Analogs of microgravity: the function of Schlemm’s canal, intraocular pressure and autonomic nervous during the head-down tilt test in healthy subjects
Department of Ophthalmology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China
Abstract
● KEYWORDS: head-down tilt; aqueous humor;Schlemm’s canal; autonomic nervous system; intraocular pressure
INTRODUCTION
Ocular physiological changes were validated in astronauts in microgravity environment, including choroidal folds,optic disc edema, the thickening of retinal nerve fiber layer,increased intraocular pressure (IOP) and decreased visual acuity[1]. During 6-month missions in space, more than half of astronauts develop with pathophysiological ophthalmic findings[2].
The astronauts IOP increasing is a pronounced finding in the microgravity environment[3-5]. The leading hypothesis suggests that increase of episcleral venous pressure (EVP) may contributes to the ocular changes[6]. However, Wenreibet al[7]demonstrated that 1 mm Hg change in IOP was only induced 0.83 mm Hg variation in EVP during the microgravity analog environment, which suggest that another possible explanation besides EVP are changing. The underlying mechanisms of the IOP variation in astronauts on the spaceflight is still unknown.
Head-down position causes a headward fluid shift similar to the environment of spaceflight, head-down tilt (HDT) test has long been used on Earth to simulate the effects of microgravity on the human body and evaluate possible countermeasures[8].Prolonged head-down bed test produces similar changes to those occurring in microgravity has been found, such as osteoporosis, muscular atrophy, basal metabolism reduction[9].Because HDT bed test is designed to simulate the effects of microgravity on the human body, it is hypothesized that microgravity-induced ophthalmological changes observed in long-duration spaceflight might occur in head-down bed test subjects. Therefore, the target of the present study was to evaluate the ocular parameters and to elucidate possible principle underlying IOP change during the HDT test.
SUBJECTS AND METHODS

Figure 1 The illustration of SC A: The schema graph of anterior chamber of the eye. SC, TM, and AC were labeled. B: The profile of SC and TM in vivo. The arrowhead shows the profile of the lumen of SC.
Ethical ApprovalThe research protocols have been approved by the Ethics Committee of Tongji Hospital (Registered Number: ChiCTR-OON-16007850). The written informed consents were obtained from all participants. The research follows the guideline of Declaration of Helsinki.
ParticipantsTotally 25 healthy staffs of Tongji Hospital,in Wuhan were enrolled from November 2017 to December 2018. All the participants received a complete ophthalmologic examination. Each eye was selected randomly.
Inclusion criteria included: IOP<21 mm Hg and normal optic nerve appearance. Age>18 years old and should not use of any topical or systemic medications in the month and caffeine ingestion was forbidden for at least 24h before the experiment commenced.
Exclusion protocol included: 1) best corrected visual acuity(BCVA) less than or equal to 0.5; 2) refractive error (RE)less than -6.0 D and RE over than +3.0 D; 3) obscure optical structure to affect imaging of optical coherence tomography(OCT), such as serious cataract and other ocular diseases;4) those with a history of ocular and systemic diseases or previously received eye operations.
Intraocular Pressure and Systemic Parameters MeasurementSubjects fasted for no less than 4h. All participants completed the upright seated condition first which began with a 5min stabilization period and the order of the 20°HDT position was performed lasted 15min. I-Care tonometer(Tiolat, Oy, Helsinki, Finland) was adopted to measure the IOP. Automatic digital blood pressure monitoring (Omron,Dalian, Liaoning, China) was used to record the systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate(HR). The mean value of arterial pressure (MAP) and ocular perfusion pressure (MOPP) were recorded using the equation below: MAP=[(2×DBP)+SBP]/3; MOPP=MAP-ΔPf-ΔPh-IOP. ΔPfis the pressure differential arise from the blood flow resistance between the heart and the eye (assumed by the author of Bill to be 4 mm Hg). ΔPhis the hydrostatic pressure differential equal to the vertical distance between the eye and the heart. In the 20° HDT position in our experiment, the vertical distance was approximately 6 cm between the heart and the eye, the ΔPhwas approximately 4 mm Hg.Five-minute electrocardiograms (ECGs) waves were depicted at two time-points (after resting 5min in upright sitting condition and after the 15min 20° HDT). The high frequency(HF) activity (0.15-0.4 Hz) was calculated by using the software of Kubios HRV (version 2.20, Finland).
Measurement of Schlemm’s CanalSerials horizontal OCT B-scans (21 scans, 15×5° retangle) of Schlemm’s canal (SC)imaging were prospectively obtained after 5min of rest in the nasal and temporal regions. All examinations were performed under standardized light and dark background (ca. 3.5 lx).
The location of conjunctival vessel was labeled as landmarks to ensure the same scanning area obtained during the HDT test.The area of SC lumen (SCAR; μm2) at different periods (after 5min of rest and after the 15min 20° HDT) were measured by the software of Image J (version 1.45S; MD, USA). A black,lucent space in the limbal was defined as the SC (Figure 1).The measurements were calculated by two experimenters(Chen W, Chen ZQ). Data discrepancy over than 15% were consulted the senior experimenter (Wang JM).
Statistical AnalysisThe SPSS software (Version 16.0, SPSS Inc., Chicago, USA) were adopted to perform the statistical analysis of the data, which were presented as the mean values(mean±SD). One sample Kolmogorov-Smirnov test and the test of homogeneity of variance were adopted. Paired samplest-test was used to found the variations of the parameters during the HDT. Linear regression analysis was adopted to calculate the correaltion between HF and SCAR. All tests were twotailed. AP-value less than 0.05 is statistically significance.
RESULTS
Twenty-five subjects (25 eyes) were involved. Four subjects were excluded because of lower quality images. Finally,21 participants (8 males, 13 females) were included. The mean age was 29.43±4.0y (range 24-37y), mean BCVA was 0.98±0.06 decimal (range 0.8-1.0, decimal) and mean RE was-2.19±1.99 D (range -3.01 to -1.28 D). All data meet the law of normal distribution and homoscedasticity.

Figure 2 SCAR decreased obviously after the 20° HDT A: The profile of SC before the HDT; B: The profile of SC after the 15min short-term HDT.
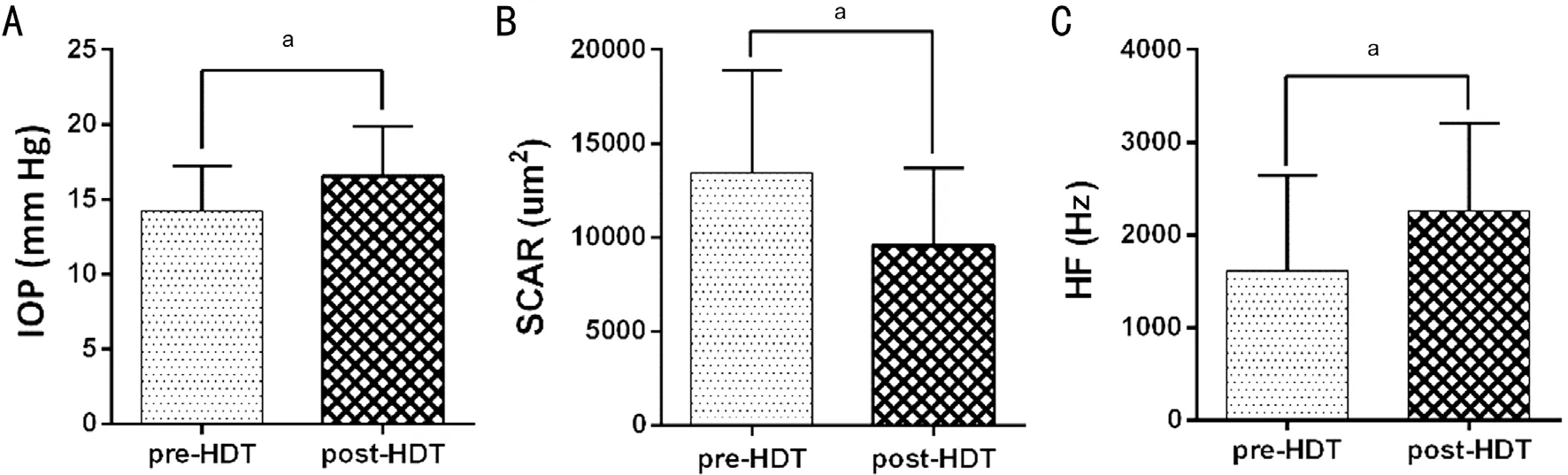
Figure 3 There’s significant differences of IOP, SCAR, and HF after the 15min short-term HRT The IOP and HF increased significantly,and the SCAR decreased obviously. aP<0.05.

Figure 4 Significant correlations by univariate regression analysis were found between ΔHF and ΔSCAR.
SCAR decreased from 13449.0±5454.9 μm2at sitting condition to 9576.6±4130.9 μm2post 15min HDT test (Figures 2A, 2B,3B). IOP increased significantly after 20° head down position from 14.0±3.03 to 17.0±3.32 mm Hg (P<0.001; Figure 3A).HF increased significantly from 1462±865 Hz at baseline to 2128±824 Hz (Figure 3C). HR decreased significantly from 76±11.49 to 70±11.52 bpm after the HDT. But there’s no significant difference of MAP and MOPP (Table 1). The linear regression analysis showed that a significant correlation of the difference of HF and SCAR during the HDT were found(R2=20%,P=0.04; Figure 4).
DISCUSSION
The increased IOP of astronauts is a pronounced phenomenon in the weightlessness environment. The fluid shift during theweightlessness was thought the main reason[10]. During the 8d manned German Spacelab Mission, it is about 5 mm Hg IOP increased after 25min of weightlessness[11]and IOP was similarly found increased 4-7 mm Hg during the first day in the microgravity enviorment[12]. However, IOP was observed normalized after the 4d of space mission[12]. During the longduration spaceflight mission, IOP was also found stablized similar to preflight values[13]. The stable of IOP during long spaceflight appears to occur despite a persistent cerebrospinal fluid flow. Huanget al[14]mentioned in his study that there seems exist a compensatory mechanism normalizes IOP during the spaceflight, but the fundamental mechanisms of IOP fluctuation is still unclear.

Table 1 Physiological parameters at baseline and 15min later following HDT test

Figure 5 The indices of HRV calculated from a ECG depicting. A: The R-R interval curve from the 5-minute recording; B: The frequency domain result before the HDT; C: The frequency domain result at 15min after the HDT. The HF of the HRV increased markedly after the HDT rest (C). HF is shown by the blue area (0.15-0.4 Hz) in B and C.
HDT test causes a headward fluid shift similar to that which occurs during space mission, and has been designed to simulate the influence of microgravity on the human body[15].In our study, IOP was found increased significantly after HDT test of 15min, the same result was found by Marshall-Goebel K[16].Autonomic nerves system maintains the balance of numerous body process, such as blood pressure, heartbeat, breathing and digestion[17]. The effect of autonomic nervous system upon IOP has been showed great interest since 1727[18]. Previous studies have showed that blocking of the superior cervical sympathetic ganglion lowered, and that electrical stimulation of the sympathetic nerve trunk raised IOP[19], decreased parasympathetic innervation reduce IOP[20]. From the above,the autonomic nerves system is responsible for the IOP regulating.
A sympathetic/parasympathetic balance is needed to regulate homeostasis. Heart rate variability (HRV) analysis is widely used to assess the function of autonomic nervous system non-invasively. The HF domains of HRV (HF, 0.15-0.4 Hz)was specifically representing the activity of parasympathetic nervous system[21]. After the HDT of 15min, we found that the HF increased significantly, which indicated that the analog test of acute tilting activating the parasympathetic nervous system (Figure 5). The similar result was found by Diedrich[22]that short term of 6° HDT causes an acute activation of the parasympathetic nerve traffic and decreased HR. In our result,the HR was also found significantly lowered. Above all, the IOP fluctuation during acute HDT might be explained by the activation of parasympathetic nervous system.
The anatomic change of SC plays a critical role in modulation of IOP. Yuanet al[23]found that SC dilation by micro-stent implanting operation significantly increased outflow facility and reduced IOP. Genaidyet al[24]found that canaloplasty (SC dilatation programme) successfully reduced the IOP after 1y in eyes with open angle glaucoma. SCAR decreased significantly and changed synchronously with IOP after 15min head down position was found in our study. In our preliminary study, the inner wall of SC containing protein gene product (PGP) 9.5 and vasoactive intestinal peptide (VIP) was validated, which reminder that vagus nerves participate in the regulation of SC[25]. After 15min HDT, we found that the SCAR decreased obviously, and contemporary the HF increased obviously.The variations of HF and SCAR during HDT were found correlated significantly, which indicated that the activated peripheral vagal nerves might be involved in modulation of the collapse of SC lumen during the HDT, and the IOP peak might be explained by the collapse of SC lumen of increased parasympathetic nervous activity after 15min of HDT.
Overall, the collapse of SC could be explained by the excitation of parasympathetic nerves after the 15min shortterm HDT, which might be involved in the regulation of IOP.
While several interesting insights were offered into ocular physiology, certain limitations of current study could be addressed. First, glaucoma patients should be enrolled in the future studies as experimental group. Second, there is no significant correlation of IOP, SCAR might be arising from the limited sample size enrolled in this study. To enlarge the sample size, some significant correlation might be found.
ACKNOWLEDGEMENTS
The authors would like to express special thanks to Zhi-Tao Wang and Jian Sun for supporting in this study.
Conflicts of Interest: Chen W,None;Chen ZQ,None;Xiang Y,None;Deng CH,None;Zhang H,None;Wang JM,None.
 International Journal of Ophthalmology2021年9期
International Journal of Ophthalmology2021年9期
- International Journal of Ophthalmology的其它文章
- Five-year results of refractive outcomes and visionrelated quality of life after SMlLE for the correction of high myopia
- Role of glycolysis in retinal vascular endothelium, glia,pigment epithelium, and photoreceptor cells and as therapeutic targets for related retinal diseases
- Predictive value of retinal function by the Purkinje test in patients scheduled for cataract surgery in Kinshasa, DR Congo
- Simultaneous pars plana vitrectomy, panretinal photocoagulation, cryotherapy, and Ahmed valve implantation for neovascular glaucoma
- Displacement of the retina after idiopathic macular hole surgery with different internal limiting membrane peeling patterns
- Association between cystatin C and diabetic retinopathy among type 2 diabetic patients in China: a Meta-analysis
