Involvement of integrin-activating peptides derived from tenascin-C in colon cancer progression
Motomichi Fujita,Hideo Suzuki,Fumio Fukai
Motomichi Fujita,Fumio Fukai,Department of Molecular Patho-Physiology,Tokyo University of Science,Noda 278-8510,Chiba,Japan
Hideo Suzuki,Department of Gastroenterology,University of Tsukuba,Tsukuba 305-8575,Ibaraki,Japan
Abstract Tenascin-C(TNC)is an adhesion modulatory protein present in the extracellular matrix that is highly expressed in several malignancies,including colon cancer.Although TNC is considered a negative prognostic factor for cancer patients,the substantial role of the TNC molecule in colorectal carcinogenesis and its malignant progression is poorly understood.We previously found that TNC has a cryptic functional site and that a TNC peptide containing this site,termed TNIIIA2,can potently and persistently activate beta1-integrins.In contrast,the peptide FNIII14,which contains a cryptic bioactive site within the fibronectin molecule,can inactivate beta1-integrins.This review presents the role of TNC in the development of colitis-associated colorectal cancer and in the malignant progression of colon cancer,particularly the major involvement of its cryptic functional site TNIIIA2.We propose new possible prophylactic and therapeutic strategies based on inhibition of the TNIIIA2-induced beta1-integrin activation by peptide FNIII14.
Key Words:Tenascin-C;TNIIIA2;Beta1-integrin;Integrin activation;Colitis-associated colorectal cancer;Colon cancer
INTRODUCTION
Extracellular matrix(ECM)proteins such as fibronectin(FN),collagen,and laminin provide a scaffold for cell adhesion and subsequently influence various physiological cellular processes,including cell differentiation,survival/proliferation,and migration.As one of the major components of the tumor microenvironment,the ECM affects the behavior of cells in the cancer microenvironment,such as cancer-associated fibroblasts(CAFs)and immune cells,resulting in cancer development[1].It therefore plays major roles in carcinogenesis and the malignant progression of cancer.
Integrins are a family of heterodimeric transmembrane glycoproteins composed of alpha- and beta-subunits that directly interact with components of the ECM.These integrins primarily mediate cell adhesion,migration,survival,proliferation,and differentiation.In contrast to membrane receptors for humoral factors such as cytokines and chemokines,integrins are unique in their ability to alter the binding affinity for ECM ligands.Integrins exist mainly in two different structural states,an inactive conformation lacking ligand-binding affinity and an active one with high affinity[2].On the other hand,integrin signaling contributes to the malignant progression of many cancers.For example,integrin alpha5beta1,a major FN receptor,is highly expressed in glioma/glioblastoma,with its expression levels reported to be associated with poor survival in glioma/glioblastoma patients[3].Alpha5-integrin promotes cell proliferation and the dissemination of glioblastoma cells[4],modulates angiogenesis[5],and contributes to temozolomide chemoresistance[6].Thus,the integrin alpha5beta1-mediated adhesive interaction of glioma cells may be associated with the acquisition of a highly aggressive phenotype in glioma/glioblastoma.Therefore,inhibition of integrin functions might be a promising therapeutic approach for cancer.
Tenascin-C(TNC)is a hexameric,multimodular ECM glycoprotein.It is poorly expressed in normal adult tissues but highly expressed in both inflammatory lesions and the tumor microenvironment[3,7-10].TNC is an endogenous activator of toll-like receptor 4,which triggers and amplifies inflammatory responses[11].In addition,TNC binds to integrin alphavbeta3 and alpha9beta1 to drive inflammatory responses by inducing the synthesis of proinflammatory cytokines,including interleukin(IL)-6,IL-1beta,and tumor necrosis factor-alpha[12].TNC is highly expressed and is thought to act as a major driving regulator of acute and chronic inflammatory diseases,including cardiac disease[13],arthritis[14],nephritis[15],sepsis[16],stroke[17],asthma[18],chronic obstructive pulmonary disease[19],and viral infections[20].Therefore,TNC may be a promising biomarker of disease activity and a therapeutic target in these inflammatory diseases.
Furthermore,the expression levels of TNC are associated with poor prognosis in patients with malignant tumors,such as glioma and breast and colon cancers[3,8,10].Accumulating evidence indicates a relationship between TNC and tumor progression.For example,TNC plays key roles in several processes of tumor progression related to proliferation[21,22],migration,invasion[23-25],angiogenesis[26,27],immunosuppression[28,29],cancer stemness[30,31],and apoptosis resistance[32],supporting the belief that TNC contributes to cancer progression and aggression.In addition,TNC has been linked to carcinogenesis[33-36].Analysis of the Rip1-Tag2 model of pancreatic beta-cell carcinogenesis,which drives a multistage carcinogenesis process,revealed that TNC contributes to multiple steps linked to carcinogenesis[34,35].Moreover,Liet al[36]revealed that the expression levels of TNC are higher in adenomatous colon polyps and colon carcinomain situthan in non-neoplastic colonic mucosa and are also correlated with TMN stages of colon cancer,further indicating that TNC might contribute to carcinogenesis and progression[36].
TNC contains several characteristic domains,such as a central domain,heptad repeats,epidermal growth factor(EGF)-like repeats,FN type III repeats(FN-III repeats),and a fibrinogen globe(Figure 1),which can interact with ECM proteins,soluble factors,and cell receptors and express various functions of TNC.In addition,human TNC contains nine alternative splicing sites in FN-III repeats,and 511 possible splice variants can theoretically be generated through alternative splicing[37].This alternative splicing could control the versatile biological functions of TNC by modulating its interaction with specific binding partners,as well as by exposing posttranslational sites and proteolytic cleavage sites[37].However,the substantial role of the TNC molecule in colorectal carcinogenesis and its malignant progression has remained elusive.
This review presents the role of TNC in the malignant progression of colon cancer and the development of colitis-associated colorectal cancer(CAC),with a particular focus on the major involvement of TNIIIA2,the cryptic functional site of TNC.We propose new possibilities for prophylactic and therapeutic strategies based on peptide FNIII14-mediated inhibition of the TNIIIA2-induced beta1-integrin activation.
PATHOLOGICAL SIGNIFICANCE OF ELEVATED TNC EXPRESSION IN MALIGNANT TUMORS
Most ECM proteins harbor functionally cryptic functional sites that are buried within their molecular structures.These cryptic sites,called matricryptic sites,are revealedviastructural/conformational changes triggered by interactions with adjacent cells or other ECM components and by remodeling/processing by ECM-degrading proteinases,including matrix metalloproteinases(MMPs)and cathepsins.The proteinases capable of degrading ECM proteins are highly upregulated in a wide variety of cancers[38-40].ECM degradation often occurs in malignant tumors,and ECM protein fragments with biological functions are released through cleavage by inflammatory proteinases[41,42].ECM fragments with functional matricryptins show unique biological functions that are not detected in their parental ECM proteins[43].ECM proteins such as TNC are proteolytically cleaved by several inflammatory proteinases,including MMPs and cathepsins[39,40,42-44].Proteolytic degradation of TNC has been detected in lung and colon cancer,and early-stage non-small cell lung cancer patients with TNC degradation show significantly worse prognosis and higher recurrence than those without TNC degradation[39,45,46].Increased MMP-2 activity has been observed in patients with degraded TNC[39],indicating that exposure of the TNC functional cryptic site by several inflammatory proteinases may be associated with the malignant progression of cancer.Saitoet al[47]previously found that TNC harbors a cryptic and functional site comprising the amino acid residues in the sequence YTITIRGV within the FN type III repeat A2[47].A 22-mer peptide containing this functional sequence of TNC,termed peptide TNIIIA2,can potently activate beta1-integrins,a state that is sustained for a long period of time[48](Figure 1).
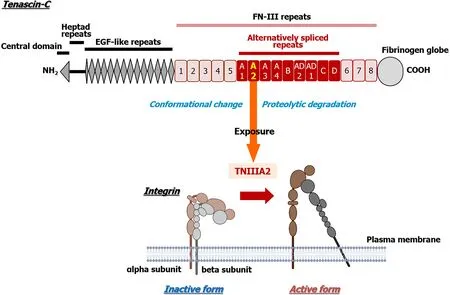
Figure 1 Schematic illustration of Tenascin-C and the amino acid sequence of peptide TNIIIA2.Conformational change in integrin activation via the lateral interaction of integrin with syndecan-4 at peptide TNIIIA2.Created with BioRender.com.
The mode of beta1-integrin activation induced by TNIIIA2 is entirely distinct from that induced by “inside-out” signaling,which is the commonly considered mode of integrin activation.Saitoet al[47]have found that syndecan-4,one of the transmembrane heparin sulfate proteoglycans,serves as a membrane receptor for TNIIIA2 and that engagement with TNIIIA2 induces a lateral association with beta1-integrins,resulting in stabilization of the active conformation of beta1-integrin[47].Based on this unique mechanism of integrin activation,this TNIIIA2-induced integrin activation is more potent and persistent than other known integrin activators,such as the various cytokines and chemokines that stimulate the “inside-out” signaling pathway[48].Because TNC variants containing the alternatively spliced domain type III-A2 are highly expressed in malignant tumors[49],the activation of beta1-integrin induced by TNIIIA2 may be related to some forms of cancer pathogenesis.We previously found that TNIIIA2 contributes to the ability of glioblastoma to acquire aggressive properties such as excessive survival/proliferation,disseminative migration,and anoikis resistance through activation of beta1-integrin[50-52].More recently,we reported that TNIIIA2 establishes inflammatory environmentsviathe NOD-like receptor family pyrin domain-containing 3/caspase-1/IL-1beta pathway[53].These findings suggest that the pathological significance of high TNC expression in inflammation and cancer may lie in activating beta1-integrins based on TNIIIA2 function.
INVOLVEMENT OF TNC IN COLON CANCER
Colorectal cancer is the third most common type of gastrointestinal tract tumor worldwide and the third leading cause of death among men and women[54].Because of recent substantial progress in diagnostic methods and advances in primary and adjuvant treatments,including standard chemotherapy and targeted treatments,the incidence and mortality of colorectal cancer has been improving[55,56],with a 5-year overall survival rate for colorectal cancer of about 60%[57,58].However,patients with metastatic colorectal cancer,which comprise 20% of patients with new colorectal cancer diagnoses,show a high mortality rate,with a 5-year overall survival rate of approximately 20%[59-62].Recently,systemic therapy involving molecular targeted drugs as well as cytotoxic drugs has been adopted for unresectable colorectal cancer.Combination with molecular targeted drugs such as bevacizumab,cetuximab,or panitumumab is recommended,depending on the RAS status[63].However,although these drugs are effective,they have various problems,including certain adverse events,and eventually become ineffective.Therefore,further investigation is still necessary to develop novel strategies for colorectal cancer,and it is important to elucidate the molecular mechanisms that enable colorectal cancer to acquire malignant properties.
TNC is highly expressed in colon cancer,and high expression levels of TNC in tissue specimens are correlated with distant metastasis,tumor recurrence,advanced TNM stage,and poor prognosis[10,36].Moreover,colon cancer cells highly expressing TNC show high metastatic potential and are associated with lymph nodes with metastasis[36].In addition,serum TNC levels,particularly those of large-spliced variants,are higher in patients with colon cancer compared with controls[64].Such levels are also correlated with tumor depth,lymph node metastasis,and disease progression[64].Therefore,the levels of TNC in tissue and serum may be a diagnostic or prognostic biomarker in colon cancer.Furthermore,the Wnt/beta-catenin signaling pathway plays a central role in carcinogenesis,and its mutation and activation are found in almost all patients with colon cancers[65].Because TNC is a Wnt/betacatenin target gene in human colon tumors[66],the deregulation of Wnt/beta-catenin signaling might lead to the overexpression of TNC in colon cancer.Experimental observations indicated that TNC secreted by myofibroblasts might act as a proinvasive factor for colon cancer cells[67].Furthermore,TNC promotes proliferation,migration,and invasion and also upregulates cancer stem cell markersviathe Hedgehog signaling pathway[31].However,the biochemical functions of TNC in the malignant progression of colon cancer have not yet been established.
MMP-2 is highly expressed in colon cancer tissues and its expression levels increase with an increase in the tumor stage[68].Furthermore,the expression levels of MMP-2 are correlated with lymph vessel invasion and disease progression in colon cancer[69].MMP-7 is another Wnt/beta-catenin target gene[70]and both MMP-2 and MMP-7 can degrade TNC[71].Furthermore,TNC variants containing the alternatively spliced domain types III-A1,-2,and -4 are highly expressed in colon cancer[49].It is presumed that the functional cryptic site TNIIIA2 of TNC may be released into the tumor microenvironment of colon cancer and contribute to its pathogenesis.Supporting this hypothesis,peptide TNIIIA2 has been shown to act directly on colon cancer cells to enhance theirin vitroinvasive potential by inducing MMP secretion[72];peptide TNIIIA2 or TNC promotes colon cancer cell invasion by upregulating MMPs[72].The cell invasion induced by peptide TNIIIA2 or TNC is completely suppressed by anti-TNIIIA2 antibody or MMP-2 inhibitor[72].Moreover,anin vivoobservation involving a spontaneous metastasis mouse model mimicking hematogenous metastasis exhibited that peptide TNIIIA2 boosted the metastasis of colon cancer cells to the lung[72].Taken together,the activation of beta1-integrin by peptide TNIIIA2(one of the biochemical functions of TNC)may help to promote colon cancer cell metastasisviainduction of MMP(Figure 2).
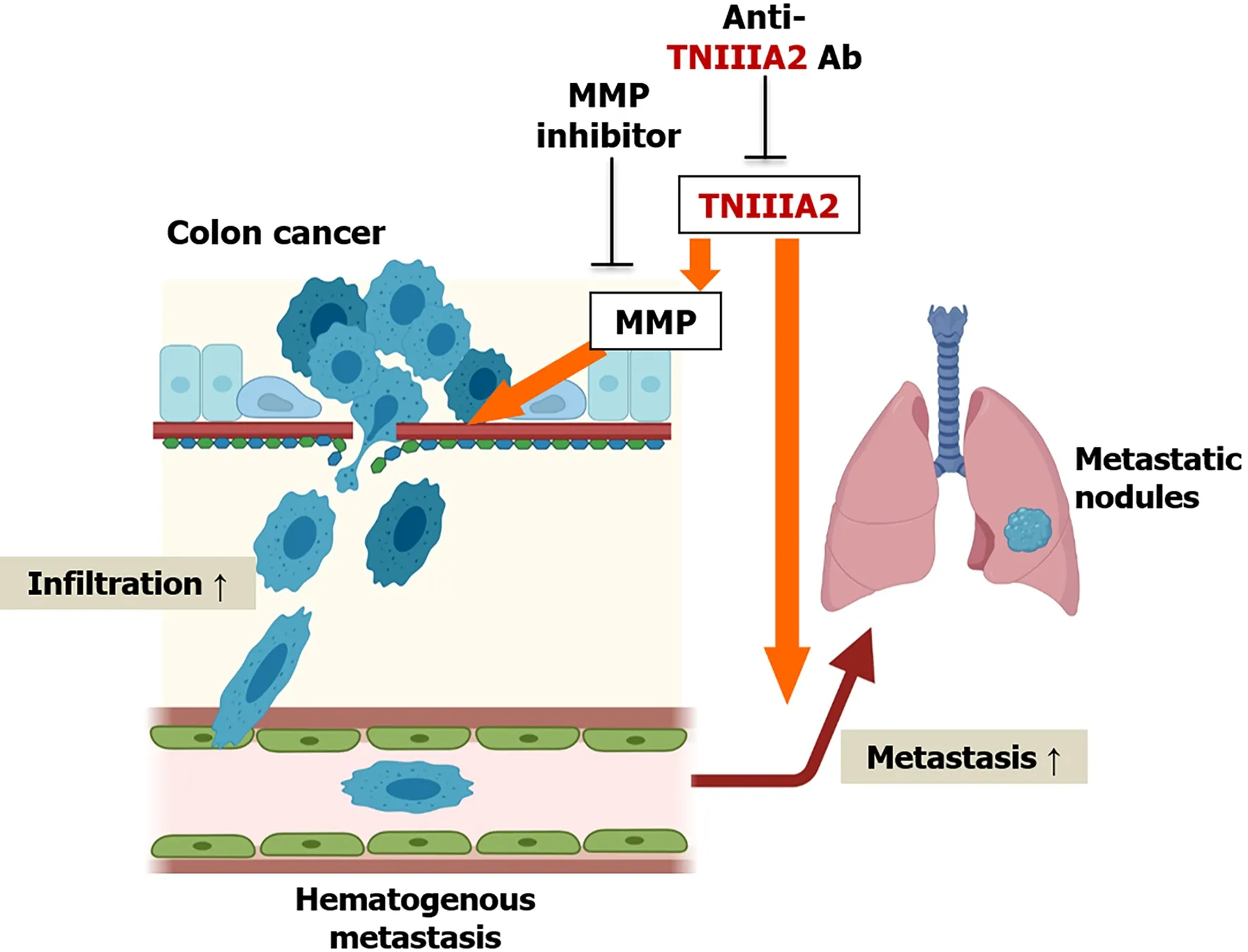
Figure 2 Schematic model of the effect of TNIIIA2 on the metastasis of colon cancer cells.Peptide TNIIIA2 boosts the infiltration of colon cancer cells via matrix metalloproteinases production in vitro and promotes pulmonary metastasis in a spontaneous metastasis mouse model.Created with BioRender.com.MMP:Matrix metalloproteinases.
Alterations in the density,distribution,and composition of the ECM are common in malignancies.This process creates the tumor microenvironment that helps to confer cancer cells with malignant properties such as tumorigenesis and metastasis[1].These alterations increase stiffness in the tumor microenvironment,which promotes protumorigenic mechanosignaling.The increased ECM stiffness of colon cancer has been associated with cancer progression[73].Through analysis of clinical specimens,a gradient of increasing ECM stiffness was observed from healthy to perilesional and colon cancer areas,which might predispose invasion[74].Furthermore,the expression levels of lysyl oxidase(LOX),which catalyzes the covalent cross-linking of collagens and elastin,are closely correlated with the progression of colon cancer[75].Compared with control cells or cells expressing a catalytically inactive LOX,colon cancer cells expressing LOX exhibit increased mechanosignaling,ECM stiffness,metastasis,and tumor burden inin vivomodelsviaactivation of beta1-integrin and the focal adhesion kinase-SRC signaling pathway[76],indicating that beta1-integrin activation might be associated with malignant progressionviaincreased ECM stiffness in colon cancer.In a recent insightful study on the role of TNC in ECM stiffness in the tumor microenvironment,Barneset al[77]demonstrated that the glycocalyx/ECM-integrin loop induces glioblastoma aggression in a tissue tension-dependent manner,with human recurrent glioblastomas showing an increase in TNC-enriched stiffened ECM and enhanced integrin mechanosignaling[77].It has also been pointed out that glioblastoma cells expressing a V737N beta1-integrin autoclustering mutant exhibit increased mechanosignaling and ECM stiffness and facilitate tumor growth[77].It is unlikely that at least the antiadhesive effect of TNC,which has been considered a major biochemical function of this protein,is responsible for the ECM stiffening and consequent enhanced integrin signaling.However,it remains unclear whether proadhesive activity(a biochemical function of TNC)is directly associated with ECM stiffness in the tumor microenvironment of colon cancer.Further investigations are required to determine whether activation of beta1-integrin by peptide TNIIIA2 could actually increase ECM stiffness in colon cancer.
Beta1-integrin is also highly expressed in colon cancer compared with normal mucosa.High expression levels of beta1-integrin have been associated with poor prognosis,and increased expression of beta1-integrin is independently correlated with decreased overall survival and disease-free survival in colon cancer patients[78].In addition,alpha5-integrin,which is coupled with beta1-integrin,also shows upregulated expression in colon cancer and is expressed mainly in the tumor stroma of clinical samples[79].Moreover,alpha5beta1-integrin expression is considered a significant independent prognostic factor.Experimental evidence indicates that overexpression of alpha5-integrin accelerates proliferation and suppresses apoptosis in colon cancer cells,with colon cancer cells overexpressing alpha5-integrin found to promote tumor growth in a murine xenograft tumor model[80].In addition,blockade of alpha5-integrin inhibits cell attachment and induces apoptosis in colon cancer cellsviaAkt suppression[81].Integrin alpha5beta1 also confers anoikis resistance in colon cancer cellsviaassociation with EGF receptor and the subsequent activation of ERK and Akt as well as suppression of the caspase signaling pathway[82].Furthermore,depletion of alpha5-integrin expression in fibroblasts suppresses the tumorigenic activity of colon cancer inin vivoexperiments,as determined by the co-injection of human colon cancer cells and human normal colonic fibroblast cells into immunocompromised mice[79].This result indicated that CAFs expressing alpha5-integrin have a tumor-promoting effect in colon cancer.Pharmacological experiments have demonstrated that the non-peptidic alpha5beta1 integrin antagonist K34c suppresses the clonogenic survival of colon cancer cells[83].In addition,ATN-161,a peptidic antagonist of integrin alpha5beta1 and alphavbeta3,reduced tumor vascularization,and combination therapy of ATN-161 and fluorouracil suppressed liver metastases in a murine model of colon cancer[84].Therefore,integrin alpha5beta1 might be a promising target for cancer therapeutics.
INVOLVEMENT OF TNC IN CAC
A link between chronic inflammation and the pathogenesis of many malignancies has been well documented.Examples includeHelicobacter pyloriinfection-associated gastric cancer and hepatitis virus infection-associated hepatocellular carcinoma[85,86].In particular,inflammatory bowel disease(IBD)patients,including Crohn’s disease and ulcerative colitis,have an increased risk of developing CAC[87-89],which is a subtype of colorectal cancer[90].Although the incidence of CAC seems to have decreased in recent years because of more frequent surveillance,improved surveillance techniques,and more effective IBD drugs for controlling inflammation,patients with IBD still have higher rates of death from colon cancer[91].Indeed,a Scandinavian population-based study recently showed that patients with IBD and colorectal cancer had an increased risk of mortality compared with those with sporadic colorectal cancer[92,93].Therefore,there is still an unmet medical need for the prevention and treatment of CAC.Unlike sporadic colorectal cancer,CAC onset does not show an adenoma-carcinoma sequence,but rather an inflammation-dysplasiacarcinoma sequence[94,95].Nonetheless,the molecular basis of CAC onset is largely unknown.Thus,research into the molecular mechanisms underlying CAC onset is urgently needed for the development of novel therapeutics.
Several studies have reported that TNC is associated with ulcerative colitis and Crohn’s disease[96-99].A genome-wide association study of African Americans found that single-nucleotide polymorphisms within the TNC gene are associated with IBD risk[100].Ninget al[101]reported that TNC is highly expressed in the inflamed stromal area of the intestinal mucosa of IBD patients[101].They also showed particularly high levels of serum TNC in patients with severe IBD compared with those with mild or moderate IBD[101].Riedlet al[96]determined that the serum levels of TNC are correlated with clinical and histological parameters of disease activity in IBD patients[96].Moreover,high levels of TNC mRNA in the mucosa of ulcerative colitis have been associated with a poor response to infliximab therapy,an effective treatment for moderate-to-severe IBD,indicating that TNC may contribute to therapeutic resistance against IBD.Therapy resistance may participate in the malignant progression of IBD due to a lack of inflammatory control,resulting in an increased risk of CAC onset.Indeed,TNC derived from intestinal myofibroblasts promotes the onset of CAC in an azoxymethane(AOM)/dextran sulfate sodium(DSS)modelviaangiogenesis[102].Thus,TNC might contribute to the development and/or malignant progression of CAC.Identification of the biological functions of the TNC responsible for the development of CAC would enable the design of agents with prophylactic and therapeutic potential for these diseases.However,the biochemical functions of TNC in CAC onset have not yet been established.
ECM remodeling is often augmented in these pathological lesions,and proteolytic cleavage of ECM proteins is performed by several inflammatory proteinases,including MMPs and cathepsins.Indeed,increased expression levels of several MMPs have been observed in IBD and are associated with disease activity in IBD,indicating that degradation of the ECM,including TNC,might occur at high levels in IBD and during CAC onset[103].Therefore,it is conceivable that the functional cryptic site TNIIIA2 might be exposed by the high levels of TNC molecules in the lesion and act as a specific pathogenic factor in the development of CAC.Supporting this assumption,our recent work demonstrated the presence of TNC and peptide TNIIIA2 in the stromal area of dysplastic lesions in AOM/DSS mice[104].Assuming that peptide TNIIIA2 acts mainly on preneoplastic epithelial cells and fibroblasts,which are abundant in the stromal area of dysplastic lesions,ourin vitroexperiments focused on the effects of beta1-integrin activation on both preneoplastic epithelial cells and fibroblasts.Interestingly,although beta1-integrin activation by peptide TNIIIA2 promoted cell adhesion,it had no direct effect on the growth of preneoplastic epithelial cells[104].Similarly,peptide TNIIIA2 had no direct effect on the growth of fibroblasts,but fibroblasts stimulated by peptide TNIIIA2 released humoral factors,or possibly factors,that drove the malignant transformation of premalignant epithelial cells in a paracrine manner,as judged by anchorage-independent cell growth and focus formation[104].These factors secreted from peptide TNIIIA2-activated fibroblasts are also able to promote the survival/proliferation of colon cancer cells[104].Furthermore,peptide FNIII14,a peptidic factor that induces a conformational change in beta1-integrin from the active to the inactive state[105],suppressed not only the TNIIIA2-induced dysregulated survival/proliferation of preneoplastic epithelial cellsin vitro,but also polyp development in an AOM/DSS mouse model[104].These results suggest that beta1-integrin activation by peptide TNIIIA2 in fibroblasts may be an important target for the prevention of CAC(Figure 3).
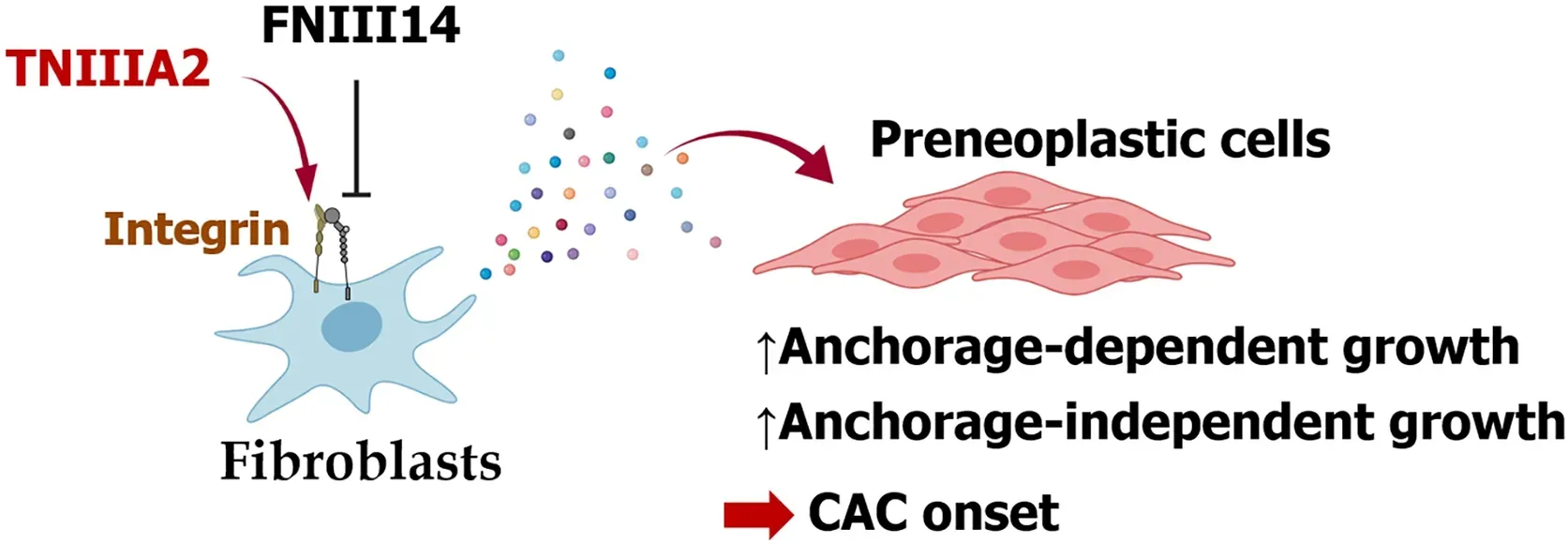
Figure 3 Schematic model of the effect of TNIIIA2 on colitis-associated colorectal cancer onset.Peptide TNIIIA2 stimulates fibroblasts to secrete humoral factors,promoting anchorage-dependent and -independent growth in preneoplastic cells.Created with BioRender.com.CAC:Colitis-associated colorectal cancer.
Several studies have demonstrated that cells in the tumor microenvironment,such as CAFs and immune cells,influence tumor progression.Among them,CAFs are key determinants of cancer development and progression[106-108].Sasakiet al[109]demonstrated that CAC incidence is abrogated in CC chemokine ligand 3- or CC chemokine receptor 5-knockout mice treated with AOM/DSS and coincides with lower accumulation of fibroblasts in dysplastic lesions compared with wild-type mice[109].These fibroblasts express heparin-binding EGF-like growth factor to stimulate the proliferation of tumor cells in CAC in mice[109].In addition,epiregulin derived from fibroblast promotes the proliferation of intestinal epithelial cells through activation of the ERK signaling pathway,augmenting CAC growth[110].These studies indicate that CAFs might be responsible for CAC development and progression.However,there is increasing evidence that TNC is upregulated in CAFs and that a high TNC expression as a CAF marker in tumor stroma is correlated with worse prognosis in several malignancies,such as breast ductal carcinoma[7],esophageal squamous cell carcinoma[9],colorectal cancer[10],and prostate cancer[111].Taken together with our results,the evidence indicates that fibroblasts produce TNC in the tumor microenvironment and that this TNC might activate CAFs to promote tumor onset and progression.
Risk factors for CAC development include pancolitis,a younger age of IBD onset,a long disease duration,chronic cholestatic liver disease,family history[112],and stricture formation[113].Intestinal fibrosis is a common complication in IBD,particularly Crohn’s disease,and the resulting clinically relevant strictures have been observed in about one-third of patients[114].Intestinal fibrosis is likely to involve increased ECM stiffness,and this stiffness could perpetuate fibrogenesis[114],leading to the development of fibrotic strictures.More recently,accumulating evidence has linked increased ECM stiffness to several malignancies,with recent studies showing that cancer progression and aggression are correlated with the stiffness of a TNCenriched ECM[115](please see the previous section).In IBD,increased ECM stiffness has been observed in strictures,and the increased ECM stiffness enhances adhesive properties,such as the formation of focal adhesion and actin stress fibers of colonic fibroblasts[116].Moreover,increased expression levels of TNC have been reported in lesions of ulceration in ulcerative colitis[98].Erdemet al[117]reported the possible involvement of increased expression levels of TNC in the development of ulcerative colitis-related strictures[117].Given that peptide TNIIIA2 can induce potent and persistent activation of beta1-integrin as well as its clustering[47,48],peptide TNIIIA2 in stromal lesions might contribute to the development of colitis-related strictures through increased ECM stiffness,leading to increased risk of CAC onset.Although further research is required to determine whether beta1-integrin activation by peptide TNIIIA2 actually increases ECM stiffness,TNIIIA2-targeting agents such as an anti-TNIIIA2 antibody might be a promising strategy for the prophylaxis or treatment of CAC development and malignant progression.
Several studies have suggested that integrin inactivation could be a promising strategy for controlling CAC development and progression.ATN-161,a peptidic antagonist of integrin alpha5beta1 and alphavbeta3,suppressed disease activity by blocking angiogenesis in IL-10-deficient mice that develop spontaneous Crohn’s disease-like colitis[118]as well as in a CD4+CD45RBhighT-cell transfer model that induced chronic pancolitis[119].Furthermore,ATN-161 also inhibits CAC developmentviainhibition of integrin alphavbeta3-mediated angiogenesis in a chemically induced AOM/DSS mouse model of intestinal and colon carcinogenesis[102],although no recent development status of ATN-161 is available.More recently,Terasakiet al[120]showed that fucoxanthin induces anoikis in colonic adenocarcinoma through attenuation of beta1-integrin signaling,which blocks CAC development in AOM/DSS mice[120].Taken together,beta1-integrin activation might become a promising target for preventing and treating CAC,and inactivation of beta1-integrin by peptide FNIII14,which can neutralize the detrimental effects of peptide TNIIIA2 on beta1-integrin activation,might be a novel and promising strategy for the management of CAC development and malignant progression.
BETA1-INTEGRINS AS POTENTIAL THERAPEUTIC TARGETS IN COLON CANCER
Several antagonists of integrin alpha5beta1 and alphavbeta3 were well tolerated in clinical testing[121,122]but failed to show therapeutic benefits in patients with malignancies.Although inhibition of integrin alpha5beta1 and alphavbeta3 might be a safe therapeutic strategy,alternative approaches should be considered,including the application of integrin inhibitors as anti-cancer drugs(reviewed in Ref.[123]).Regarding other therapeutic modalities,OS2966,a humanized monoclonal antibody targeting human beta1-integrins,is undergoing testing in a phase I clinical trial for the treatment of recurrent/progressive glioma[124].In addition,one possible strategy may be to develop drugs with modes of inhibition other than competitive inhibition of integrin.Unlike integrin antagonists,peptide FNIII14—which has the ability to induce a conformational change in beta1-integrin from the active to the inactive state[105]—has shown therapeutic efficacy against several malignancies in animal models,including CAC,glioblastoma,neuroblastoma,and acute myelogenous leukemia[105].Although further research is needed regarding its effect on the malignant progression of colon cancer,peptide FNIII14 may possess promising therapeutic properties.
CONCLUSION
Although TNC is considered a negative prognostic factor in several malignancies,the substantial role of TNC molecule in the development of colorectal cancer and its malignant progression has remained elusive.We suggest that one of the pathological roles of TNC,which is highly expressed in colon cancer,may be in activating beta1-integrins through TNIIIA2 function.This hypothesis and the previous findings open the door to prophylactic and therapeutic strategies for colon cancer that involve inhibition of TNIIIA2-induced beta1-integrin activation by peptide FNIII14.
 World Journal of Gastrointestinal Oncology2021年9期
World Journal of Gastrointestinal Oncology2021年9期
- World Journal of Gastrointestinal Oncology的其它文章
- Use of liquid biopsies in gastrointestinal cancers
- Neoadjuvant chemotherapy without radiation as a potential alternative treatment for locally advanced rectal cancer:A metaanalysis
- Prognostic value of modified Lauren classification in gastric cancer
- Scoparone inhibits pancreatic cancer through PI3K/Akt signaling pathway
- Effect of oncometabolic surgery on gastric cancer:The remission of hypertension,type 2 diabetes mellitus,and beyond
- Characterization of metabolic landscape in hepatocellular carcinoma
