Thoracic surgery in 2030
Joyce W. Y. Chan, Rainbow W. H. Lau, Aliss T. C. Chang, Ivan C. H. Siu, Teddy H. Y. Wong, Zheng Li, Calvin S. H. Ng
Introduction
Thoracic surgery has evolved at an exponential pace since the first clinical lung resection in 1882. The first pneumonectomy was performed in 1895, the first lobectomy in 1901, while double lumen endotracheal intubation for pulmonary resection did not occur until 1950. The 1990s saw the development of game-changer video-assisted thoracoscopic surgeries (VATS), while the 2000s gave witness to the explosive boom of robotic surgeries. As Abraham Lincoln once said, “The best way to predict the future is to create it”, in this article we discuss the current cutting-edge technologies and attempt to predict the future of thoracic surgery in 10 years’ time.
Advances in imaging
The drive for improving imaging capabilities of thoracoscope originates from the pursuit of fewer and smaller wounds. It has long been understood that incision length is proportional to inflammatory cytokine response (1), that video-assisted thoracoscopic surgery (VATS) result in faster patient recovery and shorter hospital stay compared to thoracotomy (2). The benefits of enhanced recovery may result from the interplay between better preserved cellular immunity and cytokine profile, attenuated stress hormone release, and improved preservation of pulmonary and shoulder function (3). Contrary to initial concerns, there is an increasing body of evidence pointing to possibly improved survival of lung cancer patients after VATS when compared with open surgery (4). It has been postulated that the betterpreserved immunity, including natural killer and cytotoxic T cells, during the early post-operative period after VATS, contributed to enhanced tumour surveillance (5). Hence, the development of thoracic surgery in the past 30 years have mainly focused on reducing the number of ports, from the standard 3-port VATS in the 1990s, to the first uniportal VATS lobectomy in 2011. Ever since, the pace of development of minimally invasive thoracic surgeries have picked up even faster, with uniportal pneumonectomy and sleeve resection performed with success by 2013, subxiphoid thymectomy and lobectomy by 2014, and non-intubated major lung resection by 2015 (6-8).
It is well-understood that by switching from multiport to uniportal VATS, the alleviation in patient sufferings are often passed on to the surgeons as technical challenges (9,10). The single port incision has to accommodate a retractor, a dissector/staple and a thoracoscope, operating in the same plane and field of vision, leading to crowded surgical site and instrument fencing, narrow field of view, and require a high level of surgeon-assistant coordination (11). New thoracoscopes are designed to overcome this, including the 3D EndoEye Flex by Olympus (12), and the Cardioscope developed by Li et al. (13). The distal flexible tip of EndoEye is able to bend up to 100 degrees in four directions, solving part of the problem by occupying less external space and providing a different plane of view from the instruments (14). However, the curved tip design, without proper position, may sometimes occupy more space than a straight scope, causing interference among instruments. The Cardioscope, with its adjustable distal flexible section, occupies less space and provides improved dexterity, able to have a maximum bending angle of 190 degrees, making it a promising endoscope for both cardiac and thoracic minimally invasive surgeries.
To eliminate the need of a thoracoscope through the surgical access all together, magnetic anchored and guided endoscopes (MAGS) have provided a near perfect solution (15) (Figure 1). A wireless high-definition camera is delivered through the single port, and is anchored to the roof of the chest wall by an external magnet placed on patient’s chest. Since the camera is placed away from the port, it provides a different plane of vision and allows triangulation between the camera and other instruments. The camera is equipped with a durable battery and its own light source. Despite initially designed for laparoscopic use, it is more suited to thoracic surgery as the rigid chest wall provides superior stability compared to the soft abdominal wall. Conventional MAGS were built from rigid materials, rendering them heavy, complex, difficult to make and costly. Our institute has dedicated years of effort in developing our soft wireless steerable MAGS (16). It composes of a 13 g steerable endoscope/camera made from printable silicon rubber with embedded magnets and a camera module. This internal component in the chest cavity is anchored and steered by a low-profile external controller on patient’s chest wall. Through the external controller, the camera can be directed at different directions, and can be repositioned or slid smoothly along the chest wall, as shown in porcine tests. In addition, a panoramic view of operative space can be obtained by incorporating the views from several cameras placed within the thoracic cavity, minimizing the need of camera repositioning for better view (17). Visual serving have also been successfully developed to automatically track either instruments or surgeon’s eye gaze, making it a semi-robotic endoscope/camera (18). With further refinements and upgrades, the system may become commercially available at reasonable cost, possessing a high potential for future widespread clinical use in uniportal VATS.
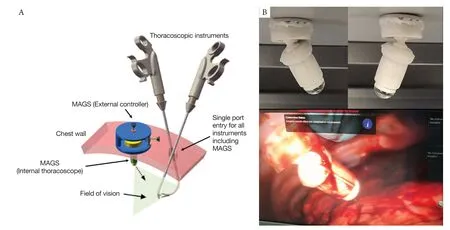
Figure 1 Magnetic anchored and guided endoscopes (MAGS). (A) The schematic diagram of MAGS use during thoracoscopic surgery. Through a single incision, the internal portion of MAGS (thoracoscope/camera) is first delivered into patient’s thoracic cavity and is anchored to the chest wall with the help of an external controller. Surgery can subsequently be performed using other instruments inserted through the previous incision; (B) the MAGS thoracoscope pointing at different directions; (C) the MAGS thoracoscope is anchored to porcine chest wall in in-vivo test.
Image overlay guided surgeries have been utilized for several years to aid structure recognition. Image overlay is a form of ‘augmented reality’ that merges computergenerated information with real world images. Position tracking system enables the semi-transparent computergenerated display to be superimposed on surgeon’s view in a real-time fashion to provide anatomical tracking. More recently, instead of projecting the image overlay on a screen monitor, submersive smart glasses or headsets have been developed, including Google Glass Enterprise Edition 2 (19), and many more. Even more futuristic would be submersive smart contact lens, experimented by big technological companies like Samsung, Google and Sony since 2014. Medical technology companies like Mojo Vision (20) and Innovega (21) are currently testing their prototypes. The former uses a scleral lens and a wearable neck device as processor, while the latter uses a soft lens with polarizer filter that pairs with a pair glasses as processor. Smart contact lens can be challenging to make-the nanotechnology necessary to pack all the components into such a small space, the estimated one million pixels needed to mimic a computer screen, and the requirement of the lens to be wearable, breathable, see-through and safe. Although their primary clients are visually impaired persons, it is predictable that smart contact lens with image overlay will be extended to future surgical adjunct market.
Future of thoracic robots
The first medical-use robots were developed in the late 1980s to perform brain biopsy, simple prostatic surgeries, and to mill out orthopaedics fittings. The prototype for da Vinci robot was initially designed by the United States military, later sold to a private company, and eventually gave rise to the da Vinci robotic system (by Intuitive Surgical Inc) (22) that is one of the most readily available robotic systems worldwide (23). However, apart from rigid robots like the da Vinci system, robotic surgery in fact encompasses 2 other types of robots - handheld semi-rigid robots, and flexible robots. Robotic assistance devices aim to improve the dexterity and accuracy of surgeon’s hands, with examples including FlexDex Surgical by FlexDex Inc. (24), ArtiSential by LivsMed (25), and a few flexible instruments designed by our institute. The majority of such tools consist of a single hand-held instrument that precisely translates the surgeon’s hand, wrist and arm movements from patient’s exterior into the corresponding movements of an end-effector inside patient’s body, based on a simple and purely mechanical design. Some instruments have a double-jointed end-effector, providing a fully articulating wristed motion. Affordability is a huge advantage due to the mechanistic design, eliminating the need for advanced computers or operative suites.
Flexible robots are made up of soft, compliant materials, and may represent the future of robotic surgery due to its ability to flex and move within patient’s body. They alleviate the problems of instrument fencing and narrow field of view commonly occurring in rigid robots, and potentially cause less trauma to surrounding tissue compared to rigid instruments, enabling conformity and safe interaction with human body (26). The first clinical use of the latest da Vinci SP robotic system was studied in our institute in 2017, where transoral robotic surgeries were successfully performed in 6 patients (27). Three fully-wristed and elbowed instruments and a fully-wristed endoscope can be inserted through a single 2.5 cm diameter cannula and entered up to 24 cm deep. Triangulation of instruments is possible due to the flexibility of instruments. Other single incision robots include Titan’s SPORT system (28) and Medrobotics’ Flex robotics system (29). Nevertheless, there are many challenges for soft robots, including low force exertion and poorer controllability due to nonlinear responses to strain.
Natural orifice surgery
To further push the boundary of non-invasiveness, there has been growing interest in natural orifice transluminal endoscopic surgery (NOTES) in the past decade. The first experience in thoracic embryonic-NOTES was reported in 2013 where transumbilical thoracic sympathectomy was performed using a 5mm ultrathin flexible gastroscopy which required diaphragmatic incision (30). Transumbilical surgical lung biopsy and pericardial window creation has been attempted (31). However, more sophisticated endoscopic platform will be needed for more complex tasks. Equipment for e-NOTES can be categorized into two groups: flexible equipment and miniaturized devices. Flexible instruments conform to natural orifice or lumen, but are less stable and have limited triangulation between scope and instrument arms due to space confinement. Recent advances in shape lock contribute to improved adjustable stiffness and therefore stability of the system. Miniature robots are delivered into the body sequentially and assembled at surgical site, saving space and avoiding tool collision. However, they are limited by the relatively low payload ability and challenging implementation. In future, a hybrid approach may provide the best solution-the main operation performed by flexible instruments while imaging and assistance provided by miniature devices. With increased blurring of treatment capabilities between surgery and endoscopy, there is a need to develop treatment-orientated endoscopes. The traditional endoscope was developed for diagnostic purpose, where the camera moves together with the effector, thus making precise surgery difficult. Our institute has developed an endoscopic surgical robot for endoscopic submucosal dissection in the gastrointestinal tract (32). The system consists of a camera, an insufflation port, and a flexible dual-arm robot (a dissector arm and a lifter arm) which can be separately advanced and moved with wrist-like motions. However, a more compact system may have to be developed for thoracic endobronchial surgeries due to the narrower lumens in the tracheobronchial tree.
Airway remains the most obvious route of access for natural orifice surgery in the thorax. In order to deliver treatment to diseases in the lung parenchyma, a myriad of complex systems is required. Firstly, accurate navigation and localization of the target lung nodule may be achieved by electromagnetic positioning (Figure 2A) [e.g., the SuperDimension Navigation system by Medtronic (33)], 3D airway reconstruction and navigation [e.g., LungPoint VBN system by Broncus Medical Inc. (34)], in combination with fluoroscopy or cone-beam CT available in hybrid theatres (35-41) (Figure 2B). This process may be further facilitated by robotic-assisted bronchoscopy, for example in the Monarch platform by Auris Health (42). Secondly, advanced tools for parenchymal access are required for peripheral lesions, these include CrossCountry tool in the Illumisite platform by Medtronic (43), or transparenchymal nodule access module in the Archimedes platform by Broncus Medical (44). A tunneled pathway is created trans-bronchially towards a lesion, under virtual bronchoscopy and CT/fluoroscopy guidance. Third is the ability to deliver treatment or execute procedures. Numerous ablative technologies for the lung have been experimented, including thermal energy like radiofrequency, microwave, cryotherapy, thermal vapor ablation; laser ablation or irreversible electroporation (45). Microwave energy is particularly well-suited as it is less affected by the high impedance of lungs and able to provide larger and faster ablation zones with little heat sink effect (46). To date, we have performed 60 transbronchial microwave ablation for lung nodules with 100% technical success rate and a low recurrence rate (47) (Figure 2C). Bronchoscopic thermal vapor ablation, initially developed as an endoscopic lung volume reduction therapy for emphysema, may also have potential for “anatomical” segmental ablation by blocking off a bronchial segment and thermally ablating the distal airways and parenchyma (48). Therapeutics may also be delivered directly into the lung nodules, including chemotherapy, brachytherapy, immune checkpoint inhibitors, or gene therapies. Potential advantages of local injection include high local dosage and little systematic side effects, and that draining lymph nodes can also be targeted. A step further, intracellular delivery of therapeutics using nanoparticles or nanoworms are under heavy research (49) and will likely be readily achievable in future. Lastly, it is possible to have real time direct visualization in small bronchioles with the latest development of thinner endoscopes. For instance, Luminus boasts an ultrathin flexible and steerable endoscope with 1mm thick optical fiber, and has a working channel with microsurgical tools (50). Its application as a bronchoscope still faces challenges including difficulty in clearance of bronchial secretions and the long route of access with high torque.
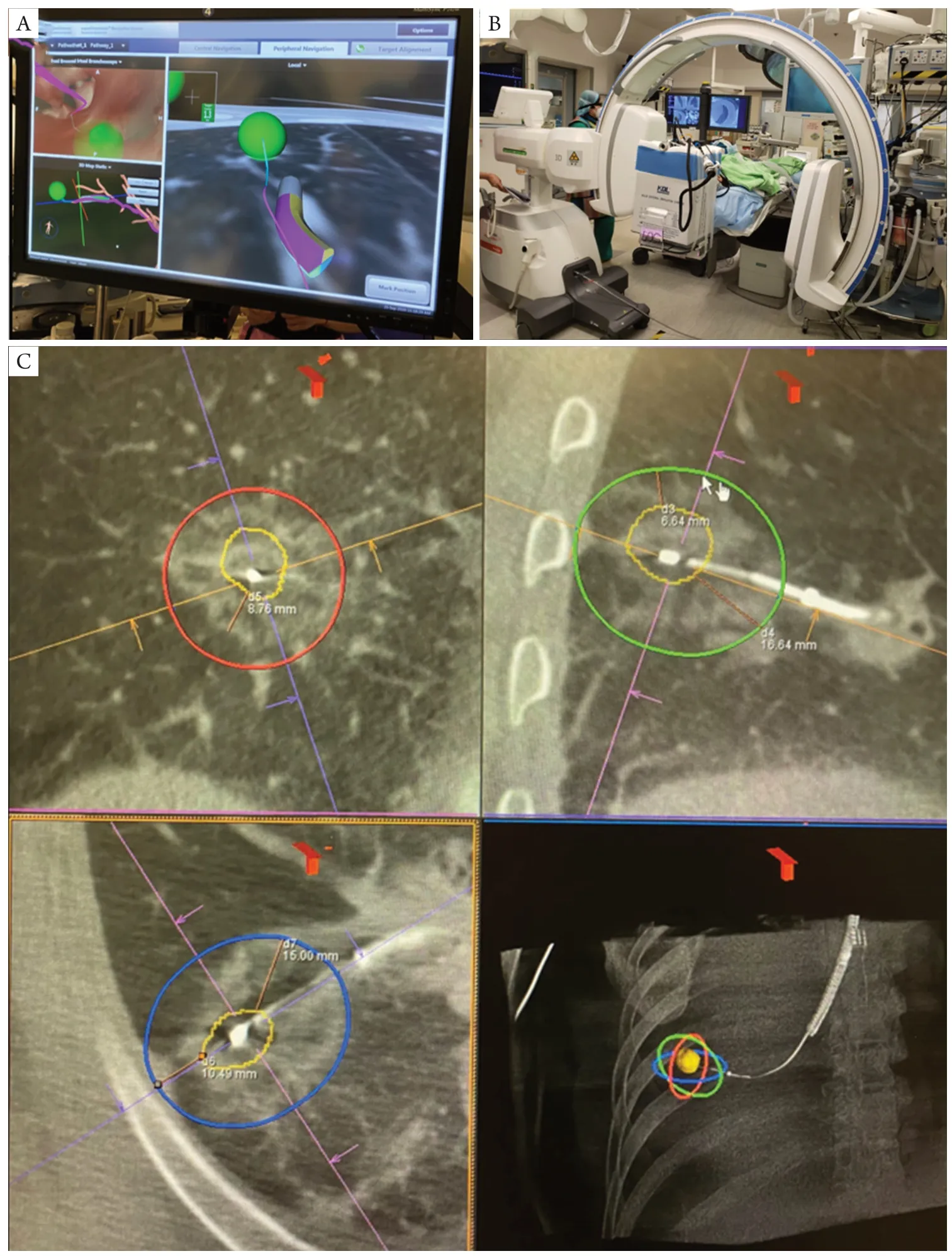
Figure 2 Electromagnetic navigation bronchoscopy microwave ablation of lung nodules. (A) A lung nodule (represented by the green ball) is navigated towards with the help of electromagnetic navigation bronchoscopy, which provides real-time locational relationship between the tip of bronchoscope locatable guide and the lung nodule. The upper left panel shows the virtual bronchoscopic view; (B) the hybrid theatre setup for lung nodule localization, utilizing cone-beam CT and fluoroscopy; (C) the 3-axes post-ablation CT images of transbronchial microwave ablation of lung nodule. The target lung lesion is outlined by orange tracings. The radio-opaque ablation catheter is seen puncturing the lesion. The red/green/blue ovals represent the predicted ablation zone at a particular energy output. The surrounding ground glass opacities around the lung nodule represents the actual ablation zone, giving a margin of 6.6 mm.
Telesurgery and automation
In 2001, the first tele-surgical robotic cholecystectomy was performed on a French patient by surgeons in New York, made possible by high-speed telecommunications and the Zeus surgical robot (51). An ultrafast feedback loop is required for smooth telesurgery, with latency <150 ms for video/audio stream and <10 ms for haptic feedback data. In 2019, Huawei supported the first 5G telesurgery in China, where the operation signal was transmitted to 50 km away to perform hepatic lobectomy in animals (52). Shortly after, surgeons working 2,500 km away have successfully implanted a deep brain stimulation implant for a patient with Parkinson’s disease. With the exponential adaptation of 5G technology worldwide, and increasing availability of surgical robotic systems, telesurgery is expected to become more popular and aid technique transferal. Improved force-feedback, or haptic technology, is essential for the further development of telesurgery, such that the surgeon can see and feel the patient in a realm of virtual reality.
The ultimate goal of robotic surgery is to achieve full automation without the need of human input or oversee. According to the society of automotive engineers, there are 5 automation levels, with the last being full automation. However, robotic surgery currently still has a long way to go. The first step in automation is robotic assistance, exemplified by the da Vinci robotic system. As of latest development, robotic surgery is at the second and third steps-task autonomy and conditional autonomy, which includes automation in logical surgical steps for example suturing or anastomosis, where a surgeon is required at most times to oversee the process. In perfect experimental situations these may be achievable, but results are much less predictable in a reallife situation, and any changes in the environment may render the process unreliable or hazardous. Automobile automation is first pioneered and heavily invested, while surgical automation is closely following. In several decade’s time, there may be no need for physical doctors at all-all diagnostics and therapeutics may be performed automatically by robots in a one-stop visit.
Conclusions
The future of thoracic surgery likely lies in better imaging qualities and techniques, more sophisticated soft robots, natural orifice surgery and enhanced level of automation. The real question is how fast these progresses can be developed safely and implemented effectively. Nevertheless, one must not limit one’s imagination to currently available technologies. As the founder of the Ford Motor Company Mr. Henry Ford had said, “If I had asked people what they wanted, they would have said faster horses.” The future of thoracic surgery may be so daring and innovative that exceeds our wildest imaginations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Chinese Journal of Thoracic Surgery for the “International Thoracic Surgery Column”. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3877/cma.j.issn.2095-8773.2021.03.02). The “International Thoracic Surgery Column” was commissioned by the editorial office without any funding or sponsorship. RWHL is a consultant for Medtronic, USA; and Siemens Healthineer. ZL possesses one pending patent in the United States entitled “wireless magnetically steerable endoscope” (No. 62/280487), and one patent in China entitled “a handheld endoscope system” (No. 20170219919.3). CSHNg is a consultant for Johnson and Johnson; Medtronic, USA; and Siemens Healthineer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the noncommercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-ncnd/4.0/.

