Ethanolic extract of Puhuang (Pollen Typhae) modulates lipopolysaccharide-induced inflammatory response through inducible nitric oxide synthase/cyclooxygenase-2 signaling in RAW 264.7 macrophages
CHEN Tzufan,CHEN Chuntzu,HUANG Yuanli,BASKARAN Rathinasamy,TSAI Jeffrey J.P.,HU Rouhmei
CHEN Tzufan,CHEN Chuntzu,HUANG Yuanli,HU Rouhmei,Department of Biotechnology,Asia University,Taichung 41354,China Taiwan
BASKARAN Rathinasamy,TSAI Jeffrey J.P.,HU Rouhmei,Department of Bioinformatics and Medical Engineering,Asia University,Taichung 41354,China Taiwan
Abstract OBJECTIVE:To evaluate the immune modulatory response of Puhuang (Pollen Typhae),ethanolic extract of dried pollens (TP-E) and charcoal activated pollens (CTP-E) were used for their phytochemical evaluation and their modulatory response against lipopolysaccharide (LPS) induced inflammatory activity on RAW264.7 macrophage cells.METHODS:Biochemical assays were carried out to quantify the 1,1-Diphenyl-2-picrylhydrazyl Radical Scavenging Activity,Reducing Power,Ferrous ion chelating ability and total polyphenol content and flavonoids.Non-toxic dose of the extract (TP-E and CTP-E) was chosen based on 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.Effect of TP-E and CTP-E on lipopolysaccharides-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression was measured by Western blot and quantitative PCR(qRT-PCR).Expression of inflammatory cytokines,such as interleukins (IL-1β and IL-6) and tumor necrosis factor α (TNF-α),was quantified using qRT-PCR.Mitogen-activated protein kinase pathway was analyzed using Western blot.RESULTS:Phytochemical analysis revealed that both TP-E and CTP-E have strong antioxidant activities and high flavonoid and phenolic contents.TP-E and CTP-E effectively inhibit the expression of iNOS and COX-2,thereby inhibiting its downstream proinflammatory regulators,the extracellular signal-related kinase-1/2,that decreases the expression of IL-1β,IL-6 and TNF-α.CONCLUSION:Phytochemical constituents present in Typha angustifolia Linn could be used for treating inflammation-related diseases.
Keywords:Typha angustifolia;lipopolysaccharides;inflammation;NF-kappa B;mitogen-activated protein kinases
INTRODUCTION
Typha angustifolia Linn (hereafter abbreviated as Typha) belonging to the family Typhaceae is an aquatic plant grown in tropical brackish locations.It is commonly called as "narrow leaf cat-tail" in English and"Puhuang (Pollen Typhae)" in Chinese.Its rhizome shoots,roots,stems,leaves and pollens are edible and possess medicinal values.1In traditional medicine,scrofula,abscesses and abdominal pain can be treated using Typha pollens.Rhizome and root hair powder of Typha can be used to treat kidney stones,sores,inflammation and diarrhea.2The rhizome powder was used as nutritional supplement to inhibit intestinal inflammation and oxidative stress induced by trinitrobenzenesulphonic acid.2Typha pollens possess various constituents,such as flavonoids,3stigmasterols,4fatty acids,5nucleosides6and cerebrosides.7Pharmacological effects of the Typha pollens are mainly due to the strong antioxidant capacity of flavonoids.8The pollens were used as an analgesic agent,9to prevent uterus contraction10and to protect adrenaline-induced endothelial cells damage.11
In Chinese Pharmacopoeia,the dried pollen was listed for treating hematuria,blood stagnation,secondary-dysmenorrhea and metrorrhagia.12Recent studies reported different medicinal uses of Typha pollens,like anti-obesity,anti-inflammatory,antiplatelet aggregation and anti-atherosclerosis.The active components are flavonoid glycosides,sterols,terpenoids and cerebrosides classes.7,8,13-15Other pharmacological benefits of the pollens are preventing cardiovascular diseases,such as myocardial infarction,hyperlipidemia and coronary heart diseases,by increasing microcirculation and cAMP levels.16,17
Tissue injury during any pathological condition could leads to local inflammation response which scavenge pro-inflammatory signaling molecules.A prolonged inflammation (chronic) response is deleterious and may cause damage to vital organs that results in cardiovascular diseases,18neurodegenerative disorders,19infarction,20and lung injury.21-24
Macrophages,are the predominant immune cells involving in the inflammatory response.25Lipopolysaccharide(LPS)can stimulate the immune responsein vitroandin vivo.26Exposure to LPS triggers macrophages to express inducible nitric oxide synthase (iNOS) and cyclooxygenase-2(COX-2),and results in the release of nitrite oxide (NO) and prostaglandin E2 (PGE2),which are strong inflammatory mediators.Activation of iNOS and COX-2 can also stimulate the expression and release of other pro-inflammatory cytokines,such as tumor necrosis factor-α (TNF-α),interleukin-1β(IL-1β),and interleukin-6 (IL-6),27,28and finally results in inflammation responses.
Nuclear factor-κB(NF-κB)is a key transcriptional regulator which plays a central role in the inflammatory signaling.NF-κB can be activated by any external stress or reactive oxygen species (ROS) which induce the expression of its downstream regulator iNOS,COX-2 and pro-inflammatory cytokines.27Mitogen activated protein kinases (MAPKs) are downstream regulators of NF-κB and are activated during inflammation.Extracellular signal-related kinase (ERK-1/2),p38,and c-Jun NH2-terminal kinase (JNK1/2) are some of the MAPKs that can be phosphorylated and activated during inflammation.29Inhibiting the phosphorylation of MAPKs can inhibit enormous inflammatory cytokines expression,which could help in preventing and treating inflammation related disease.30
In the present study,we quantified the antioxidant activities of ethanol extracts of the dried pollens (TP-E)and charcoal activated pollens (CTP-E) of Typha angustifolia Linn and their immune-modulatory effect in LPS-induced inflammation in Raw 264.7 macrophage cells.
MATERIALS AND METHODS
Reagents
Dulbecco's modified Eagle's medium (DMEM),fetal bovine serum(FBS),L-glutamine,penicillin,and streptomycin were purchased from Gibco (Thermo Fisher Scientific,Waltham,MA,USA).Dimethyl-sulfoxide(DMSO) and LPS were purchased from Sigma (Burlington,MA,USA).Primary antibodies against COX-2,iNOS,β-actin and the horseradish peroxidase-conjugated secondary antibodies were purchased from Abcam (Cambridge,UK).ERK1/2,phospho-ERK1/2,JNK1/2/3,phospho-JNK1/2/3,p38,phospho-p38 antibodies were purchased from GeneTex (Irvine,CA,USA).Chemiluminescent HRP Substrate (ECL) was purchased from Advansta (Irvine,CA,USA).
Typha pollens extractions
Forty grams of Typha pollens(dried or charcoal activated) were extracted with 2 liters of deionized water by 2 h of incubation in a 60 ℃water bath with sonication,followed by autoclaving at 121 ℃for 20 min.The insoluble materials were removed by centrifugation at 5000 rpm for 10 min.The extract was freeze dried,then redissolved in deionized distilled water(50 mg/mL),aliquoted and stored at-20 ℃for further utilization.
Forty grams of Typha pollen (dried or charcoal activated) was extracted with 2 liters of 95% ethanol by 2 h of incubation in a 60 ℃water bath with sonication.The insoluble materials were removed by centrifugation at 5000 rpm for 10 min.The extract was filtered twice,dried at 55 ℃,then redissolved in 95% alcohol(50 mg/mL),aliquoted and stored at-20 ℃for further utilization.
1,1-Diphenyl-2-picrylhydrazyl(DPPH)radical scavenging activity
Typha extracts were diluted (0.002-1 mg/mL) and mixed with 0.8 mL of an ethanolic solution containing 0.2 mM DPPH(SIGMA,Burlington,MA,USA).The mixtures were shaken vigorously and left to stand in the dark for 30 min.The absorbance was read at 517 nm.Butylated hydroxytoluene (BHT) (SIGMA,Burlington,MA,USA) was used as the positive control.The scavenging activity (%) was defined as the percentage of the reduced absorbance caused by the treatment.
Reducing power
The reducing power was measured on the activity of reduction of colorless Fe3+-tripyridyltriazine (TPTZ)(SIGMA,Burlington,MA,USA) complex to the blue color Fe2+-TPTZ.In brief,6 L of pollen extract was mixed with 180 L of FRAP solution (300 mM sodium acetate,10 mM Fe3+-TPTZ (in 40 mM HCl) and 20 mM FeCl36H2O in the proportion of 10∶1∶1 vol/vol/vol)at 37 ℃and left to stand for 5 min.The absorbance was measured at 593 nm.
Ferrous ion chelating ability
Ferrous ion chelating assay was performed by mixing 250 μL of different quantities of Typha extracts or EDTA(a positive control)(J.T.Baker,Phillipsburg,NJ,USA)with 25 μL of FeCl2solution(2 mM)and 50 μL of ferrozine(5 mM)(SIGMA,Burlington,MA,USA).The mixtures were left at room temperature for 10 min.The absorbance of the mixtures was measured at 562 nm.The chelating activity of samples was expressed as the inhibitory percentage calculated by the following formula:(Ao-As)/Ao ×100%,where As is the absorbance of the sample and Ao is the absorbance of equivalent quantity of EDTA.
Total polyphenol and flavonoids content
The total polyphenol content was determined by the Folin-Ciocalteu assay.Fifty μL of Typha extracts was mixed with 1 mLNa2CO3(2%) and then reacted with 50 μL Folin-ciocalteu's reagent (50 %) (Merck &Co.,Kenilworth,NJ,USA) at room temperature for 30 min.The absorbance of products was read at 750 nm.Gallic acid (Bio BASIC Inc.,Amherst,NY,USA) was used for drawing a calibration curve.The total phenolic content was expressed in terms of milligrams of gallic acid equivalent per gram of extract.Flavonids contents were determined by Aluminum chloride colorimetric method using Quercetin (SIGMA,Burlington,MA,USA)as a standard.Typha extracts(100 μL)were mixed with 0.3 mL of methanol,0.02 mL of 10%aluminum chloride,0.02 mL of 1 M potassium acetate and 0.56 mL of distilled water.After 30 min of incubation at room temperature,the absorbance of the mixtures was measured at 415 nm.
Cell culture
Murine macrophage cell line RAW 264.7 was purchased from the Food Industry Research and Development Institute (Taiwan Chian).Cells were grown in DMEM medium supplemented with 10% Fetal bovine serum(FBS),1%penicillin/streptomycin/Amphotericin B and 4 mM L-glutamine in a humidified atmosphere containing 5%CO2at 37 ℃.
Cell viability assay
The effects of pollen extracts on cell viability were evaluated using the MTT assay.Briefly,RAW 264.7 cells were seeded at a density of 4 × 105cells/well in a 96 well-plate and incubated at 37 ℃for 24 h in serum-free medium.Extracts were added and incubated for further 24 h.Reactions were carried out by adding 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to each well at a final concentration of 5 mg/mL and then incubated at 37 ℃for 24 h,following by adding 0.1 mL of 10% sodium dodecyl sulfate and incubated for 16 h.The absorbance was read at 570 nm on an ELISA reader (Perkinelmen,Waltham,MA,USA).
Nitric oxide assay
Nitrite,a stable oxidation product of nitric oxide,was assayed as an indicator of NO production.Experiments were carried out according to previously described.31
Western blot analysis
RAW 264.7 cells were pretreated with pollen extracts for 2 h and then stimulated by LPS (1 μg/mL) (SIGMA,Burlington,MA,USA) for a specified time periods described in the figure ligands.The nuclear,cytoplasmic and total cellular proteins were prepared according to the Membrane,Nuclear and Cytoplasmic Protein Extraction Kit's protocol(Bio BASIC Inc.Amherst,NY,USA),and quantified using Bradford protein assay (Bio-Rad,Hercules,CA,USA).Equal amounts of protein(20 μg)were used for blotting.The blots were developed by using the ECL detection kit(Advansta Inc.,Menlo Park,CA,USA) and detected with ImageQuant LAS 4000 (GE Healthcare,Chicago,IL,USA).
Quantitative Reverse-transcription polymerase chain reaction(qRT-PCR)analysis
RAW 264.7 cells in 100-mm dishes (106cells/mL)were incubated for 24 h and pretreated with Typha pollens extracts for 2 h and then stimulated with LPS(1 μg/mL) for 4 h.Total RNA was isolated using the Total RNA Reagent (Bioman Scientific Co.,Ltd.,New Taipei City-Taiwan) according to the manufacturer's instructions.An aliquot of 1 μg was reverse-transcribed(RT) using iScript ™cDNA Synthesis Kit (Bio-Rad,Hercules,CA,USA).All qRT-PCRs have been carried with the following conditions:denaturing at 95 ℃for 30 secs,annealing at 60 ℃for 1 min,and elongation at 72 ℃for 1 min,for a total of 40 cycles.The cycle threshold (Ct) of each gene was normalized with that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) to get difference of Ct (ΔCt of target-GAPDH).The differential expression between treated and control samples (ΔΔCt) was calculated as the fold difference(2-ΔΔCt).GAPDH,IL1-β,IL-6,COX-2,iNOS and TNF-α gene expressions were detected by PCR using a thermal cycler (Applied Biosystems,Waltham,MA,USA).The sequences of primers were listed in Table 1.
Statistical analysis
The results in this study were presented as the mean ±standard deviation (±s) and evaluated using one-way analysis of variance (ANOVA) with Tukey multiple comparison test (GraphPad Prism 7,San Diego,CA,USA).ThePvalue less than 0.05 was considered to be statistically significant.
RESULTS
Antioxidant activity assays
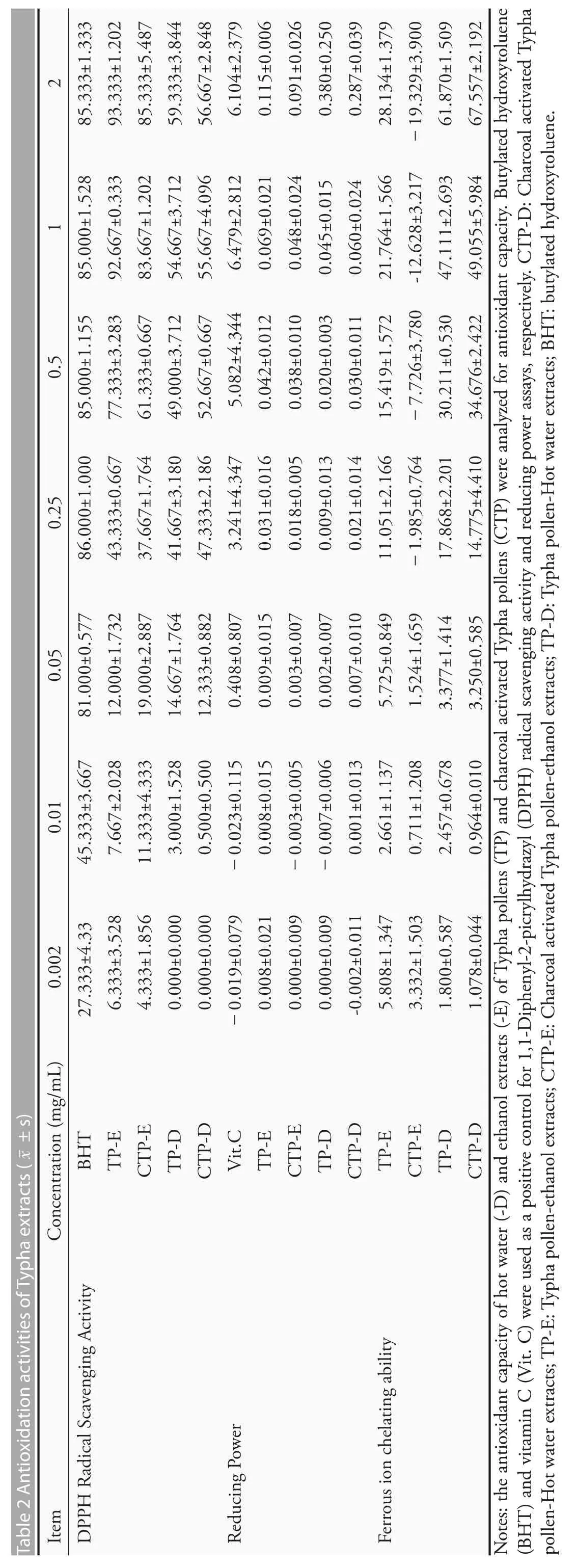
The antioxidation activity of Typha extracts were shown in Table 2.The DPPH free radical scavenging rates showed that the positive control,BHT,has a strongest free radical cleaning activity.All of the aqueous extracts of dried or charcoal activated pollens(TP-D and CTP-D,respectively) and their ethanolic extracts (TP-E and CTP-E,respectively) have moderate free radical cleaning activities.The ethanolic extracts have a higher activity than the aqueous extracts.The reducing power was also tested and the results showed that the pollen extracts do not have reducing power.The ferrous ion chelating ability of Typha extracts was expressed as the percentage of inhibition using an equivalent quantity of EDTA,a strong divalent cation chelator.Our data demonstrated that TP-D and CTP-D has a higher chelating activity (about 50% of that of EDTA),while TP-E exerted only 25%of the inhibitory activity of EDTA,and CTP-E did not have a ferrous ion chelating ability.
Polyphenol and flavonoids contents in Typha pollen extracts
The flavonoids contents were 30.32 ± 2.63,4.82 ±3.62,1.83±1.31 and 5.18±1.31 milligrams of Quercetin equivalent per gram of extract for TP-E,CTP-E,TP-D and CTP-D,respectively.The ethanolic extraction of pollen has a highest flavonoids content.The polyphenols contents were 51.92±0.99,34.81±1.81,63.04 ± 1.87 and 64.14 ± 1.71 milligrams of gallic acid equivalent per gram of extract for TP-E,CTP-E,TP-D and CTP-D,respectively.Hot water could recover more phenolic compounds from the sample than the ethanol.
Effects of Typha pollen extracts on the viability of RAW264.7 macrophages
To find an appropriate concentration range of the extracts for cellular assay,an MTT assay was preformed to study the toxicity of Typha pollen extracts to the macrophage RAW 264.7 cells.The result shown that only a higher concentration (more than 0.01 mg/mL)of CTP-D was slightly toxic to RAW 264.7 cells.For TP-E,CTP-E and TP-D,all concentrations (below 0.1 mg/mL) used in this research did not affect with the survival of RAW264.7 cells(data not shown).
Effects of Typha pollen extracts on LPS-stimulated NO productions
To study the effect of extracts on LPS-triggered NO production,RAW264.7 cells were pretreated with nontoxic concentration(0.002,0.01,0.05 and 0.1 mg/mL)of extracts for 2 h before treatment with LPS(1 μg/mL)for further 24 h,and the concentration of NO was measured.As shown in Table 3,NO production surged after LPS treatment.Pretreatment of 0.1 mg/mL TP-E,CT-E and CPT-D decreased significantly the production of NO to 76%,11%and 71%,respectively,in comparison to the untreated group.Nevertheless,TP-D did not decrease the NO production.
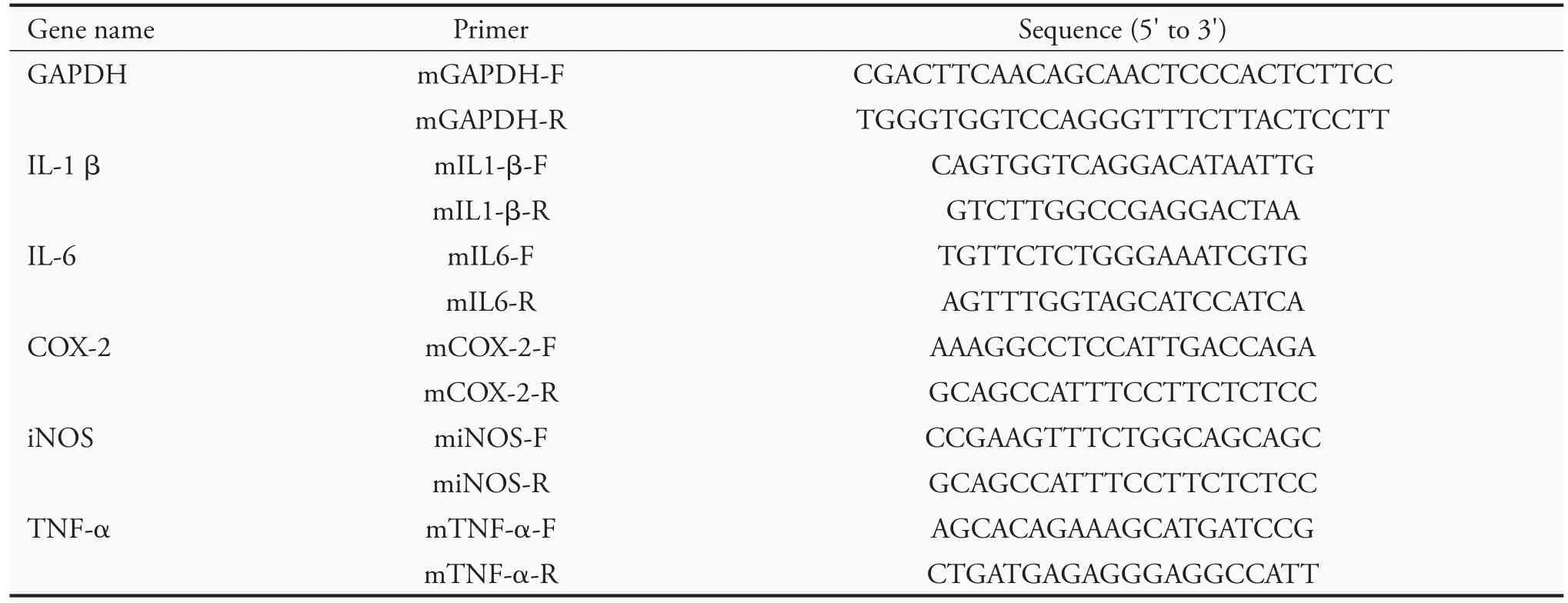
Table 1 Primers used in reverse-transcription polymerase chain reaction analysis
Effects of Typha pollen extracts on LPS-stimulated iNOS expression
Since TP-E and CT-E showed a more promising result in inhibiting NO production,we used these two samples in the following experiments.The protein and mRNA level of iNOS were assessed by Western blot and qRT-PCR,using β-actin and GAPDH as internal controls.The results were showed in Figure 1.TP-Esuccessfully decrease the expression of LPS-induced iNOS mRNA and iNOS protein in a dose dependent manner.CTP-E also reduce the protein expression of iNOS.The iNOS mRNA expression levels were increased in the groups treated with differentconcentrations ofCTP-Eexcept0.05mg/mL.
Effects of Typha pollen extracts on LPS-stimulated COX-2 expression
In addition to iNOS,COX-2 is another important mediator of inflammatory pathway.The effect of Typha extracts on the LPS-induced COX-2 expression was studied.The results shown in Figure 2 demonstrated that the COX-2 mRNA expression levels have decreased in the groups treated with different concentrations of TP-E and CTP-E.COX-2 protein levels were decreased in groups treated with TP-E and CTP-E.
Effects of Typha pollen extracts on the LPS-stimulated IL-1β,IL-6 and TNF-α mRNA expressions
We further investigated the effect of TP-E and CTP-E on expression of pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells.Cells were pretreated with TP-E and CTP-E for 2 h before adding LPS (1 μg/mL) and total RNA were isolated 4 h after LPS stimulation.IL-1β,IL-6 and TNF-α mRNA were determined by quantitative reverse-transcription PCR.As shown in Table 4,the mRNA levels of IL-1β and IL-6 increased in samples treated with 0.002 and 0.01 mg/mL of TP-E,and no significant difference with control in those treated a higher concentration of TP-E.The IL-1β mRNA levels decreased in samples treated with CTP-E,however,the difference is not significant.Expression level of IL-6 was decreased when treating with a low concentration of CTP-E,but increased when we increased the concentration of CTP-E.The TNF-α mRNA levels decreased in the samples treated with TP-E and CTP-E in a concentration-dependent manner,except for the samples treated with 0.1 mg/mL of TP-E.
Table 3 Effects of Typha extracts on NO production in LPS-induced RAW264.7 cells(±s)
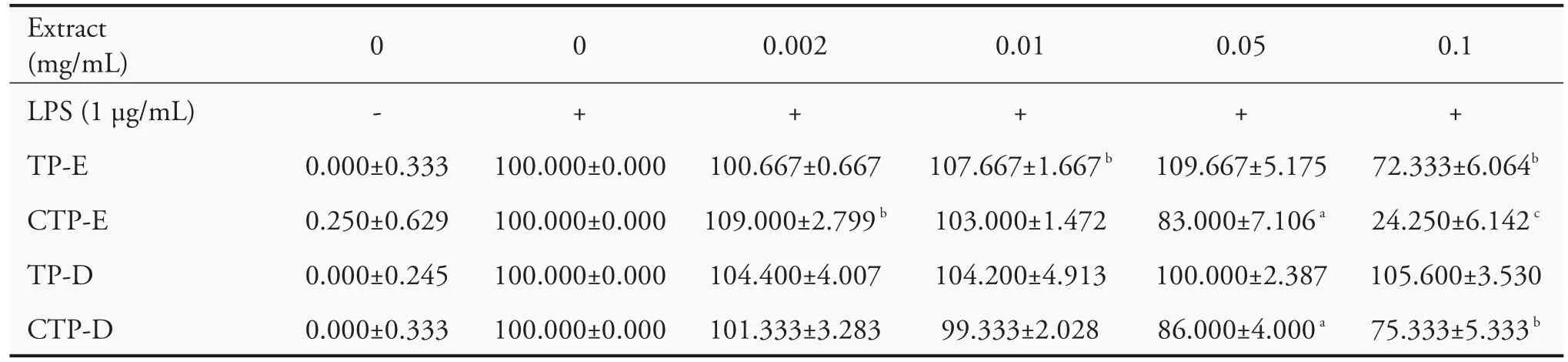
Table 3 Effects of Typha extracts on NO production in LPS-induced RAW264.7 cells(±s)
Notes:CTP-D:Charcoal activated Typha pollen-Hot water extracts;TP-E:Typha pollen-ethanol extracts;CTP-E:Charcoal activated Typha pollen-ethanol extracts;TP-D:Typha pollen-Hot water extracts;NO:nitrite oxide;LPS:lipopolysaccharides.Results are shown as the ratio in percentage of NO production in comparison with that of LPS-induced cells(100%).A statistical significance probability(P)-value(≤0.05)was labeled asaP<0.05,bP<0.01 andcP<0.001.
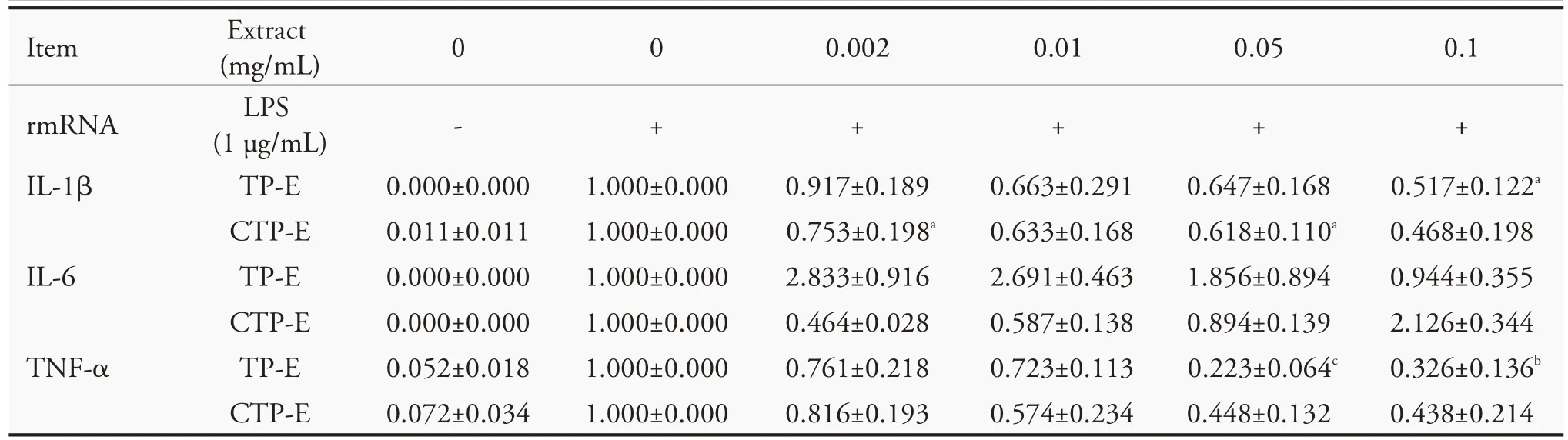
Table 4 Effects of Typha extracts on the expression of LPS(Lipopolysaccharides)-induced pro-inflammatory cytokines
Effects of Typha pollen extracts on MAPK signaling
To evaluate the effect of Typha pollen extracts on the expression and phosphorylation of MAPKs,RAW264.7 cells were treated with different concentration of Typha extracts for 2 h before treatment with LPS (1 μg/mL).Total cellular proteins were purified 15 minutes after addition of LPS.Antibodies against ERK 1/2 (phospho-Thr202),JNK 1/2 (phospho-Thr183/Tyr185),and p38 (phospho-Thr180/Tyr182) were utilized to detect the level of phosphorylation of MAPKs.As shown in Figure 3,the phosphorylation level of ERK was slightly decreased in a concentration-dependentmanner in the groups treated with different concentrations of TP-E and CTP-E but no significant difference for those of JNK1/2 and p38.
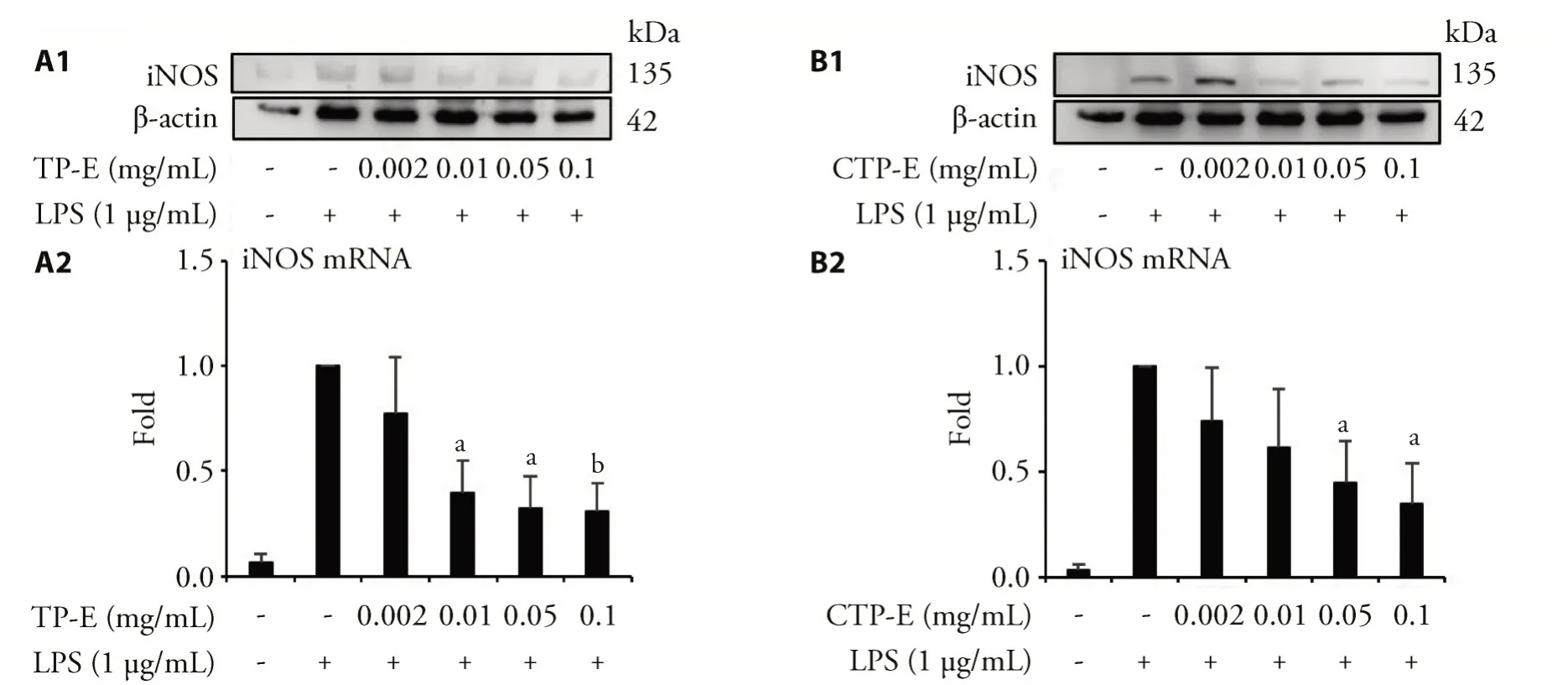
Figure 1 TP-E and CTP-E inhibit iNOS protein and mRNA expression induced by LPS
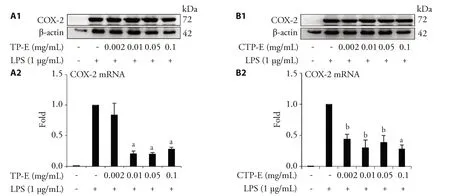
Figure 2 Effects of Typha pollen extracts on COX-2 protein and mRNA expression induced by LPS (Lipopolysaccharides) in RAW264.7 cells
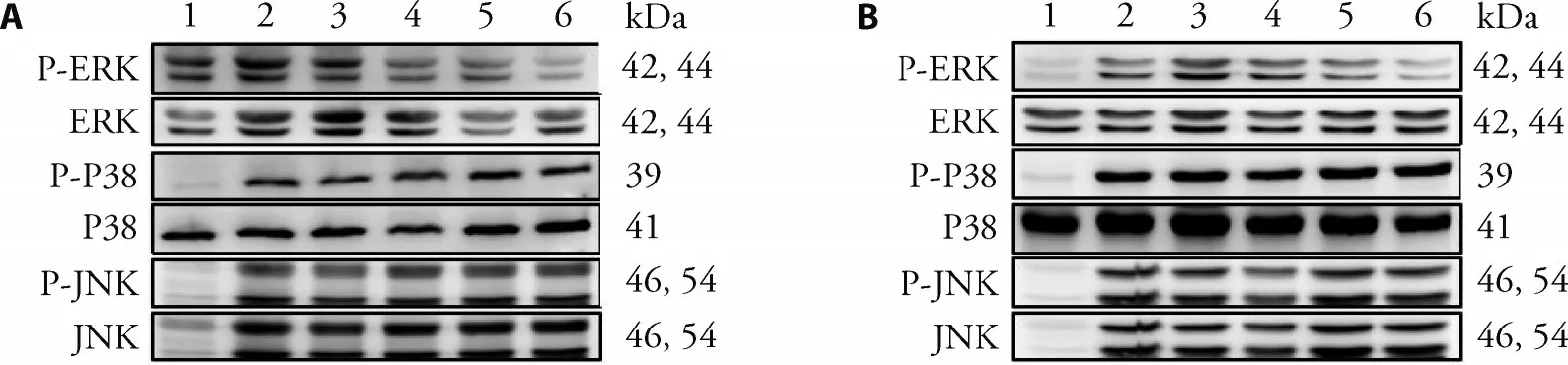
Figure 3 Effect of Typha pollen extracts on LPS-induced activate MAPKs
DISCUSSION
NO is a key intracellular messenger playing crucial role in the host defense,cellular homeostasis and pathogenesis of inflammatory response.32Low concentration of NO synthesis by endothelial nitric oxide synthase is essential for the normal immune function,while excessive NO production mediated by iNOS could be deleterious by hyper generation of ROS.33Activated iNOS expression in macrophage will continuously triggers the overproduction of NO.34Inhibiting NO overproduction by down regulating iNOS expression might be promising strategy preventing inflammatory disease.34,35COX-2,one of downstream regulator of NF-κB,was known to be involved in inflammation.COX-2 catalyze the synthesis of PGE2 from arachidonic acid at low concentration in normal condition.Excess synthesis of PGE2 by COX-2 over expression induce inflammation which might be life threaten if persist for long time.36Our results demonstrated that TP-E and CTP-E significantly down regulated the expression of two major inflammatory mediators iNOS and COX-2 at both mRNA and protein level in LPS-induced RAW264.7 murine macrophage cells,also suppress NO release induced by LPS treatment.From this it was clear that,TP-E and CTP-E inhibit LPS-induced iNOS and COX-2 expression thereby modulated its downstream immune regulators NO and PGE2.
Upon any external stimuli,macrophages are the first line immune defense cells to induce immune response during inflammation.TNF-α,IL-1β and IL-6 are pro-inflammatory cytokines produced by the immune activated macrophage.28In macrophage,LPS treatment trigger TLR4 receptors,which in turn activate the expression and release of pro-inflammatory cytokines TNF-α,IL-1β and IL-6.37Increased expression and release of this pro-inflammatory cytokines are early event observed in many inflammatory diseases.TNF-α is the key pro-inflammatory cytokine expressed at initial phase upon any immune stimuli,which could also act as a signaling molecule for the expression of other cytokine molecules and aggravate the immune response.38IL-1β act as a regulator of immune response at local and systemic levels and signaling molecule for the expression of inflammatory cytokines.39Pro-inflammatory cytokine IL-6 can stimulate PGE2 expression and activate inflammatory signaling cascade for production of excess inflammatory cytokine.28In our study,LPS-treatment induces the expression of pro-inflammatory cytokines TNF-α,IL-1β and IL-6 in RAW264.7 macrophage cells,on the other hand,TP-E and CTP-E effectively down regulated the LPS-induced expression of TNF-α,IL-1β and IL-6,suggest the anti-inflammatory effects of TP-E and CTP-E.
MAPKs are the kinase proteins family which includes ERK1/2,JNK1/2 and p38,are activated by redox signaling cascade which could leads to inflammation,oxidative stress and apoptosis.29,40Phosphorylate MAPKs trigger to produce more ROS in the cells.41LPS treatment could trigger the phosphorylation of ERK1/2,JNK1/2 and p38 in many types of cells including macrophages.42Our data also suggest that LPS treatment in RAW264.7 macrophage cells the phosphorylate ERK1/2,JNK1/2 and p38.TP-E and CTP-E treatment effectively inhibit the phosphorylation of the MAPKs.The results from our present study clearly displayed that the MAPK signaling pathway was involved in mediating the anti-inflammatory effects of TP-E and CTP-E in RAW264.7 cells.
Immuno-modulatory effect of TP-E and CTP-E on LPS-induce inflammatory pathogenesis and their molecular mechanism behind their activity was demonstrated in RAW264.7 macrophage cells.
In conclusion,results from our study revealed that TP-E and CTP-E effectively ameliorate LPS-induced inflammatory response through modulating iNOS/COX-2 and ERK1/2.Taken together,anti-oxidant and anti-inflammatory activity of Typha angustifolia Linn extract could be used for preventing inflammation related diseases.
 Journal of Traditional Chinese Medicine2021年6期
Journal of Traditional Chinese Medicine2021年6期
- Journal of Traditional Chinese Medicine的其它文章
- In vivo anti-diarrheal activity of jujube honey on castor oil-induced diarrhea in mice
- Target prediction and activity verification for the antidepressant action of Huangqin(Radix Scutellariae Baicalensis)
- Pingchuan formula (平喘方) improves allergic asthma in mice through inhibiting nuclear factor-kappa B/mitogen-activated protein kinase signaling pathway
- Can Fig and Olive Ameliorate the toxicity Induced by 2-nitropropane in some organs of mice? role of inflammatory versus anti-inflammatory genes
- Efficacy of Renshen(Radix Ginseng)plus Fuzi(Radix Aconiti Lateralis Preparata)on myocardial infarction by enhancing autophagy in rats
- Anti-hypertensive and endothelia protective effects of Fufang Qima capsule (复方芪麻胶囊) on primary hypertension via adiponectin/adenosine monophosphate activated protein kinase pathway
