaloutcomes,their use in guiding decisions in the management of CRLM has not been validated.
Newer scoring systems that includeRASmutational status as a prognostic factor have outperformed traditional scoring systems[59].KRASmutation is present in approximately 15%-36% of patients with CRLM and has been found to be an independent predictor of decreased RFS[hazard ratio(HR)=1.89]and OS(HR=2.24)[60].In patients treated with FOLFOX4 plus bevacizumab,radiographic and pathologic rates of response were worse inKRASmutants(10.5% and 36.8%)compared to wild-type(32.9% and 58.9%)[61].Among patients treated surgically for CRLM,those withKRASmutation had lower five-year disease-specific survival than their wild-type counterparts(29%vs21%,P=0.024)[62].BRAFis another commonly implicated gene in the pathogenesis of CRC,with the V600E mutation occurring in approximately 9% of patients with CRC[63].RFS was 5.7 mo in patients withBRAFmutation,compared to 11 mo in those withKRASmutation and 14.7 mo in those with wild-typeKRASandBRAF[64].Although the presence ofBRAFmutation is now considered when choosing a chemotherapy regimen,it is not currently factored into any clinical risk algorithms.With an increasing understanding of cancer biology and ability to detect molecular markers,future scoring systems will likely evolve to incorporate individual tumor mutational status and may be able to better select which patients stand to benefit from NCT.
Monitoring response to therapy
The Response Evaluation Criteria in Solid Tumors(RECIST)is used to objectively measure radiographic response to treatment for most solid organ cancers.However,conventional size-based RECIST criteria have been poor to predict pathologic response for CRLM[65].Modified criteria based on morphologic changes(mRECIST)has been superior to RECIST in assessing response to NCT,though neither were predictive of residual tumor burden[66].Optimal morphologic response to chemotherapy is defined as a change toward homogenous,hypoattenuating lesions with thin,sharply defined tumor-liver interface(Figure 1).While morphologic response correlates with pathologic response,the majority of treated CRLM(83%)that appear to have complete radiographic response to chemotherapy are subsequently found to have viable tumor on pathologic review[65,67,68].
Computed tomography(CT)scan remains the most common imaging study used in the initial diagnosis of CRLM.The PROMETEO-01 study demonstrated,however,that magnetic resonance imaging(MRI)was more sensitive than CT scan(91%vs82%,P=0.002),and that after NCT,the sensitivity of CT for detection of CRLM dropped to 71%[69].The use of positron emission tomography(PET)scan is not recommended as a tool for routine staging or surveillance,though it may have a role in the detection of occult extrahepatic disease[70].A meta-analysis comparing multiple imaging modalities in the preoperative detection of CRLM reported pooled sensitivity estimates of MRI,CT,PET,and PET-CT at 85.7%,69.9%,54.5%,and 51.7%,respectively[71].The investigators noted that,in the neoadjuvant setting,MRI was the most appropriate imaging modality for preoperative assessment of CRLM.In addition,they found that the diagnostic accuracy of PET and PET-CT scans were strongly affected by the use of chemotherapy.
Among laboratory work-up for CRC,CEA is commonly used for prognostication before resection,as well as for postoperative surveillance for recurrent disease.The use of CEA levels has also been studied in conjunction with imaging to monitor response to systemic therapy in metastatic CRC and can accurately predict disease nonprogression[72-76].In one large analysis of 2643 patients with metastatic CRC,a reduction in CEA levels by at least 7.5% from baseline differentiated non-progressive from progressive disease with a sensitivity of 71.6% and specificity of 76.2%.Using this cutoff,it was concluded that progressive disease could be excluded in 97.3% of patients and 72.8% of initial restaging CT scans obtained 3 wk after initiation of systemic chemotherapy could be avoided[77].It was therefore concluded that CEA levels,which are inexpensive and easy to obtain,are a useful adjunct to imaging for initial monitoring of response to systemic therapy in patients with metastatic CRC.
DLM
With the development of increasingly effective chemotherapeutic agents,dramatic tumor reduction has been observed following initiation of therapy in many cases of CRLM.The phenomenon in which there is a complete radiologic response in hepatic tumors on cross-sectional imaging is referred to as DLM.DLM can represent a unique problem in patients treated with NCT because although the tumor may become undetectable radiologically,this does not necessarily equate complete pathologic response(Figure 2).A number of imaging modalities can be used to detect CRLM both prior to and during surgery.In the preoperative setting,MRI,CT,and PET had sensitivity estimates of 85.7%,69.9%,and 54.5%,respectively[71].Meanwhile,the use of contrast-enhanced intraoperative ultrasound(IOUS)had the highest sensitivity(99%)compared with IOUS without contrast(88%),contrast-enhanced MRI(83%),and contrast-enhanced CT(81%)[78].While DLM has been described in 7%-37% of patients undergoing preoperative systemic therapy,residual disease,either macroscopic microscopic,or early disease recurrencein situhas been observed in 61%-83% of CRLM that appear to have a complete response on imaging[67,79-84].For patients with DLM leftin situ,the intrahepatic recurrence rate is significantly higher.Patients with untreated DLM had one- and three-year intrahepatic RFS of 40.2% and 16.1%compared to 68.8% and 35.1% for those whose original disease sites were resected(P=0.04)[80].Lesions at greatest risk for disappearance are those that are <2 cm in diameter or deeper than 1 cm into the liver parenchyma,while the likelihood of developing DLM increases with longer duration of NCT[80,85].In these situations,the placement of a fiducial marker can facilitate the localization of metastatic disease that has otherwise become undetectable on imaging[84,85].Alternatively,patients at highest risk for DLM may benefit from upfront surgery rather than NCT if clinically feasible.

Figure 1 Radiographic response to neoadjuvant chemotherapy.A:A 70-year-old man presented with a T2-hyperintense metachronous rectal liver metastasis,seen on magnetic resonance imaging;B:Computed tomography scan performed after 6 cycles of FOLFOX plus bevacizumab showed partial Response Evaluation Criteria in Solid Tumors response with decrease in maximum diameter from 9.6 cm to 7.0 cm,as well as optimal morphologic response.The section planes displayed above show the tumor in maximum diameter.The patient subsequently underwent right posterior sectionectomy with no evidence of disease 3 yr after surgery.
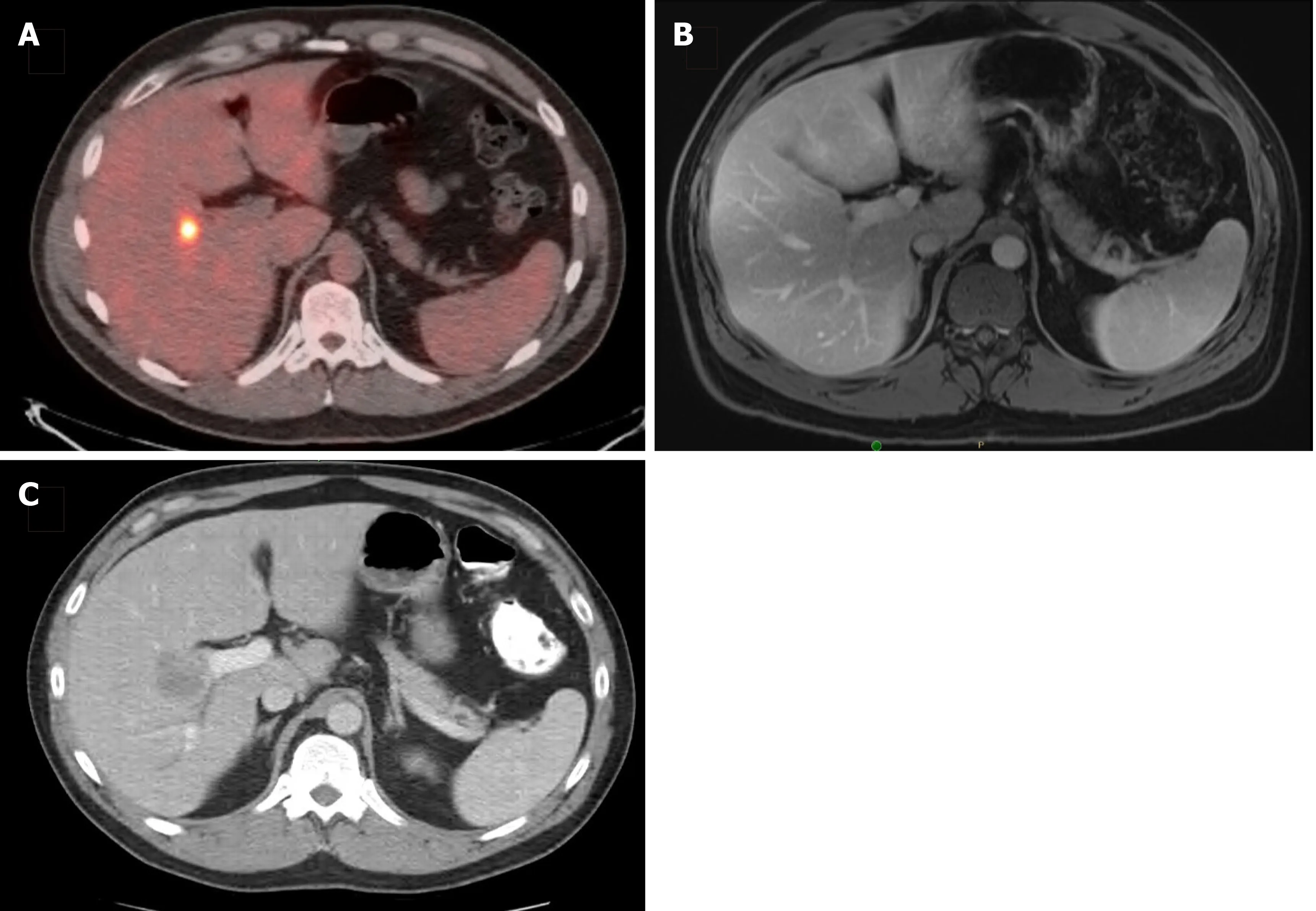
Figure 2 Disappearing liver metastasis.A:A 36-year-old man presented with a solitary synchronous colorectal liver metastasis to segment 5,seen on staging positron emission tomography-computed tomography scan;B:After 6 cycles of CAPOX plus bevacizumab,followed by pelvic radiation(5040 cGy in 28 fractions),subsequent magnetic resonance imaging(shown)and intraoperative ultrasound were unable to localize the lesion;C:He was then lost to follow-up and returned 18 mo later with an intrahepatic recurrence,seen on surveillance computed tomography scan and confirmed with tissue biopsy.
SURGICAL CONSIDERATIONS
Resection margins
Achieving negative margins at the time of resection remains an important determinant of survival for patients undergoing hepatectomy for CRLM.Data on the minimum margin width needed to optimize survival and avoid disease recurrence have been controversial.In one large meta-analysis,resection margins >10 mm were associated with significant improvement in OS at 3 years[relative risk(RR)=0.86],5 years(RR=0.91),and 10 years(RR=0.94),as well as in DFS at 3 years(RR=0.93)and 5 years(RR=0.88)after surgery[86].However,multiple large retrospective studies have failed to demonstrate a survival benefit in patients who underwent resection with margin width between 1-10 mmvs>10 mm[87-89].Nonetheless,the need for margins at least 1-mm wide has been well-established as significantly decreased OS has been associated with submillimeter margins(36 movs65 mo,P=0.03)[90].Notably,the negative impact of positive margins was most pronounced in patients with a suboptimal response to NCT[91].
Future liver remnant
Currently,CRLM are defined as resectable if it is anticipated that the disease can be completely resected,two adjacent liver segments can be spared,adequate vascular inflow and outflow and biliary drainage can be preserved,and the functional capacity/volume of the future liver remnant(FLR)will be sufficient[92].The FLR is most commonly measuredviaCT volumetry.Importantly,given the impact of chemotherapy on liver function,the threshold FLR volume may be higher in patients who have received NCT.For example,while a FLR ≥ 20% of total liver volume(TLV)has been proposed for patients with normal liver function,the presence of pre-existing liver disease or liver injury from systemic therapy necessitates increased preservation or augmentation of liver tissue.For patients with marked steatosis or patients who have received extensive chemotherapy prior to hepatic resection,a FLR volume >30%of TLV is recommended,while patients with frank cirrhosis require FLR volume >40% of TLV[93].
For patients projected to have insufficient FLR volume post-hepatectomy,preoperative portal vein embolization(PVE)can be pursued to induce hypertrophy of the non-involved portion of the liver.PVE has also been found to reduce the incidence of postoperative liver insufficiency among patients who experienced hepatic atrophy secondary to prolonged NCT[94].In patients with extensive bilateral liver metastases,the use of NCT to decrease tumor burden in combination with PVE and two-stage hepatic resection has been shown to improve survival.After a median follow-up of 50 mo,patients with advanced bilateral CRLM who completed two-stage hepatic resection following initial treatment with chemotherapy were found to have significantly increased OS compared with individuals who received chemotherapy only(64%vs15% at 5 years,P<0.001)[95].
ALTERNATIVE APPROACHES
Immunotherapy
Immune checkpoint inhibition directed against programmed cell death-1(PD-1)and cytotoxic T-lymphocyte antigen-4(CTLA-4)has revolutionized the treatment of CRC with microsatellite instability.Immunotherapeutic agents such as pembrolizumab and nivolumab(monoclonal antibodies targeting PD-1),and ipilimumab(a monoclonal targeting CTLA-4),have been introduced as alternative treatment options to chemotherapy for patients with metastatic CRC and high microsatellite instability(MSI-H)or mismatch repair deficiency(dMMR).Due to deficits in mismatch repair,these tumors have a high mutation burden that create abundant tumor-specific neoantigens,drawing T-cell infiltrates to the tumor microenvironment.In a phase III clinical trial,pembrolizumab monotherapy was found to be superior to chemotherapy when given to patients who had tumors with MSI-H and dMMR.Patients who were treated with pembrolizumab experienced longer PFS(16.5 movs8.2 mo,P<0.01)and higher rates of partial or complete response(43.8%vs33.1%)compared to those treated with established chemotherapeutic regimens,including mFOLFOX6 ±bevacizumab or cetuximab and FOLFIRI ± bevacizumab or cetuximab[96].Although the use of immunotherapy has not been well studied in the neoadjuvant setting,early investigations indicate that neoadjuvant immunotherapy either alone or with chemotherapy can be a viable treatment strategy for CRC with dMMR[97,98].Furthermore,several trials are currently underway to evaluate the safety and efficacy of combined immuno- and chemotherapy regimens[99].It is likely that in the future,immunotherapy may have a significant role in the neoadjuvant management of CRLM with dMMR.
Intra-arterial therapies
Strategies including hepatic arterial infusion(HAI)and trans-arterial chemoembolization(TACE)allow for the delivery of chemotherapy to a more targeted distribution within the liver while minimizing systemic effects.These locoregional treatment methods take advantage of the fact that liver metastases derive their blood supply predominantly from the hepatic artery circulation and allow for direct infusion of chemotherapeutic drugs at much higher concentrations than could be administered with systemic therapy.The perioperative use of HAI in patients with CRLM has been associated with increased rates of complete pathologic response and conversion to resectability,as well as improved OS[81,100-102].Despite these advantages,the practice of HAI carries a relatively high rate of technical failure.TACE,which arises from the same basic principles as HAI,does not have the same level of technical difficulty.The use of irinotecan-loaded drug-eluting beads(DEBIRI)has emerged as a safe and effective technique for the delivery of TACE to hepatic metastases.Patients treated with DEBIRI experienced improved survival and tumor response,as well as higher rates of conversion to resectability,compared with individuals treated with systemic therapy alone[103-105].The ability to deliver locoregional chemotherapy to hepatic metastases continues to be a topic of high interest given the potential to limit adverse systemic effects and preserve patient functional status prior to surgical resection,as well as administer more potent and targeted doses of cytotoxic agents.Comprehensive reviews of perioperative trans-arterial therapies have been recently published[106,107].
CONCLUSION
The treatment of CRC is unique from most other cancers in that surgery for curative intent is routinely performed for stage IV disease.Over the last two to three decades,innovations in medical therapies and surgical techniques,based on an increasingly individualized approach to oncological care,have dramatically expanded the number of treatment options for patients with metastatic CRC.For patients with CRLM,surgical resection remains the only chance for cure,though optimization of patients preoperatively and the prevention of disease recurrence postoperatively requires a multidisciplinary approach.The initial evaluation of patients with CRLM involves determining the resectability of their disease.For those with borderline resectable or unresectable CRLM,systemic chemotherapy is recommended as conversion to resectability can occur in a substantial proportion of these patients.In contrast,the routine use of NCT remains controversial for patients with resectable CRLM given the lack of existing data showing definitive benefit in these cases,while hepatotoxicity is a known risk of current standard chemotherapeutic agents.Future endeavors should seek to determine the optimal selection criteria for patients with high-risk resectable CRLM,as well as standardize the definition of resectability,establish universal criteria for conversion to resectability,and examine outcomes regarding quality of life in patients receiving NCT.
Overall,the landscape of oncological treatment continues to trend toward more personalized and targeted therapy,and efforts in characterizing the biochemical profile of individual tumors are underway with the goal of discovering novel chemoor immunotherapeutic agents with higher potency and lower risk for adverse effects.Advancements in recent years have expanded the opportunity for curative-intent treatment to an increasing number of patients,as well as resulted in significantly improved survival rates for those with CRLM.In face of the continuously growing number of possible therapies available for metastatic CRC,consensus statements have come to agree that these patients are best served at tertiary centers where a multimodal treatment strategy can be facilitated by an expert multidisciplinary team.Ultimately,outcomes for patients with metastatic CRC are expected to continually improve moving forward.
 World Journal of Gastrointestinal Oncology2021年9期
World Journal of Gastrointestinal Oncology2021年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Use of liquid biopsies in gastrointestinal cancers
- Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer:A metaanalysis
- Prognostic value of modified Lauren classification in gastric cancer
- Scoparone inhibits pancreatic cancer through PI3K/Akt signaling pathway
- Effect of oncometabolic surgery on gastric cancer:The remission of hypertension,type 2 diabetes mellitus,and beyond
- Characterization of metabolic landscape in hepatocellular carcinoma
