Efficacy of topical dorzolamide 2% in diabetic cystoid macular edema
Department of Ophthalmology, Mansoura Ophthalmic Center,Faculty of Medicine, Mansoura University, Mansoura 35516,Egypt
Abstract
● KEYWORDS: cystoid macular edema; diabetic maculopathy; dorzolamide; retina
INTRODUCTION
Diabetic macular edema (DME) is among the most prevalent causes of visual loss in diabetic retinopathy[1],whereas its global occurrence ratio is about 6.8% among diabetic patients[2]. The exact pathophysiology of DME is still unclear, but it is believed to be multifactorial and complicated,caused mainly by blood-retinal barrier (BRB) disruption due to functional damage and necrosis of retinal capillaries. This disruption of BRB leads to abnormal leakage and accumulation of intravascular fluid into the neurosensory retina and in the intraretinal layers of the macula[3]. The resulting macular thickening deforms photoreceptors, thus causing vision loss[4].Laser photocoagulation has been proved effective in minimizing blindness from DME by at least 60%[5]. However,it is usually associated with retinal necrosis resulting in a reduction of pericentral-sensitivity[6]. That is why treatment should also include drugs capable of reduction or elimination of the edema without permanent destruction of the retinal anatomy.
Earlier studies concluded the benefits of intravitreal triamcinolone acetonide injection in the treatment of DME either alone or versus laser therapy[7]. Despite these benefits,the associated high incidences of cataracts and consistent intraocular pressure (IOP) increase are considered major drawbacks of intravitreal triamcinolone acetonide therapy[8].
Currently, anti-vascular endothelial growth factor (anti-VEGF) substances are widely used and have revolutionized diabetic retinopathy treatment. They have been demonstrated as effective alternatives to laser photocoagulation and have replaced it as first-line therapy[9]. Although the results of anti-VEGF agents in diabetic retinopathy are promising,each intravitreal injection has the risk of post-injection and/or drug-class-associated devastating adverse effects as endophthalmitis[10], intraocular inflammation[11], IOP elevation[12], and systemic side events[13].
Systemic carbonic anhydrase inhibitors (CAIs) have been efficient in treating maculopathy present in retinal diseases as uveitis[14], retinitis pigmentosa[15], or postoperatively after cataract surgery[16], as well as that associated with epiretinal membranes[17]. However, the use of systemic CAIs is further limited by frequent and hazardous side effects[18]which are thought to result from inhibition of intracellular carbonic anhydrase (CA) isoenzymes[19]. Dorzolamide, a topical CAIs, well-tolerated and effective as an ocular hypotensive drug[20], has been used for macular edema due to retinitis pigmentosa[21-22], retinoschisis[23], and choroideremia[24]. It has a comparable effect to acetazolamide in macular edema due to retinitis pigmentosa[25]. In fact, few studies[26-27]have investigated the efficacy of CAIs, especially the topical dorzolamide, in resolving cystoid macular edema (CME) in diabetic retinopathy. This study was carried out to study the efficacy of topical dorzolamide 2% in the treatment of CME in diabetes patients.
SUBJECTS AND METHODS
Ethical ApprovalThe study protocol wa s reviewed and approved by the Ethical Committee of Mansoura University,Faculty of Medicine, and was performed in accordance with the rules of the Helsinki Declaration (R/16.04.65). The study was registered in UMIN clinical trial register system (ID issued: UMIN 000022608 Receipt No: R000026059). Written informed consent was obtained from all patients after a clear explanation of the purpose of the study, the nature of the procedure, conceivable benefits, and possible risks.
This was a prospective, nonrandomized, open, and interventional study carried out on diabetic patients with CME recruited at Mansoura Ophthalmolic Center, Mansoura University in Mansoura city, Egypt.
Patients’ Selection
Inclusion criteriaThe study included adult diabetic patients(both type 1 and 2) recently diagnosed with diabetic CME(of duration less than 3mo). CME was diagnosed by slit lamp biomicroscopy with non-contact lens, fluorescein angiography,and optical coherence tomography (OCT; Topcon Corp, Tokyo,Japan).
Exclusion criteriaPatients were excluded from the study if they had: 1) advanced diabetic retinopathy; 2) serous retinal detachment; 3) vitreo-retinal traction syndrome; 4) Mixed maculopathy (macular ischemia by angiography ); 5) prior laser therapy or intravitreal injections (IVI); 6) additional ocular diseases, including uveitis, glaucoma, or neoplasms; 7)history of other medications with potential effect on the visual acuity and/or retinal function.
Initial assessmentAll subjects underwent an ophthalmic examination that included: 1) best-corrected Snellen visual acuity (BCVA) measurement; 2) standard slit-lamp biomicroscopy; 3) IOP measurement by Goldmann applanation tonometry; 4) dilated fundus examination using non-contact Volk 90 lens, and indirect ophthalmoscope; 5) the severity and the level of diabetic retinopathy were evaluated through the fundus examination and fluorescein angiography based on the International Clinical Diabetic Retinopathy Disease Severity Scale[28]; 6) OCT: 3D-OCT 2000 (Topcon Corp,Tokyo, Japan) was used in this study for macular scanning.It was performed at a resolution of 512×128 with six linear scans oriented radially 30° apart and centered on the fovea.Central macular and foveal thicknesses were measured within a 6 mm diameter circle centered on the foveola and the circular map was subdivided into 9 regions. The 6 mm ring was divided into 3 rings, with the inner ring 1 mm in diameter,the middle ring 2 mm and the outer ring 3 mm in diameter. It was corresponding to the foveal, perifoveal, and parafoveal areas respectively. Color-coded graphs and numerical maps were used for quantitative evaluation. CME was diagnosed as an absence of central foveal contour, diffuse thickening of the foveal/or perifoveal area more than 250 μm, and a presence of intraretinal cysts. Scans with the image quality of 55 or more were included for analysis; 7) all participant’s glycemic control was ascertained pretreatment and at 3mo post-treatment through glycosylated hemoglobin A1C (HbA1c) measurement.Treatment protocolThis comprised administration of topical dorzolamide 2% eye drops (Trusopt 2% eye drops, Merck &Co., Inc., NJ, USA) three times daily for 30d.
Post-treatment evaluation and follow-up scheduleThe first follow-up visit was at one week, then each patient was seen after one month, and three months to evaluate the patient’s response to treatment. At each follow-up visit, BCVA, IOP,fundus biomicroscopy, and OCT images were obtained and recorded.
Study outcomes and data analysisThe main primary outcome was the changes in macular morphology posttreatment. OCT findings were evaluated by measuring the changes in the central foveal zone (CFZ) thickness and then all study participants were graded as responders or nonresponders. The non-responders to the topical dorzolamide 2%were further classified as non-responders without worsening,or non-responders with worsening.
Secondary outcomes measurements included changes in BCVA, HbA1c variables, and IOP. Snellen visual acuity measurements were converted into logMAR equivalents for statistical analysis.
Statistical AnalysisThe baseline and the outcome data were classified, scheduled, and then analyzed using the computer program SPSS (Statistical package for social science) version 17.0 (SPSS Inc. Chicago, Illinois, USA). To compare the mean values and changes from pretreatment values, the paired Student’st-test was used. ThePvalue of less than 0.05 was considered statistically significant. Independentt-test was conducted to measure the difference between mean values of HbA1c variables among the responders and non-responders and to assess its impact on the treatment outcomes.
RESULTS
Ninety-three patients fulfilled the inclusion criteria; however, 9 patients were excluded from the study as they did not complete the 3mo follow-up period. Accordingly, 84 patients (93 eyes)were included in the study; with a mean age of 54.22±5.8y.Patients’ demographic and basic clinical data are shown in Table 1.
There was a statistically significant improvement in BCVA mainly one month after treatment (Figure 1). The mean±SD baseline logMAR BCVA was 1.08±0.26. It improved to 1.02±0.28 (P=0.116), 0.66±0.24 (P<0.001), and 0.87±0.26(P<0.001) at 1wk, 1, and 3mo after treatment respectively(Table 2). At the end of 3mo, sixty-four eyes (68.8%) had a subjective improvement in the BCVA. Twenty-two eyes(23.7%) had no change from the initial pretreatment BCVA,while 7 eyes (7.5%) showed a decline in their BCVA (Table 3).The mean±SD IOP changed from16.52±2.57 mm Hg at baseline to15.42±1.37 mm Hg at 1wk, to 14.66±1.74 mm Hg after 1mo and to 15.84±1.49 mm Hg at the end of 3mo. The changes were statistically significant (P=0.002, <0.001,<0.001) respectively (Table 4).
Detailed OCT images analysis showed that all post-treatment central foveal thickness (CFT) values were significantly decreased compared to baseline values (P<0.05; Figure 2).The mean±SD baseline CFT was 535.27±97.4 μm, which decreased at one week to 420.87±115.2 μm and further decreased at one month to 357.43±125.8 μm, then slightly increased to 376.23±114.5 μm at the end of 3mo, but remained significantly lower than the pretreatment value (Table 5).
OCT analysis revealed that, 56 eyes (60.02%) showed improvement in response to topical dorzolamide 2% through the follow-up period (3mo), 27 eyes (29.03%) did not show any improvement and the macular thickness did not worsen,while 10 eyes (10.57%) did not show any improvement but the macular thickness worsened when compared to the baseline thickness. The mean baseline and post-treatment HbA1c values were significantly higher in non-responders as compared to responders (P<0.001; Table 6).
All patients completed the follow-up period of at least 3mo(mean±SD follow-up 3.95±1.3mo; range: 3-7mo). There were no significant ocular or systemic side effects observed during the follow-up period.
DISCUSSION
The pathogenesis of DME seems to be attributable to inflammatory processes which play a main role in functional and morphological changes of DME[29]. Several previous studies have handled and evaluated the various methods for the treatment of diabetic maculopathy[3,5-9,30].
Lately, clinical interest has focused on the effect of acetazolamide, a systemic CAI, on the macular edema fromvarious retinal disorders[14-17]. Unfortunately, acetazolamide may lead to serious side effects[18-19]. That is why it cannot be administered for a long time (3-5 days maximum).Dorzolamide is a topical CAI that can be used as an alternative prolonged therapy and can be administered to these patients constantly to prevent their visual acuity impairment[26-27].
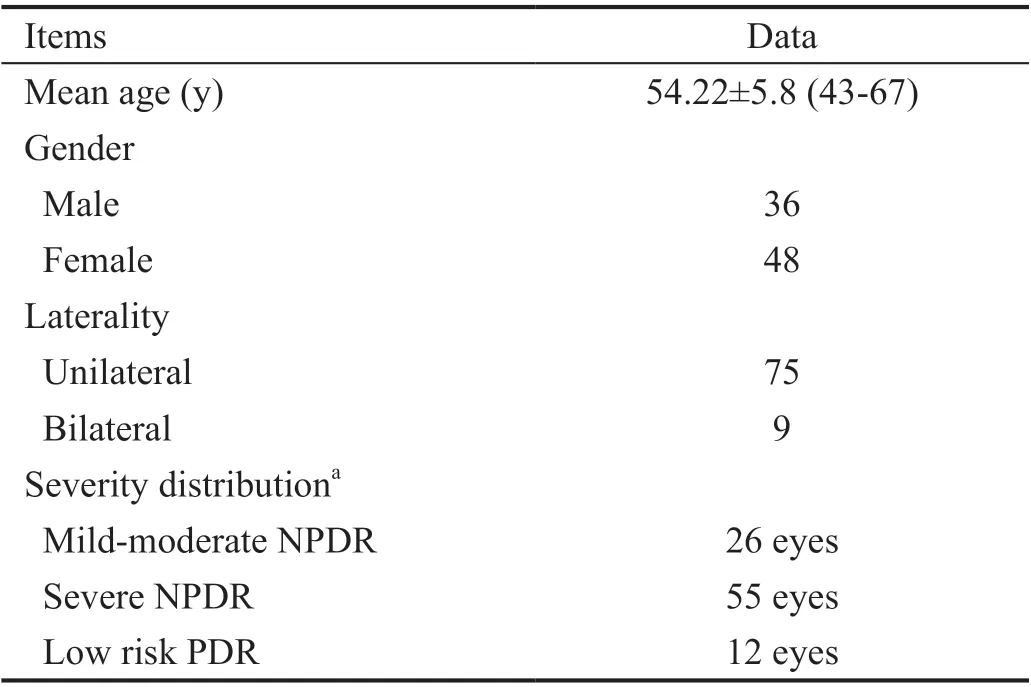
Table 1 Patients demographics
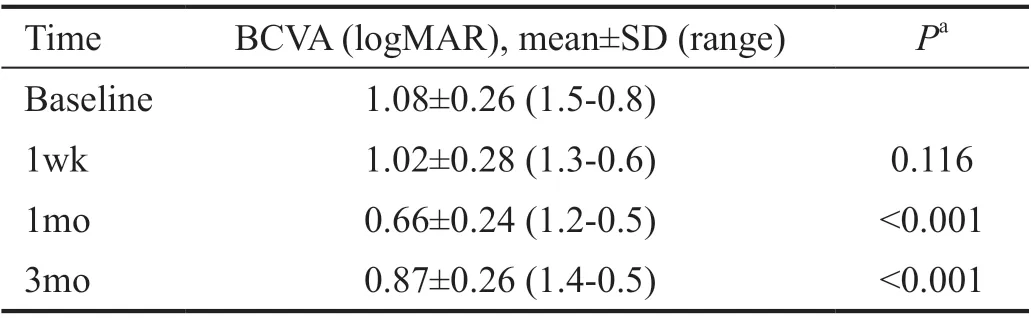
Table 2 The mean BCVA prior and after treatment
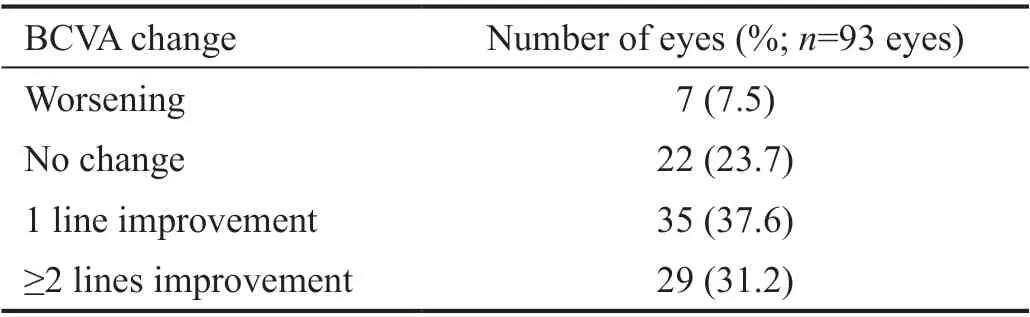
Table 3 Change in BCVA of 93 eyes at the end of follow-up

Table 4 The mean IOP
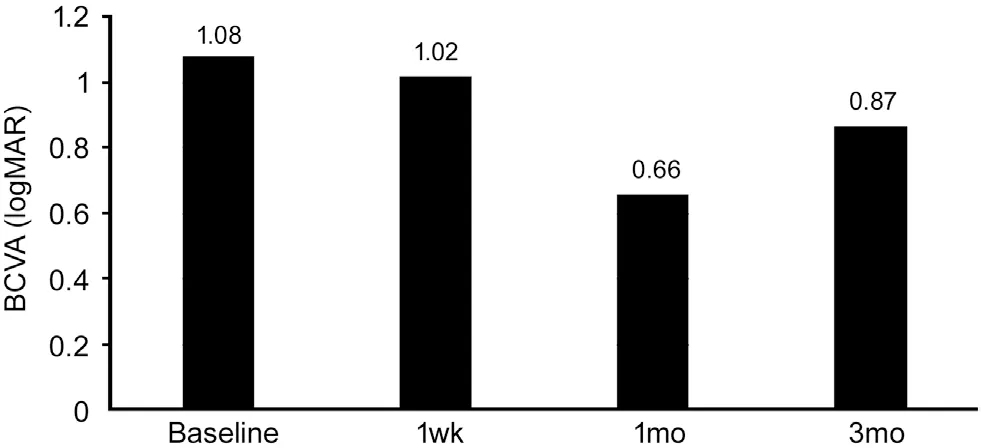
Figure 1 Mean BCVA prior and after treatment with topical dorzolamide 2% BCVA: Best corrected visual acuity.
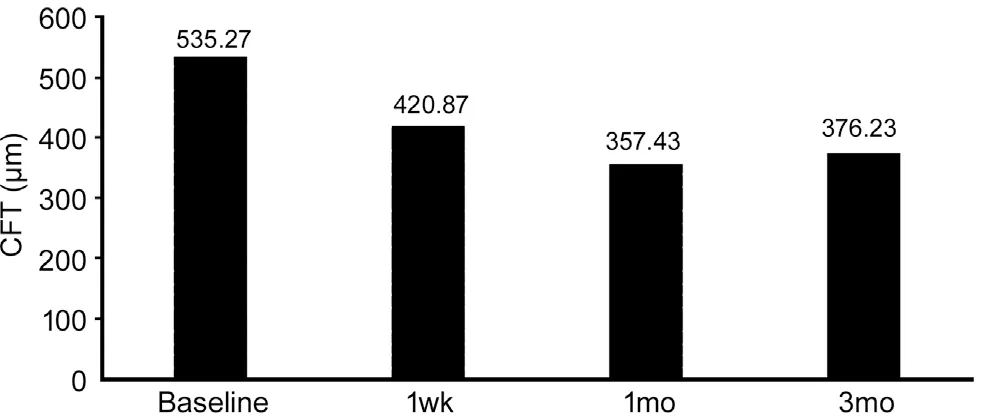
Figure 2 Mean CFT prior and after treatment with topical dorzolamide 2% CFT: Central foveal thickness.
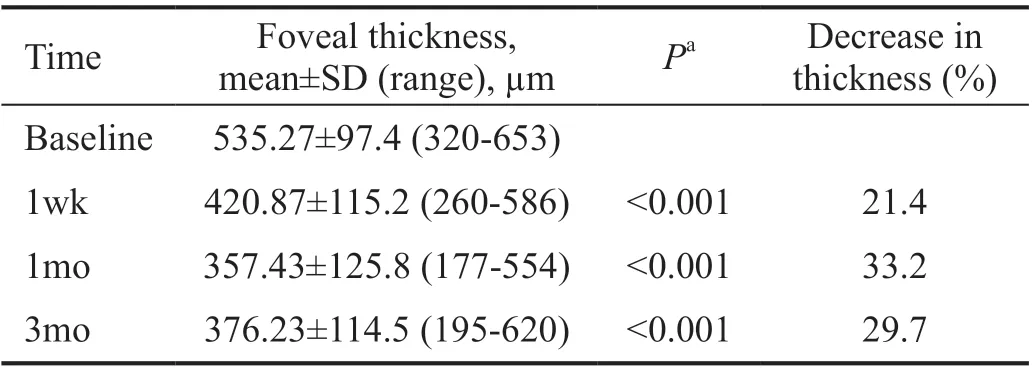
Table 5 The mean central foveal thickness
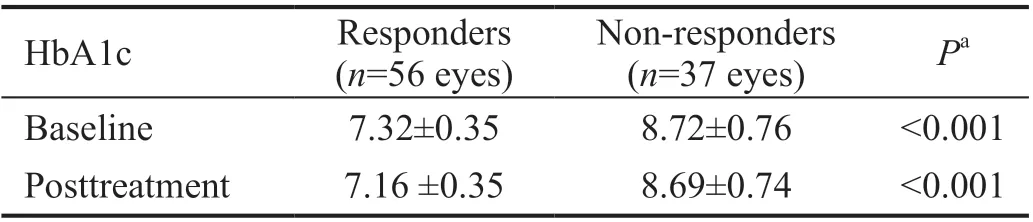
Table 6 The mean HbA1c among the responders and non-responders
It has been reported that the response to CAI therapy is better in patients with diffuse retinal pigment epithelial (RPE) disease than in those with primary retinal vascular disease, such as diabetes or retinal vein occlusion[31]. This was explained by alteration of RPE membrane-bound CAIs, which may have lost their polarized distribution due to accumulation of macular edema, with resultant stimulation of ion and fluid-removal across the RPE from the retina to the choroid[32]. However,some reports suggested that CAIs affect the BRB as they stimulate at least one ion-pump transport mechanism, resulting in an increase in passive permeability and outward-active transport across the BRB[33]. The exact mechanism for fluid transport across broken-down BRB in diabetic retinopathy remains obscure.
As the anti-inflammatory effect of dorzolamide had been reported before[34], the authors assumed that it would help in endogenous intraretinal fluid absorption through RPE and thus can improve the vision and prevente further retinal photoreceptor loss and neurodegeneration. The purpose of the current study was to evaluate the anatomical and functional effects of topical dorzolamide 2%, on CME in diabetic patients.
Based on analysis of the current series OCT, it was found that 56 eyes (60.2%) showed a significant improvement in central macular thickness (CMT) in response to topical dorzolamide,29% did not show an improvement or worsening, and 10.8%showed an increase in macular thickness compared with pretreatment values. The mean CFT was significantly lower at all post-treatment time points with the best-recorded results at one month and minimal deterioration at three months, but still better than pre-treatment levels. So, it can be assumed that sustained therapy may be required. Among the study cohort,68.8% of eyes demonstrated a subjective improvement in the BCVA, 23.7% did not show any change from the pre-treatment BCVA value, while only 7.5% showed a decrease in their BCVA.
Several prior studies have investigated the effect of HbA1c on the treatment outcomes of diabetic edema with variable designs and conclusions. Some reported a strong correlation between the high HbA1c and the persistent macular edema[35-36]; by contrast, others found there was no relation[37]. In the current cohort, patients who had lower HbA1c levels either baseline or post-treatment showed a better response to treatment,suggesting that HbA1c is one of the sensitive variables to predict final treatment outcomes.
Lima-Gómezet al[27]studied the effect of topical dorzolamide,in comparison to placebo on the reduction of retinal thickness after focal laser photocoagulation in DME. They reported a significant reduction in CMT after topical dorzolamide, which was absent in eyes that received a placebo. But the reduced CMT was not followed by a significant improvement in visual acuity. In their study, topical dorzolamide was used three times daily for only three weeks. Moreover, the duration of CME was not specified; patients could have prolonged edema which would have caused irreversible functional damage to retinal photoreceptors, even if anatomical architecture has been restored. In the current study, patients with recently diagnosed diabetic edema were selected as the duration of DME, which could affect the macular cells’ function either mechanically or toxically with more photoreceptor damage during the evolution of the disease.
One of the interesting questions here is the maintained response to treatment at the end of three months in spite that patients were off-treatment for two months. Understanding the pharmacokinetics of topical dorzolamide can interpret this topic point and might be a valuable guide. Following chronic topical application of dorzolamide, the drug reaches the systemic circulation and accumulates as well as its metabolite (N-desthyl) in red blood cells (RBCs). After the cease of the drug, its RBCs concentration initially shows a sudden decline followed by slower nonlinear wash-out of RBCs resulting in a prolonged half-life of the elimination phase up to four months[38].
The prolonged effect of dorzolamide is mostly related to the incomplete washout of the drug from the eye. Until now, there is no consensus regarding the complete washout period of topical anti-glaucomatous drops. According to the published literature; it varies from 4 to 8wk[39]. In the current work, the IOP decreased until the first month then started to increase again in the third month.
The current study confirms a promising effect of topical dorzolamide in visual improvement and CMT reduction within three months. To the authors’ knowledge, there have not been published trials of the efficacy of topical dorzolamide 2% as a first-line treatment for diabetic CME. Recent trials investigated its additive role to the intravitreal anti-VEGFs and reported promising effects[40-41]. This novel study also has attempted to evaluate a less invasive approach than the intravitreal one in non-refractory diabetic edema with fewer potential complications. The absence of a randomized design and a control group are the limitations of the present study. Further randomized investigations of a larger number of cases with longer follow-up will be needed.
In conclusion, the current study suggests that topical dorzolamide 2% can be promising, effective, affordable, and safe therapy in treating non-refractory diabetic CME. The poor control of diabetes mellitus and elevated serum HbA1c might contribute to unsatisfactory treatment outcomes.
ACKNOWLEDGEMENTS
The manuscript was accepted in part at 10thInternational Conference on Clinical & Experimental Ophthalmology(Dubai, UAE) as oral presentation and the abstract published as a conference paper inJ Clin Exp Ophthalmol.DOI:10.4172/2155-9570.C1.048.
Conflicts of Interest:Badawi AE,None;Mokbel TH,None;Elhefney EM,None;Hagras SM,None;Abdelhameed AG,None.
 International Journal of Ophthalmology2021年9期
International Journal of Ophthalmology2021年9期
- International Journal of Ophthalmology的其它文章
- Five-year results of refractive outcomes and visionrelated quality of life after SMlLE for the correction of high myopia
- One-step viscoelastic agent technique for lCL V4c implantation for myopia
- miRNA-26b suppresses the TGF-β2-induced progression of HLE-B3 cells via the PI3K/Akt pathway
- Pediatric ocular trauma with pars plana vitrectomy in Southwest of China: clinical characteristics and outcomes
- Socio-economic disparity in visual impairment from cataract
- Disseminated hydatid disease in the orbit and central nervous system
