Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis:A contemporary narrative review
Saberio Lo Presti,Tarec K Elajami,Mohammad Zmaili,Reza Reyaldeen,Bo Xu
Saberio Lo Presti,Reza Reyaldeen,Advanced Cardiac Imaging Fellows,Sydell and Arnold Miller Family Heart Vascular and Thoracic Institute at Cleveland Clinic,Cleveland,OH 44195,United States
Tarec K Elajami,Department of Cardiology,Mount Sinai Medical Center,Miami Beach,FL 33140,United States
Mohammad Zmaili,Department of Internal Medicine,Cleveland Clinic Foundation,Cleveland,OH 44195,United States
Bo Xu,Section of Cardiovascular Imaging in the Robert and Suzanne Tomsich,Department of Cardiovascular Medicine,Sydell and Arnold Miller Family Heart Vascular and Thoracic Institute at Cleveland Clinic,Cleveland,OH 44195,United States
Abstract Infective endocarditis is one of the leading life-threatening infections around the world.With the exponential growth in the field of transcatheter interventions and advances in specialized surgical techniques,the number of prosthetic valves and cardiac implantable devices has significantly increased.This has led to a steep rise in the number of cases of prosthetic valve endocarditis (PVE) comprising up to 30% of all cases.Clinical guidelines rely on the use of the modified Duke criteria;however,the diagnostic sensitivity of the modified Duke criteria is reduced in the context of PVE.This is in part attributed to prosthesis related artifact which greatly affects the ability of echocardiography to detect early infective changes related to PVE in certain cases.There has been increasing recognition of the roles of complementary imaging modalities and updates in international society recommendations.Prompt diagnosis and treatment can prevent the devastating consequences of this condition.Imaging modalities such as cardiac computed tomography and 18-fluorodeoxyglucose positron emission tomography/computed tomography are diagnostic tools that provide a complementary role to echocardiography in aiding diagnosis,pre-operative planning,and treatment decisionmaking process in these challenging cases.Understanding the strengths and limitations of these adjuvant imaging modalities is crucial for the implementation of appropriate imaging modalities in clinical practice.
Key Words:Prosthetic valve endocarditis;Multimodality cardiac imaging;echocardiography;Cardiac computed tomography;18-fluorodeoxyglucose photon emission tomography/computed tomography
INTRODUCTION
Infective endocarditis (IE) is the third most common life-threatening infection in the world with a reported in-hospital mortality as high as 14%-22% and 1-year mortality of up to 40%[1].The volume of prosthetic valve replacement procedures has dramatically increased over the last decades[2].This has led to an increase in the incidence of prosthetic valve endocarditis (PVE),accounting for 20% to 30% of all cases of IE[2-4].Traditionally,the diagnosis of IE is based on the modified Duke Criteria which relies on echocardiography[5].Initially,transthoracic echocardiography (TTE) is utilized to assess for PVE;however,due to technical limitations and acoustic shadowing,transesophageal echocardiogram (TEE) is generally mandated when it is difficult to evaluate the prosthetic structures or the clinical suspicious remains high despite an apparently unremarkable TTE,according to the contemporary guidelines from the European Society of Cardiology (ESC) and American Heart Association/American College of Cardiology guidelines (AHA/ACC)[3,5,6].In cases where TEE yields a negative result and clinical concern persists,guidelines recommend to either repeat the study in 3-7 days or to complement the evaluation with an alternative imaging modality such as 18-fluorodeoxyglucose photon emission tomography/computed tomography (PET/CT18F-FDG) or cardiac computed tomography (CCT)[3,5,6].Although TEE has generally good diagnostic performance,it is limited by prosthetic material-related artifacts,and certain complications of PVE,such as abscesses and pseudoaneurysms may be missed in some cases by TEE[7].
Patients with PVE are at a higher risk of developing complications and worse outcomes when compared with native valve endocarditis (NVE) patients,even when the causative organism are similar[3,8].Therefore,it is of uttermost importance to accurately diagnose this condition and institute prompt treatment to ameliorate its deleterious consequences.There has been increased recognition of the pivotal role of multimodality imaging in the diagnosis and treatment of PVE[9,10].The purpose of this narrative review is to focus on the diagnosis of PVE with a special emphasis on the emerging complementary use of multimodality imaging modalities.
EPIDEMIOLOGY
The incidence of PVE ranges between 1% to 4% in the first year after surgery followed by approximately 1% per year thereafter[11].The risk of developing PVE is higher within the first 5-years post-surgery (1.4% to 5.7%),and half of the patients with PVE develop a prosthetic valve abscess and pseudoaneurysm,which are associated with increased mortality of 30%-54%[7,12,13].The reported incidence of PVE is heterogeneous,reflecting valve-related,patient,and geographical factors.
Valve-related factors
In a study from the Danish national registry of 18041 patients,the overall incidence of PVE was 69.8/10000 person years in patients undergoing surgical valve replacement[14].When examined based on the anatomical location,the incidence was 65/10000 person years for mitral valve replacement (MVR),70/10000 person years for aortic valve replacement (AVR),which increased to 89.4/10000 person years when both mitral and aortic valves were replaced[14].Despite this difference,the cumulative incidence of PVE at 10-years was similar for both MVR and AVR (5.2%)[14].
PVE comprises 11% of all cases of tricuspid valve endocarditis and 43% of all cases of pulmonary valve endocarditis (Figure 1)[15].In a cohort of congenital heart disease patients,924 surgical pulmonic valve replacement were performed with 19 (2%) cases attributed to PVE,corresponding to an incidence of 333/100000 person years[16].A large single-center cohort of 2124 adult patients (median age 41.5 years) with IE reported 24 cases of pulmonary valve endocarditis,of which 54.2% of cases occurred in the context of prosthetic valves[17].
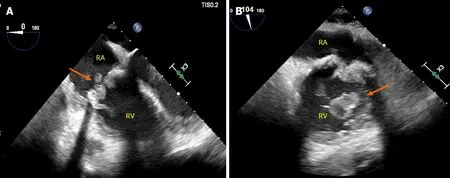
Figure 1 Prosthetic valve endocarditis complicating prior bioprosthetic tricuspid valve replacement secondary to intravenous drug abuse relapse.
PVE is also a feared complication in transcatheter aortic valve replacement (TAVR)[18].In a meta-analysis by Andoet al[19] in 3761 patients undergoing percutaneous or surgical AVR (SAVR),the overall incidence of PVE was not significantly different between TAVR and SAVR at 1,2 and 3.4 years follow-up[19].Over this period of time,there was a trend towards a higher incidence of PVE in the TAVR group (0.86% to 2%)compared to the SAVR group (0.73% to 1.3%),and this was enhanced in patients with intermediate surgical risk [2.3%vs1.2%;odds ratio (OR)=1.92,95% confidence intervals (CI):0.99 to 3.72,P=0.05].In the Nordic Aortic Valve Intervention(NOTION) trial,280 Low-surgical risk patients with severe aortic stenosis were randomized to TAVR (self-expanding CoreValve) or SAVR (stented bioprosthesis)[20].This trial showed a non-significant difference in the 5 year-cumulative incidence of PVE between these two approaches (6.2% for TAVRvs4.4% SAVR)[20].Similar results were reported by Summeret al[21],in a pooled cohort of all patients from PARTNER I and PARTNER II trials (8530 patients,107 cases of PVE),where the incidence over time of PVE was similar for TAVR [5.21 PVE per 1000 person-years(95%CI:4.26-6.38)] and for SAVR [4.10 per 1000 person-years (95%CI:2.33-7.22);incident rate ratio,1.27 (95%CI:0.70-2.32);P=0.44][21].Andoet al[19] also reported a subgroup analysis in TAVR patients which demonstrated comparable risk between balloon-expandable valves (BEV) and self-expandable valves (SEV)[19].Similar findings were described by Regueiroet al[22] in a cohort of 6363 patients undergoing TAVR,where the incidence of PVE at 1-year did not significantly differ (0.95% SEVvs1.25% BEV;P=0.33)[22].When other complications were analyzed,the rate of systemic stroke and embolism was higher in patients with BEV (8.7%vs20.0%adjusted OR=2.46,95%CI:1.04-5.82,P=0.04)[22].
Furthermore,percutaneous edge to edge mitral valve repair is an increasingly relevant transcatheter intervention,where post clip implantation endocarditis has been described only in case reports,remaining an extremely rare presentation[23].
In terms of the type of valve prosthesis,the Danish National Registry demonstrated in 18041 patients undergoing left-sided valve replacement that the use of bioprosthetic valves was associated with an increased risk for prosthetic infection in patient undergoing either MVR (HR=1.91,95%CI:1.08-3.37) or AVR (HR=1.70,95%CI:1.35-2.15) over 10 years follow-up[14].These results were similar to those reported by Brennanet al[24] in a large cohort of patients undergoing SAVR (bioprosthetic=24410;and mechanical=14789) followed up for 12 years where the risk of PVE was higher in those patients undergoing bioprosthetic valve replacement (HR=1.60;95%CI:1.31-1.94).However,a limitation of these studies was their retrospective nature [14,24].Conflicting evidence arises from 3 randomized clinical trials,comprising a total of 40207 patients that underwent left sided valve replacement,which showed no significant difference between bioprosthetic and mechanical prosthesis [22-24].A major limitation of these trials is the lack of power to detect meaningful differences since IE was not specified as a major endpoint[25-27].
Patient factors
There is conflicting data regarding the age group most susceptible to develop PVE.In the Grupo de Apoyo al Manejo de la Endocarditis infecciosa en España (GAMES)Database registry,which included 3120 patients with IE,patients 65-79 years old(elderly) had a significantly higher incidence of PVE compared with those<65 years(young) and ≥ 80 years old (octogenarian) (37.3%vs24.4% and 26.3%,respectively,P<0.001)[28].This observation was also demonstrated by Lópezet al[29],studying a cohort of 600 Left-sided IE patients 40% of whom had PVE,showing a similar age distribution[29].In contrast,a smaller observational study of 72 patients with IE by Menchi-Elanziet al[30] demonstrated that elderly patients (65-79 years old) had a significantly lower prevalence of PVE compared to the young and octogenarians[30].In patients with PVE,there is male predominance with 3:1 ratio across various studies[14,28-30].This ratio changes towards 1:1 in the octogenarian group[28-30].Interestingly,in an observational study of 621 patients with left-sided IE,mitral mechanical valve PVE was more common in women than in men[31].The mechanism behind these findings remain unclear,and further studies are required to examine the age and gender influence on PVE.
Geographical factors
In an observational,prospective multicenter cohort of 2670 patients from 28 countries with IE,556 patients (20.1%) had PVE[1].The highest percentage of PVE cases was in Southern Europe,Middle East and South Africa (26.10%),and it was the lowest in South America (11.9%)[2].In the United States,the incidence of PVE was 20.9%,with the highest source corresponding to health care associated infections (44.8%),followed by intravascular device-related infection (27.6%),non-nosocomial heath care-associated PVE (21.1%),and hemodialysis (12.9%)[2].
CAUSATIVE AGENTS
The prevalence of the most common causative organisms causing PVE according to the timing and technique of valve surgery are shown in Figure 2.Within 60 days from surgical valve replacement,the most common causative organism of PVE is Staphylococcus Aureus (30%) followed by Streptococcus species (28%).Between 2 to 12 months after surgery,coagulase-negative Staphylococci are the most common organism (36%),and after one year,Streptococci predominantly viridians group,are the leading cause[2,32-35].After TAVR,Enterococci and Staphylococcus Aureus (25% and 16%-24%,respectively) are the predominant organisms (Figure 2)[36,37].

Figure 2 Prevalence of causative organisms according to timing and technique of valve intervention.
CLINICAL FEATURES
The clinical diagnosis of PVE is challenging as patients often manifest non-specific symptoms,such as fever,weakness and poor appetite in the early post-operative period[38].Therefore,the presence of a new murmur,new or worsening congestive heart failure (CHF),conduction abnormalities and stroke should all raise suspicion for PVE[38].Data from the International Collaboration on Endocarditis (ICE) Prospective Cohort Study (PCS) reported the occurrence of CHF in 32.9%,intra-cardiac abscess in 29.7%,stroke in 18.2% and other systemic embolization in 14.9%,among 556 patients with PVE[2].When comparing NVE and PVE,both had a similar incidence of CHF,stroke and persistent bacteremia;however,the incidence of systemic embolization was lower in PVE[2].
From the TAVR international registry consisting of 245 patients who developed PVE after TAVR (BEV and SEV),the most common initial symptom was fever (approximately 80%),followed by CHF (approximately 40%),and cutaneous manifestations(approximately 3%) with a median time to onset after TAVR of 5.3-5.5 months[22].In this study,stroke was significantly higher with BEV compared to SEV (24.6%vs7.8%,P<0.01)[22].The lower rate of cutaneous manifestations in PVE,such as Osler’s nodes,Janeway lesions and Roth’s spots could be attributed to a more acute course of the disease in PVE,compared to a more protracted course commonly seen in NVE[38].In Reguiero’s cohort,patients with PVE following TAVR had no significant difference in mortality between BEV and SEV (37%vs36%,respectively)[22].
In terms of mortality,Wanget al[2],described in-hospital mortality was significantly higher in the PVE group (127/556 patients) compared to NVE (310/1895 patients)(23%vs16%,P<0.001).After multivariate analysis,the key drivers of increased mortality were CHF (OR=2.33,95%CI:1.62-3.34),intracardiac abscess (OR=1.86,95%CI:1.10-3.15),and stroke (OR=2.25,95%CI:1.25-4.03).Østergaardet al[14] in a cohort of 18,041 undergoing left sided valve replacement (AVR 88.8%,MVR 9.7%,and both 1.5%) demonstrated that PVE in AVR patients was associated with higher mortality than in MVR at 10 years (44%vs39%,P<0.01)[14].Moreover,they also divided these results according to the prosthesis type showing a significantly higher mortality with bioprosthetic compared to mechanical valve in both AVR and MVR at 10-years.However,when both groups were matched,there was no significant difference in mortality[14].
DIAGNOSTIC IMAGING MODALITIES
Transthoracic and transesophageal echocardiography
Although acoustic shadowing and reverberation artifacts from prosthetic material hamper the imaging resolution,echocardiography remains the forefront diagnostic modality in suspected PVE[5].Wide availability,low cost,rapid acquisition and interpretation,and a lack of radiation are some of the important qualities that make echocardiography the first-line imaging modality[6].The echocardiographic examination should focus on identifying infection-related changes,such as vegetations,perivalvular abscess,prosthesis dysfunction or dehiscence,fistulas,or unexpected and premature structural degeneration of the valves[3] (Figure 3).
The sensitivity of TTE in PVE ranges from 17% to 36%;in comparison,it increases to 82% to 96% with TEE,suggesting the importance of TEE for better assessment of all cases of suspected PVE[39,40].Despite the enhanced temporal and spatial resolution of multiplanar TEE,its ability to identify prosthetic valve abnormalities can be challenging[41].Another commonly encountered limitation is an inability of echocardiography to differentiate between active and healed vegetations following antibiotic treatment.To overcome this limitation,serial studies are required to assess for size progression of the vegetation[41].
Three-dimensional (3D) echocardiography is a complementary modality which provides valuable information regarding the anatomy of the prosthetic valves and adjacent structures from different angles.Novel 3D-rendering software aid in the characterization of the vegetation size and location,destructive changes,perforations,abscess characterization,prosthetic dehiscence and associated regurgitant jets[42-44].Chahineet al[45] described in 242 patients,an improved sensitivity over the recent decade for the detection of PVE (70.8%vs93.7%,P=0.009) with contemporary TEE technology including an increased use of 3D imaging (Figure 4)[45].

Figure 3 Para-valvular fistula in a patient with complicated endocarditis involving prior stentless aortic valve.
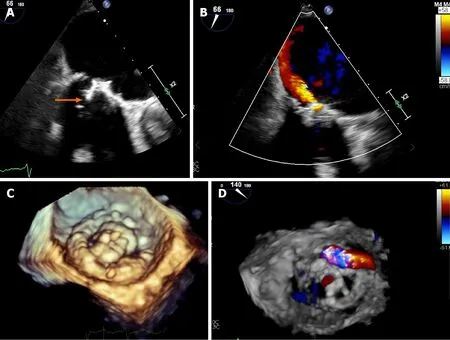
Figure 4 Transesophageal echocardiogram in a patient with mitral valve replacement endocarditis with 3D-reconstruction.
Echocardiography has been shown to predict outcomes in PVE.Wanget al[46]studied 115 patients with surgically proven IE (52% with bioprosthetic valves;15.5%with metallic valves) and recognized that abscess or pseudoaneurysm detected by TEE were independently associated with increased in-hospital mortality and morbidity[OR:3.66 (95%CI:1.76-7.59);P=0.001][46].
In cases where the clinical suspicion remains high despite an initial negative result,short-term interval follow-up is a strategy that can enhance imaging sensitivity at the expense of prolonging the time to diagnosis.This can usually be performed 3-7 days following the initial evaluation[3].In contrast to this “watch and wait approach”,adjuvant imaging modalities play a complementary role in the diagnosis of PVE,potentially expediting patient care[7].
CCT
CCT has become an increasingly important imaging tool for the diagnosis and preoperative planning of patients with PVE.CCT offers a number of technical advantages over echocardiography including higher spatial resolution and imaging window independence[47].CCT has demonstrated similar diagnostic yield for the detection of perivalvular complication[7].Feutcheret al[47] compared CCT with TEE in 37 patients with IE,6 of whom had PVE.The study showed that CCT had an excellent correlation with TEE in determining vegetation size (vegetation size by TEE 7.6±5.6 mm) (r=0.95;P<0.001).In addition,vegetation mobility was accurately diagnosed by CCT in 96% of the patients,and both modalities had similar detection rates for abscesses and pseudoaneurysms with the caveat that CCT provided more detailed anatomical location and extension[47].Fagmanet al[48] compared ECG-gated CT and TEE with surgical findings including abscess,vegetation,and dehiscence in 27 patients with aortic PVE.The agreement was good between surgical findings and ECG-gated CCT(kappa 0.66,95%CI:0.49-0.87) and TEE [0.79 (0.62-0.96)],but the combination of both TEE and ECG Gated CCT provided even better diagnostic performance [0.88(0.74-1.0)][48].In a more recent study by Koneruet al[49] in 122 patients with PVE undergoing pre-operative evaluation,the performance of high-resolution ECG synchronized 4D-CT was similar to TEE for the detection of abscess/pseudoaneurysm in prosthetic valves,independent of the type of prosthesis (70vs68 %;P=0.82) and anatomical location with a synergistic effect seen when both modalities were combined (sensitivity:CT alone,70%;TEE alone,68%;CT + TEE,86%)[49].This incremental benefit of combining both modalities for PVE assessment was also described in a metanalysis by Habetset al[40] who reported a pooled sensitivity/specificity of 36/93% for TEE,86/98% for CCT,and 100/94% for both modalities together.The authors also described improved detection of peri-annular PVE complications with CT scan,especially when occurring towards the anterior aspect of the aortic root where acoustic artifacts affect visualization with TEE[40].In a contemporary metanalysis of 872 patients with definite endocarditis,the subgroup analysis for PVE showed that TEE demonstrated a higher sensitivity when compared with CCT for the detection of vegetations (89%vs78%) at the expense of lower specificity (74%vs94%,P<0.05)[50].Although CCT showed a trend towards improved detection of periannular complications (%vs89%;P=0.06),TEE was more sensitive for the detection of leaflet perforation (79%vs48%;P<0.05)[50].
CCT has also proven to be useful in surgical planning by providing additional diagnostic information regarding the anatomy of coronary arteries and aorta,and degree of calcification[3,51].The major advantage of CCT over coronary angiography,is the ability to demonstrate coronaries and bypass graft patency non-invasively,avoiding risk of vegetation embolization during catheter manipulation[47].It may also be valuable in the urgent evaluation of hemodynamically unstable patients who are unable to undergo TEE (Figures 5 and 6)[52].
A potential weakness of CCT is its relatively low temporal resolution,resulting in decreased sensitivity for the detection of small vegetations (<4 mm) and leaflet perforation (<2 mm);however,it has a comparable diagnostic performance to TEE for detecting fistulas,paravalvular leaks and prosthetic valve dehiscence[47,50] (Figure 5).Therefore,TEE remains a superior technique for the detection of small vegetations that are<5 mm (Table 1)[53].Some inherent technical challenges include the need for use of contrast which may exclude patients with iodine allergy or advanced kidney disease;the presence of arrhythmia that impairs the quality of image acquisition and the non-negligible amount of radiation exposure[54].Similar to echocardiography,CCT has prognostic value in PVE.In a cohort of 155 patients with surgically proven IE,112(72.3%) corresponding to patients with previous valve replacement (metallic and bioprosthesis) or repair,the presence of pseudoaneurysm,abscess,and fistulas detected on CCT independently predicted mortality (HR=3.82,95%CI:1.25-11.7,P<0.001;and 9.84,95%CI:1.89-51.0,P=0.007 respectively)[46].
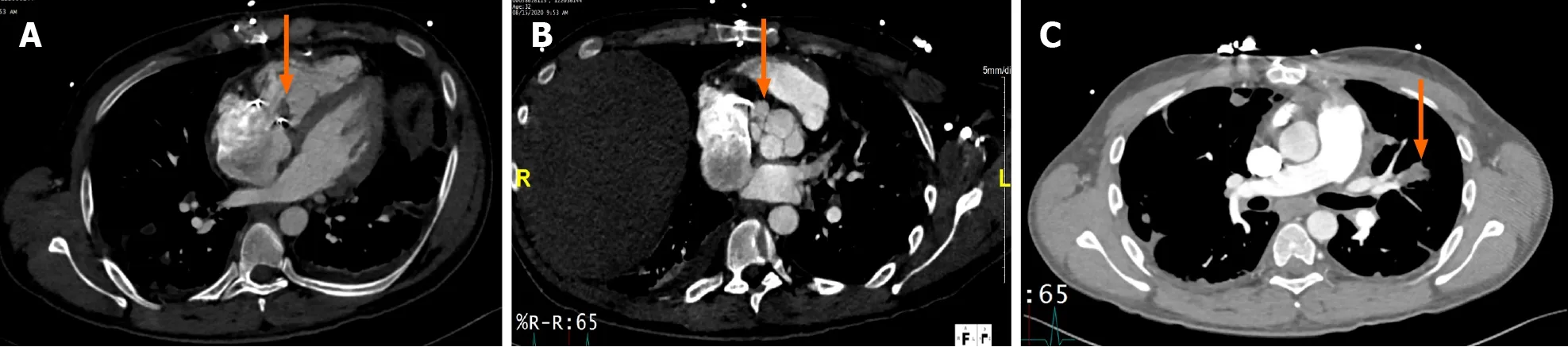
Figure 5 Cardiac computed tomography in a patient with tricuspid valve replacement endocarditis with peri-valvular extension.

Table 1 Comparison between different imaging modalities in the evaluation of prosthetic valve endocarditis
Cardiac magnetic resonance imaging
The roles of cardiac magnetic resonance imaging (MRI) in IE,and more specifically in PVE,are currently limited,and less well defined.In general,this imaging modality offers several unique advantages such as improved 3D-visualization of cardiac structures compared to TEE,the ability to identify inflammatory changes in the myocardium and pericardiumviadelayed enhancement imaging,differentiation of vegetations from intracardiac masses,ability to diagnose infiltrative cardiomyopathies,accurate quantification of regurgitant valvular lesions and the ability to be used in patients unable to receive iodine-based contrast[54,55].However,the role of MRI for evaluation of infective changes,especially in PVE is limited.Some of the factors that account for its limitations include incompatibility with some implantable cardiac devices,reduced availability and significant artifacts caused by metallic leaflets[53,54].
As part of the pre-operative work up,MRI does not provide as accurate information regarding the anatomy of the chest wall and its proximity to cardiac structures as does CCT,and thus is not usually favored for this purpose[56].Nevertheless,MRI of the brain is recommended in pre-operative patients who have neurologic deficits and may also be reasonable in high-risk left-sided IE to screen for subclinical embolic events[3,56].The ability of brain MRI to detect subclinical cerebral lesions,which may be found in up to 70% of patients who are neurologically intact clinically,has substantial clinical implications,as presence of systemic embolization represents one minor Duke criterion[5].This in turn may allow earlier diagnosis and the implementation of therapeutics[57,58].
PET/CT
Hybrid modalities such as leucocyte scintigraphy and18F-FDG PET/CT have also been recognized as important complementary diagnostic imaging modalities.18F-FDG PET/CT relies on the administration18F-FDG radioisotope,which is taken up by active inflammatory cells at the site of the infection.On the other hand,leucocyte scintigraphy isolates and labels granulocytes with99mTc that can be localized and quantified at a specific acquisition point in time[3,59].The steps in preparation of leucocyte scintigraphy involving the drawing and reinjection of leucocytes,makes the utilization of18F-FDG radioisotope more favorable in clinical practice[1].
The modified Duke criteria only considers echocardiography as the diagnostic imaging modality for IE.The AHA guidelines,despite acknowledging the usefulness of PET/CT for the detection of extracardiac complications,have not yet recommended its routine use for diagnosis[5].In contrast,in the latest iteration of the ESC guidelines for the management of IE,the presence of abnormal activity of18F-FDG PET/CT or leucocyte scintigraphy SPECT/CT (>3 months after implant) around the perivalvular region of a prosthetic valve was upgraded as a major imaging criterion for diagnosis of IE[3].As a result,Sabyet al[60] reported an improvement of the modified Duke criteria sensitivity from 70% to 97% without trading-off its specificity,with the use of PET/CT[60].Although similar findings in terms of sensitivity were found by Philipet al[61] in 115 patients with PVE (91 definite cases and 24 rejected cases) where the sensitivity increased from 57.1% to 83.5%;there was a decrease in specificity from 95.8% to 70.8%,with an overall improvement in accuracy from 65.2% to 80.9%.Wanget al[62] also reported in 333 patients with PVE an enhanced sensitivity of 86%,however,the sensitivity of the test decreased to 72% in the presence of cardiac implantable electronic devices (CIED)[62].
18F-FDG PET/CT can be utilized early in the evaluation of suspected PVE,especially if microbiologic cultures and echocardiographic imaging are unrevealing[63].18F-FDG PET/CT is a useful complimentary imaging modality for the diagnosis of IE that has demonstrated improving performance over time,especially in challenging cases of PVE and CIED related infections[62,64].It has also been suggested to have a potential role in monitoring the response to antibiotic therapy[63,65].The enhanced diagnosis of PVE with PET/CT has important clinical implications,helping to re-classify up to 90%of the “possible IE” cases by modified Duke Criteria,and providing a conclusive diagnosis (definite/rejected) in 95% of the cases[66].It has also significantly altered the treatment plan in up to 35% of the cases by virtue of antibiotic treatment prolongation(27.5%),surgical referral (15%) and prevention of unnecessary device extraction(17.7%)[67].This is attributed in part to its ability to detect extracardiac foci of infection,either septic emboli or other sources of infection,in around 17% of the cases with whole body PET/CT (Figure 7)[68].

Figure 6 Transesophageal echocardiogram and cardiac computed tomography in a patient with metallic aortic valve replacement endocarditis,complicated by aortic dissection.

Figure 7 18-fluorodeoxyglucose photon emission tomography/computed tomography in a patient with prosthetic aortic valve endocarditis complicated by septic emboli.
Cautious interpretation of18F-FDG PET/CT results must be entertained,especially in the early postsurgical period during the first 3 months.Following the implantation of a prosthetic valve,an inflammatory response to the foreign body occurs,which is reactive in nature without necessarily implying the presence of infection[69].Other causes of false positive results include:soft atherosclerotic plaques and active thrombi,cardiac tumors (whether primary or metastatic),and inflammatory conditions such as vasculitis and myocarditis[70,71].Rouzetet al[59] described in a cohort of 39 patients with prosthetic valves and absence of clinical infection that approximately half of these patients will continue to have a homogenous uptake on18F-FDG PET/CT in the perivalvular area that may persist years after surgery,and therefore should not be confused with infective changes[59].On the other hand,false negative may still occur in the presence of small vegetations (<5 mm),recent antibiotic administration,metastatic brain lesions and high glucose states[3,72].Although contemporary data reported equipment availability in 70.3% of European centers and 56.3% non-ESC centers,the availability,cost,and expertise needed with this imaging modality impose additional limitations on its employment in routine clinical practice[4,73].
Real world data from the ESC-EORP EURO-ENDO (European infective endocarditis) registry in 3116 adults with IE from around the globe (2470 from Europe,646 from non-ESC countries),identified 939 (30.1%) cases of PVE and 308 (9.9%) with device related infection[4].18F-FDG PET/CT was implemented in 518 cases (16.6%)and leucocyte scintigraphy in 38 (1.2%)[4].Around 25% of the18F-FDG PET/CT were obtained in patients with PVE,and 26% in patients with device infections,which were significantly higher when compared with NVE (9.5%) (P<0.0001) [4].The test performance was superior in patients with PVE with a reported sensitivity of 66.8% (vs28% for NVE and 16.3% for device infections) [4].Extracardiac foci were observed in close to 40% of patients (34.5% in PVE,42.3% in NVE,and 43.8% in device infections),most frequently seen in the lungs (27.1%)[4].
MANAGEMENT
In this section,a brief overview of the general management principles will be discussed;however,a detailed discussion of antimicrobial therapies and surgical techniques is beyond the scope of this article.Treatment of PVE consists of broadly surgical and/or medical management.Randomized controlled trial data comparing combined treatment to medical treatment alone are lacking.However,several large cohorts examined outcomes in surgical and medical therapy group.In a large meta-analysis by Mihoset al[74] of 32 studies including 2636 patients with PVE,surgical management was associated with lower 30-day mortality and higher survival at 22 months and similar rate of recurrence compared to medical therapy alone (25%vs34% and 69%vs58%,respectively)[74].The limitation of this study is lack of adjustment analysis for risk factors and time from medical therapy to surgery[74].In another large prospective study of 1025 patients with PVE,early surgery was associated with lower 1- year and in-hospital mortality compared with medical therapy alone (HR=0.57,95%CI:0.49-0.67 and HR=0.44,95%CI:0.38-0.52,respectively)[8].However,this benefit with early surgery was absent after adjustment for survivor bias and clinical factors[8].In several observational studies,the benefit of surgery was mostly seen in patients with PVE complications that carry high mortality rate such as valve regurgitation or dehiscence,paravalvular abscess or fistula,heart failure,and coagulase negative or Staphylococcus Aureus PVE[75-77].In this context,a 2015 scientific statement from the AHA,2015 ESC and 2016 American Association for Thoracic Surgery guidelines recommended as class I indication surgical intervention after weighing risks and benefits based on operative risk profile and overall outcome in the following scenarios:PVE with complications,such as new or worsening CHF,prosthetic valve dehiscence,hemodynamically significant valvular or paravalvular regurgitation,obstruction or intracardiac abscess[3,5,56].It is reasonable to proceed with surgery as class II indication in cases of persistent bacteremia,infection-relapse despite appropriate antibiotics treatment,aggressive infection by Staphylococcus Aureus or fungi,non-HACEK gram negative organisms,multi-resistant organisms or in the presence of fastidious organism clustered as “culture negative” PVE[3,5,56,78].PVE vegetations are at risk for embolization,especially when the size exceeds 10 mm,or there is increasing in size despite antibiotics[3,34,56].In these situations,surgical intervention should be considered[3,34,56].
Antibiotic therapy should be initiated in all cases of PVE with consultation of an infectious disease specialist for guiding the antibiotic choice[3,5,38,56].Three sets of blood cultures separated by 30-60 minutes should be obtained before antibiotic initiation and at least every 24-48 h until the blood culture is negative[3,5,38,56].All patients should be monitored for side effects of antibiotics,clinical response and symptoms/signs that suggest PVE complications.In the latter case,an echocardiogram should be repeated.The treatment duration is generally 6 week starting from the first negative blood culture,and it can be extended for an additional 6 week if surgical specimens demonstrate a positive gram stain and culture or positive polymerase chain reaction.Antibiotic therapy should subsequently be tailored according to the culture results[3,5,38,56].
CONCLUSION
The diagnosis of PVE remains challenging due to its often non-specific clinical presentations and prosthesis-related artifacts that impair the optimal visualization of cardiac structures by echocardiography.Echocardiography continues to be the first-line imaging modality in suspected cases of PVE due to is wide availability,low cost,rapid interpretation,and safety.TEE is mandated in most PVE cases due to the reduced sensitivity of TTE in this context.The consequences of missing prosthesis-related infections are serious,and therefore,evaluation of PVE requires the optimal complementary use of imaging modalities to achieve the best outcomes.Adjuvant imaging modalities,particularly CCT and18F-FDG PET/CT have important niche roles.These imaging modalities improve the ability to accurately and timely diagnose PVE,contribute to the pre-operative planning of appropriate patients,and guide decisionmaking for therapies.
 World Journal of Cardiology2021年8期
World Journal of Cardiology2021年8期
- World Journal of Cardiology的其它文章
- Associations of new-onset atrial fibrillation and severe visual impairment in type 2 diabetes:A multicenter nationwide study
- Role of coronary angiogram before transcatheter aortic valve implantation
- Nutritional supplement drink reduces inflammation and postoperative depression in patients after off-pump coronary artery bypass surgery
- Association of marital status with takotsubo syndrome (broken heart syndrome) among adults in the United States
- Angiotensin receptor blocker neprilysin inhibitors
- Surgical strategies for severely atherosclerotic (porcelain) aorta during coronary artery bypass grafting
