Improvement of human embryonic stem cell-derived retinal pigment epithelium cell adhesion, maturation, and function through coating with truncated recombinant human vitronectin
Xin-Yue Zhu, Yu-Hong Chen, Ting Zhang, Su-Jun Liu, Xin-Yue Bai,Xian-Yu Huang, Mei Jiang, Xiao-Dong Sun
1Department of Ophthalmology, Shanghai General Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai 200080, China
2National Clinical Research Center for Eye Diseases, Shanghai 200080, China
3Shanghai Key Laboratory of Fundus Diseases, Shanghai 200080, China
4Shanghai Engineering Center for Visual Science and Photomedicine, Shanghai 200080, China
Abstract
INTRODUCTION
H9 human embryonic stem cell-derived retinal pigment epithelial (hES-RPE) cells, a potential cell source for cell transplantation therapy of retinal degenerative diseases,are especially useful in atrophic ‘dry’ age-related macular degeneration (AMD) and retinitis pigmentosa (RP). These retinal degenerative diseases are characterized by RPE damage,degeneration, loss and progressive cellular dysfunction of the neuroretina[1-2]. A challenge in the clinical transformation of cell transplantation is to generate optimized hES-RPE cells on a large-scale to meet clinical use requirements and ensuring their quality, quantity, consistency, and safetyin vitro[3].hES-RPE cell culturein vitrorequires coating substrates to facilitate cell adhesion, growth, and mature, as they can affect cell integration and survival after cell transplantation[4].Previously devised systems have used complex and undefined substrates, such as feeder fibroblast layers or Matrigel.Matrigel is currently the conventional coating substrate for hES-RPE cell culture. However, Matrigel is extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma, a tumor rich in extracellular matrx (ECM) proteins, exhibits lotto-lot variability and can introduce unwanted xenogeneic contaminants. Thus, the use of Matrigel is not safe for clinical transplantation[5]. Therefore, there is a need to explore human recombinant cell culture matrix proteins as the coating substrates. Currently, the lack of xeno‐free, defined coating substrates and standardized culture system has hampered the clinically application of functional and safe hES-RPE cells[6].
To explore defined, purified ECM proteins as coatings,researchers have tried to use single ECM protein components as coating substrates. The effects of defined ECM proteins as coating substrates for human embryonic stem cell (hESC)differentiation or hES-RPE cell maintenance remain elusive,but important for clinical translation. It has been reported that multiple purified ECM proteins, such as synthemax II-SC[7],collagens (I and IV)[8], fibronectin[8], and vitronectin[9]in fully‐defined, feeder‐free culture systems support the growth of induced pluripotent stem cells (iPSCs), the differentiation of pigmented regions from iPSCs and the maintenance of iPSC‐RPEs. Laminin521 (LN-521) is ubiquitous in human basement menbranes and can support stem cells without feeder and rock inhibitor[10]. To date, LN-521 is another promising ECM proteins for future clinical-grade coating substrates[11-13]. A recent study had shown successfully RPE differentiation with preclinical efficiency using LN-521 matrix[13]. LN-521-based RPE culture and differentiation methods are likely to find extensive use in generating therapeutic stem cell-derived RPE for regenerative medicine. However, evaluation of the effects of LN-521 on stem cell-derived RPE adhesion, maturation,and function after differentiation has not been performed yet. Vitronectin has been used to culture stem cell derived-RPE cells in many clinical trials so far[1-2]. To the best of our knowledge, which coating substrates of those two is more conducive to hES-RPE cell culture has not been described.Hence, in the present study, we aimed to investigate the effect of LN-521 on hES-RPE adhesion, maturation, and function after differentiation, and compare it to vitronectin.
In this study, we selected commercially available human recombinant laminin cell culture matrices, LN-521 (one isoform of laminin) and truncated recombinant human vitronectin(VTN-N)[14], to identify their adhesion ability for hES-RPE cells, and their effects on the morphology, maturation and function of the cellsin vitro.First, we verified the optimal coating concentrations of LN-521 and VTN-N for hES-RPE cell adhesion. Αs the first step in the interaction between cells and a material, cell adhesion seems to play a key role in cellular characteristics and determines the growth of the cells[15-17]. This will be very helpful for establishing a standardized large-scale hES-RPE cell culture protocol. Furthermore, we evaluated the morphology, maturity, and phagocytic function of hES-RPE cells on both coating substrates. Our results demonstrated that hES-RPE on VTN-N matured more rapidly and showed better phagocytic ability. Taken together, our findings may serve as the basis for the future transplantation of healthy hES-RPE cells into the subretinal space for successful RPE functional repair[8].
MATERIALS AND METHODS
LN-521/VTN-N CoatingFirst, we calculated the stock solution concentrations and amounts of LN-521 (Biolamina,LN521) and VTN-N (Gibco, A14700) needed for the experiments and slowly thawed the coatings at 4℃ before use.The LN-521 and VTN-N stock solutions were diluted with 1×dulbecco’s phosphate-buffered saline (DPBS, with Ca2+and Mg2+) and 1×DPBS (without Ca2+and Mg2+) to different concentration (0, 0.25, 0.5, 1, 2, 4, 8 µg/cm2). For the doseresponse adhesion assay, 96-well black glass bottom plates were used. For other experiments, we used 24-well plates and Millicell EZ Slide 4-well glass (Millipore, PEZGS0416).The dilute solutions were added to each culture vessel at the recommended volumes. The coated plates were incubated at 4℃ overnight. Prior to use, the culture vessels were prewarmed to room temperature for at least 1h. LN-521 coated vessels needed to be rinsed with 1× DPBS (with Ca2+and Mg2+) before use.
hES-RPE Cell Culture and SeedingH9 hESCs were spontaneously differentiated into RPE (hES-RPE) cells as described previously[18]. hES-RPE cells were cultured with X-VIVO 10 medium for at least 30-35d at passage 1 and frozen at passage 2 on day 5[19]. Cultures were free of mycoplasma as evaluated by polymerase chain reaction(PCR; data not shown). The hES-RPE cells were plated at a density of 1.0×105cells/cm2in culture medium. The cells were cultured at least 2-3wk in X-VIVO 10 media except for phagocytosis analyses, for which the cells were cultured in MEM-Nic medium[20][MEM alpha with GlutaMAX, 1% fetal bovine serum (FBS), 1% penicillin/streptomycin, 0.1 mmol/L non-essential amino acids (NEAA), 1% N1 supplement,0.25 mg/mL taurine, 20 ng/mL hydrocortisone, 0.013 ng/mL triiodo-thyronin, and 10 mmol/L nicotinamide]. The cells were maintained at 37℃, 5% CO2, and the medium was replaced with fresh medium three times per week.

Table 1 The sequence of primers used in the real-time PCR
Cell Adhesion AssayDose-response adhesion assays were performed with a previously used protocol with some modifications[9,21]. Briefly, hES-RPE cells were seeded into each well of 96-well plates coated with LN-521 and VTN-N at 1.0×105cells/cm2. After 6h of incubation, the nonadherent cells were removed by rinsing 3 times with 1× DPBS (with Ca2+and Mg2+), and the adherent cells were fixed with 4%paraformaldehyde (PFA) for 15min at room temperature. Cells were then stained with DAPI for 10min at room temperature.The cells were quantified and analyzed with a high content imaging system (PE, Operetta).
Quantitative Real-time Polymerase Chain ReactionCells were collected and total RNA was isolated using the RNAsimple Total RNA Kit (Tiangen, DP419). cDNA was synthesized from 500 ng of total RNA using PrimeScript RT Master Mix (Takara, Japan, RR0036A). TB Green Fast qPCR Mix (Takara) and the ViiA7 real-time PCR system (Thermo Fisher) were used to perform quantitative real-time polymerase chain reaction (qRT-PCR). The primer sequences used for qRT-PCR are shown in Table 1. Triplicate reactions were run in a 96-well plate for each sample. Cycle threshold (Ct)values were obtained from the thermal cycler, and 2‐ΔΔCtwas calculated to determine the expression level of target genes.
Immunofluorescent StainingThe hES-RPE cells were cultured for 20d after being plated on Millicell EZ Slide 4-well glass coated with Matrigel, LN-521 or VTN-N. The cells were fixed with 4% PFA for 15min at room temperature and then permeabilized with 50% ethanol for 5min at room temperature.Αfter washing 3 times with blocking buffer (5% goat serum,0.5% BSA and 0.05% saponin in PBS), the cells were incubated with primary antibody in blocking buffer overnight at 4℃. Primary antibodies against the following were used for immunostaining: ZO1 (Invitrogen, 33-9100; 1:200), BEST1(Abcam, ab2182; 1:1000), phalloidin-iFluor488 (Abcam,ab176753; 1:1000), and phalloidin-iFluor647 (Abcam,ab176759; 1:1000). Then, the cells were washed 3 times with blocking buffer and incubated with Alexa Fluor-conjugated secondary antibodies for 1h at room temperature. Nuclei were stained with DAPI. Images were taken using a confocal microscope (Leica, DMi8).
Isolation of Photoreceptor Outer SegmentsPhotoreceptor outer segments (POS) were isolated from fresh porcine eyes according to established protocols with minor modifications[22].Briefly, set up continuous sucrose gradients (27%-50%) in ultra-clear centrifuge tubes before the procedure and keep on ice until ready to use. Each gradient is 22 mL in volume and can hold a maximum of 8 retinas. Then, detached the retinas and homogenized with glass homogenizer, spin at 1000 RPM for 3min. Collect the supernatants and add a maximum of 4 mL to each gradient. Spin at 24000 RPM in a SW40-Ti rotor for 1h and 10min at 4℃. Αt the end of the run, there should be 3 bands on the gradient. The middle band is orange and contains most of the POS. Quickly mark and draw up everything in the middle orange band only and transfer to a new tube. Dilute the mixture with isolation medium and spin at 9500 RPM for 10min at 4℃. There should be an orange pellet off to the side of the tubes. Decant the supernatant and suspend the POS in DEME medium with 2.5% sucrose to determine the yield uner the hemocytometer or store at ‐80℃ for future use.
Phagocytosis Assay and Flow Cytometry AnalysisA phagocytosis assay was performed as previously described with some modifications[18]. Briefly, hES-RPE cells were seeded on Matrigel, LN-521 and VTN-N coated plates at a density of 1.0×105cells/cm2and cultured in MEM-Nic medium for at least 20d until the RPE cells reached confluence.Prelabeled (AlexaFluor 555/647 NHS Ester, Invitrogen,A20109/A20006) POS were added to the hES-RPE cells(10 POS/per cell) and incubated for 4h at 37℃ in 5% CO2.The cells were vigorously washed 5 times with 1× DPBS(with Ca2+and Mg2+) to remove unbound POS. Images were captured by fluorescence microscopy. The phagocytic ability of hES‐RPE cells coated with different substrates was also be identified by flow cytometry. Αfter incubation with prelabeled(ΑlexaFluor647) POS for 4h at 37℃ in 5% CO2and complete washing, the cells were harvested with TrypLE Select Enzyme(1×, Thermo Fisher) and resuspended in PBS to produce a single‐cell suspension. Α FΑCS scan flow cytometry machine(Beckman Coulter, CytoFLEX) was used to measure the ΑlexaFluor647 fluorescence‐positive cells.
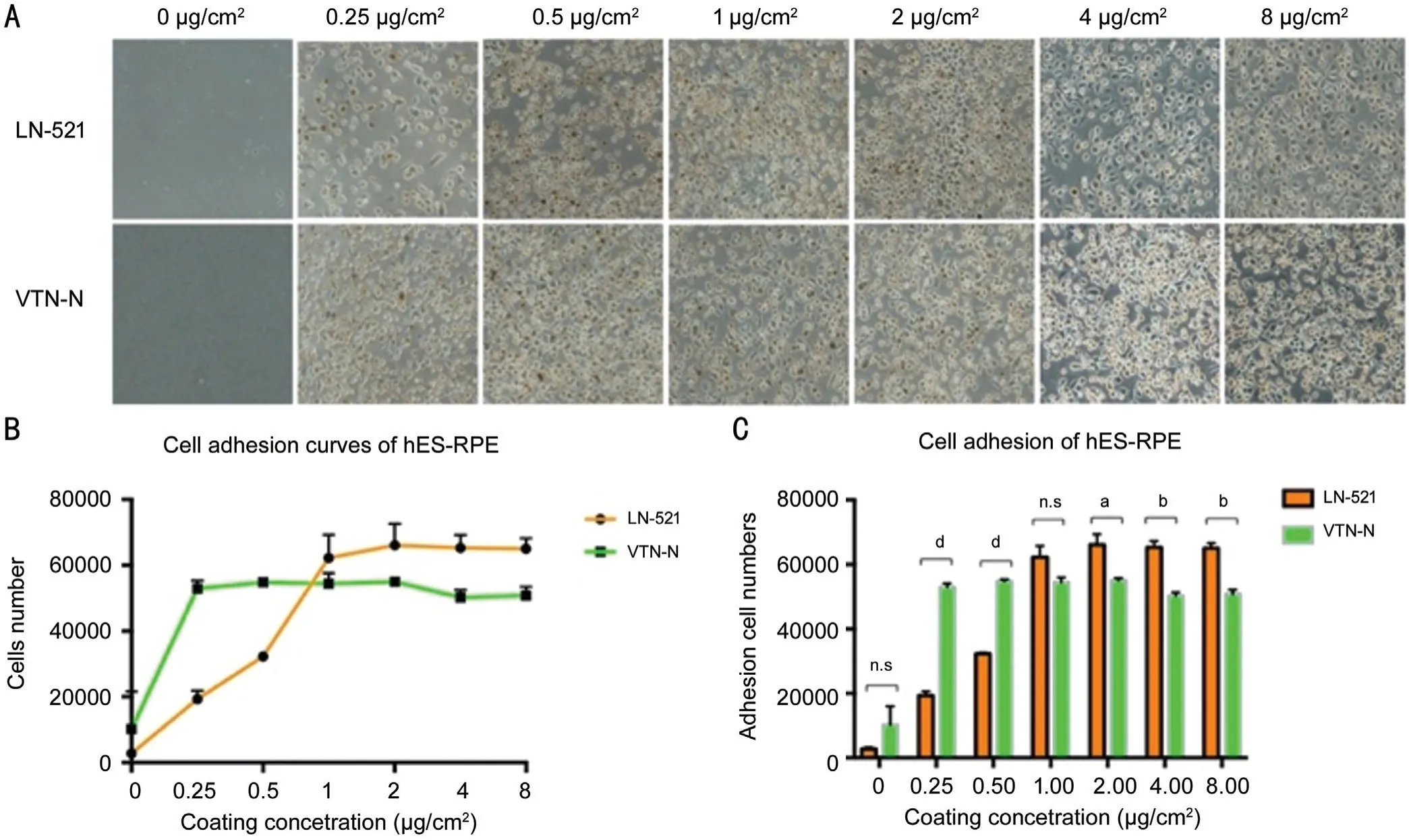
Figure 1 hES-RPE cell adhesion on LN-521 and VTN-N Α: Brightfield images of hES‐RPE cells adhered to LN‐521 and VTN‐N coated wells after 6h of incubation with different concentrations (0, 0.25, 0.5, 1, 2, 4, and 8 µg/cm2). B: Cells were quantified and analyzed with a high content imaging system. The number of adhesion cells on LN-521 increased until reaching the optimal concentration 2 µg/cm2, while cell adhesion on VTN‐N was not significantly different from the concentration of 0.25 µg/cm2. C: Comparison of the number of adherent cells with the two coating substrates at each concentration tested. Unpaired t-tests were performed. aP<0.05, bP<0.01, dP<0.0001.
Statistical AnalysisAll results presented were obtained from at least three times independent experiments. GraphPad Prism 7 was used for mapping and statistical analyses. The results are presented as the average value±SD. Differences were considered statistically significant if thePvalue<0.05.
RESULTS
Comparison of Adhesion of hES-RPE Cells on LN-521 and VTN-NIn investigating the adhesion of LN-521 and VTN-N to hES-RPE cells, we found that LN-521 and VTN-N can both enhance cell adhesion. After 6h of incubation, very few hES-RPE cells adhered to wells without a coating. With increasing coating concentration, increasing numbers of cells attached to the surface of the coated wells (Figure 1A). As shown in Figure 1B, the optimum concentration of LN-521 for hES-RPE cell adhesion was 2 µg/cm2. When the concentration exceeded 2 µg/cm2, there was no significant increase in cell attachment. However, VTN-N at a concentration of only 0.25 µg/cm2already showed good hES-RPE cell adhesion,and with increasing concentration, the number of attached cells did not obviously increase. In addition, when the coating concentration was less than 1 µg/cm2, the adhesion of VTN-N to the cells was significantly better than that of LN-521(P<0.05), however, when the coating concentration was higher than 1 µg/cm2, the total number of hES-RPE cells adhered to the LN-521 was superior to that of cells adhered to VTN-N(P<0.05; Figure 1C). The optimal concentrations of LN-521 and VTN-N for hES-RPE cell adherence were 2 and 0.25 µg/cm2,respectively, which is inconsistent with the manufacturers’recommendations (0.5 µg/cm2for LN-521; 0.9 µg/cm2for VTN-N). This result will be very valuable for the culture of hES-RPE cells under the best conditions and at the lowest cost.

Figure 2 Morphology and protein localization in hES-RPE cells on Matrigel, LN-521, and VTN-N A: Brightfield image of cultured passage 3 hES-RPE cells under a 20× objective on day 20; B: Matrigel; C: LN-521; D: VTN-N. hES-RPE cells express properly localized apical phalloidin (purple), basal BEST1 (green) and ZO‐1 (red) at tight junctions. Scale bars: 25 μm.
Impact of LN-521 and VTN-N on Cell Morphology and Polarity of hES-RPETo evaluate hES-RPE cell maturity,we compared the cell morphology and polarity of hESRPE cells on LN-521, VTN-N, and Matrigel. Three kinds of substrates all induced a cobblestone-like morphology in the hES-RPE cells, which showed evidence of tight junctions at approximately 2-3wk after seeding (Figure 2A). RPE cell function is closely related to cell polarity. Cell polarity at day 20 was assessed by immunostaining for F-actin, an apical marker[23-24], basal marker BEST1[25], and tight junction marker,ZO-1. hES-RPE cells grown on the three kinds of coating substrates had predominantly apical expression of F-actin(purple, phalloidin) and basal expression of BEST1 (green),indicating polarization (Figure 2B-2D). Also those cells all showed strong ZO-1 staining, which was highly visible at the margins of the hexagonally shaped RPE cells (Figure 2B-2D,red). These results revealed that the coating substrates LN-521 and VTN-N were suitable to support hES-RPE cell polarity and maturity, at least at the optimum coating concentration and with sufficient cultivation time.
Effect of LN-521 and VTN-N on RPE Maturation After PassageAn examination of gene expression in hES-RPE cells was performed by qPCR analysis. We evaluated and compared gene expression levels with the two coating substrates relative to those with Matrigel midway (day 10) and at the end (day 20)of the experimental time course. Expression levels of the RPEspecific markers, retinal pigment epithelium specific protein 65 (RPE65)[26], bestrophin1 (BEST1, a calcium-activated chloride channel)[25], melanin-producing tyrosinase (TYR)[27],microphthalmia-associated transcription factor (MITF)[28]and premelanosome protein (PMEL)[29]were evaluated. As shown in Figure 3, all of these RPE‐specific markers were expressed at higher levels in the VTN-N group than in the LN-521 group on day 10. However, the expression of these markers did not significantly differ between the two substrates groups on day 20. This result suggests that hES-RPE cells cultured on VTN-N substrate matured and showed RPE characteristics faster than those cultured on LN-521.
Abilities of LN-521 and VTN-N to Affect RPE PhagocytosisThe phagocytic abilities of hES-RPE cells cultured on the coating substrates Matrigel, LN-521, and VTN-N were compared to further verify the cell function. After incubation with prelabeled POS for 4h at 37℃ in 5% CO2, total POS(red) bound and ingested by hES-RPE cells cultured on different coating substrates was detected, as shown in Figure 4A. Phalloidin-iFluor 488 (green) was used to show the apical zone of the cells. Flow cytometry analysis also showed that 93.4%, 96.5%, 98.2% of hES-RPE cells on Matrigel, LN-521,and VTN-N respectively were POS-positive (Figure 4B). At this stage, more than 78.15% of the total POS were bound POS (data not shown). Based on that, the difference in the total amount of phagocytosed POS were from bound POS not ingested POS. Therefore, this result indicated that hES-RPE cells on the VTN-N presented the highest phagocytic activity.In addition, with both LN-521 and VTN-N, the fluorescence intensity of combined POS was greater than that with Matrigel.This result suggests that a defined coating substrate is more effective than undefined complex substrates.
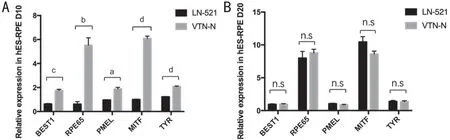
Figure 3 Gene expression in hES-RPE cells on days 10 and 20 qPCR analysis was carried out to compare gene expression levels in hESRPE cells cultured on LN-521 or VTN-N relative to those cultured on Matrigel as a control at day 10 (A) and day 20 (B). Unpaired t-tests were performed. aP<0.05, bP<0.01, cP<0.001, dP<0.0001.
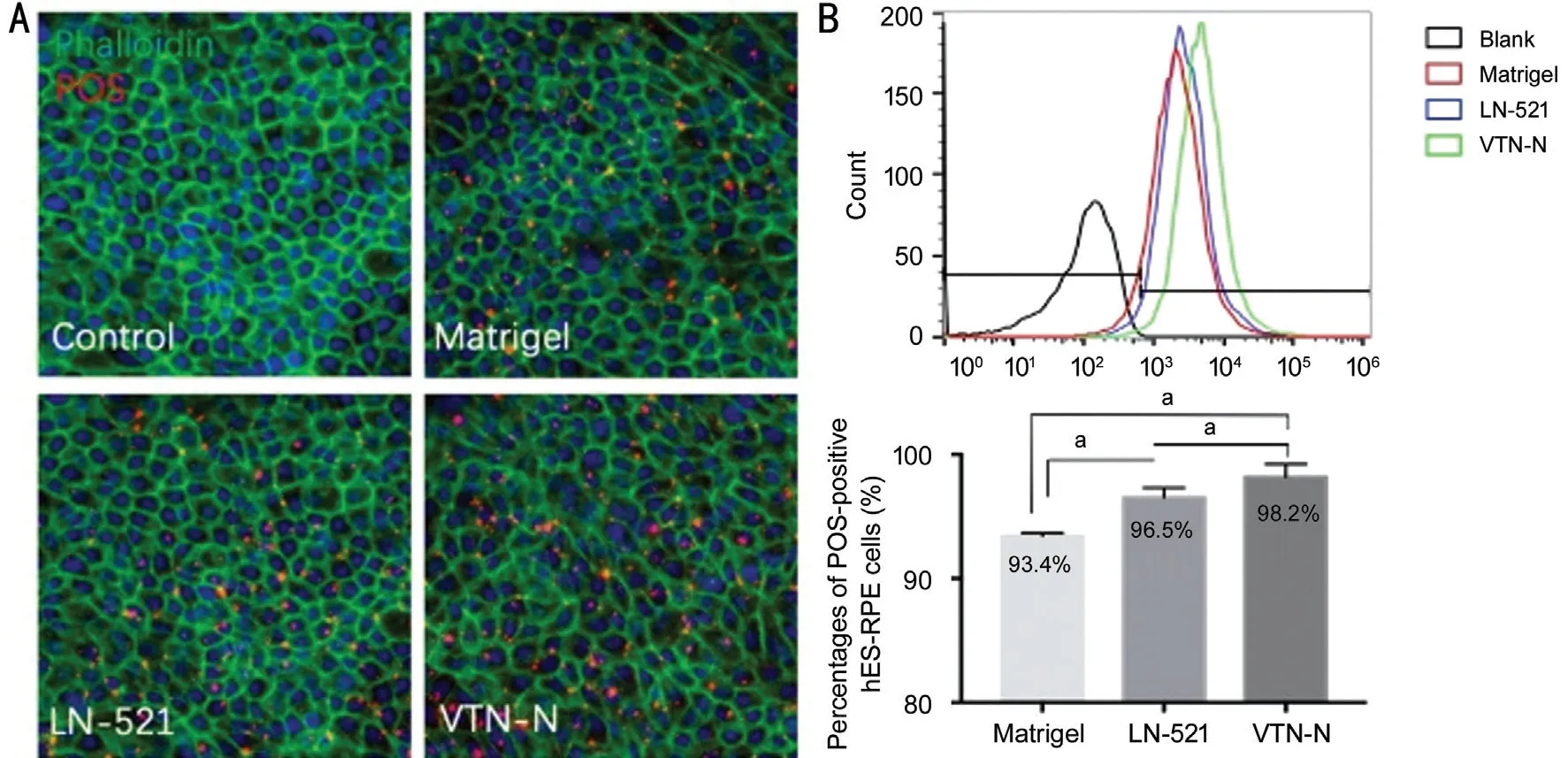
Figure 4 VTN-N coated hES-RPE cells bound more POS than Matrigel or LN-521 coated hES-RPE cells A: Prelabeled (AlexaFluor555)POS (red) were bound to hES‐RPE cells. Immunofluorescent staining with phalloidin‐iFluor 488 showed a cobblestone‐like cellular morphology.B: Flow cytometry analysis showed that the percentages of POS-positive hES-RPE cells on Matrigel, LN-521, and VTN-N were 93.4%, 96.5%,98.2% respectively. Paired t-tests were performed (aP<0.05).
DISCUSSION
Stem cell-based therapy is a promising therapeutic treatment for late-stage AMD, which is one of the leading causes of blindness. Multiple phase I/II clinical trials for stem cellbased therapies primarily designed to examine the safety of stem cell-derived RPE transplantation in late-stage AMD have begun[30-32]. However, the quality of the transplanted hES-RPE cells is very important for long-term survival and function after transplantation. While Matrigel has been a very common ECM for stem cell‐based RPE differentiation and culture, it is critical to establish an alternative ECM substrate, which is animal-free and non-xenogeneic for cell therapy development and clinical trials. Two of the most popular defined matrices routinely employed in hES-RPE culture are laminin and vitronectin[33-34].In this study, we provide the first comprehensive analysis of those two substrates (LN-521 and VTN-N) on cell adhesion,morphology, polarity and phagocytic function of hES-RPE cells. The results showed that the adhesion of LN-521 to the cells was dose-dependent, however, VTN-N presented a good adhesive ability even at low concentrations. This indicates that the ability of VTN-N to adhere to hES-RPE cells is relatively stable and that VTN-N is less affected by changes in concentration or batch-to-batch variation than LN-521. It is very important for industrial Good Manufacturing Practice(GMP) production to maintain good stability and quality. The low concentration of the coating VTN-N needed and its high stability for cell adhesion will be the significant advantages for the large-scale industrial production of hES-RPE cells for future clinical applications. Although the number of adherent cells in the LN-521 group was slightly greater than that in the VTN-N group when the coating concentration was above 2 µg/cm2, this difference was not obvious, and on the day after seeding, there was no difference in the number of adherent cells between the two groups.
Cells can respond to the culture surface to which they adhere[16]. Therefore, their morphology, polarity and function may differ with different coating substrates. RPE-specific markers in hES-RPE cells on three kinds of coating substrates were probed midway (day 10) and the end of the experimental time course (day 20). We found that hES-RPE cells on VTN-N showed polarity and maturation more rapidly than those on other coating substrates. However, at the end of the culture period, the cells were all sufficiently mature, and no significant difference in RPE-specific gene expression levels or cell morphology were observed. Additionally, the speed with which mature hES-RPE cells can be acquired is another advantage of VTN-N.
Phagocytosis is one of the most important functions of RPE cells. After incubation with POS 4h, the percentages of POS-positive cells on each coating substrate were 93.4%(Matrigel), 96.5% (LN-521), and 98.2% (VTN-N), meaning that VTN-N coated hES-RPE cells had a greater phagocytic ability than LN-521 or Matrigel. VTN-N can recognize Arg-Gly‐Αsp (RGD) motif and bind to αvβ5, which is one of the key proteins involved in RPE phagocytosis[35]. The vitronectinbinding αvβ5 can localize to focal adhesion and activate focal adhesion kinase (FAK)[36-38]. This may explain why VTN-N facilitated phagocytosis.
The ultimate goal of hES-RPE cell culture is to obtain high quality hES-RPE cells on a large-scale for clinical applications in treating retinal degenerative diseases. VTN-N had a stable effect on cell adhesion and worked effectively at a low concentration. Thus, it is more cost‐effective than LN‐521 for large-scale clinical applications.
In conclusion, to obtain optimum quality of hES-RPE cells for clinical transplantation, our results suggested that VTN-N is a superior culture substrate for xeno-free culture and maintenance of hES-RPE as its lower required concentration and ability to promote faster maturation and better phagocytic function. This research may help to establish a standardized culture method with xeno-free and defined coating substrate that supports the clinical use of stem-cell derived RPE cells.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81730026; No.81970816); the National Key R&D Program (No.2017YFA0105301); Science and Technology Commission of Shanghai Municipality(No.20Z11900400; No.19495800700).
Conflicts of Interest: Zhu XY,None;Chen YH,None;Zhang T,None;Liu SJ,None;Bai XY,None;Huang XY,None;Jiang M,None;Sun XD,None.
 International Journal of Ophthalmology2021年8期
International Journal of Ophthalmology2021年8期
- International Journal of Ophthalmology的其它文章
- Macular density alterations in myopic choroidal neovascularization and the effect of anti-VEGF on it
- ldentification and validation of tumor microenvironmentrelated lncRNA prognostic signature for uveal melanoma
- Factors associated with axial length elongation in high myopia in adults
- Visualizing the intellectual structure and recent research trends of diabetic retinopathy
- Therapeutic effect of Keap1-Nrf2-ARE pathway-related drugs on age-related eye diseases through anti-oxidative stress
- Newly-found functions of metformin for the prevention and treatment of age-related macular degeneration
