Progress of ubiquitin-proteasome system in the pathophysiology of heart failure and the intervention of traditional Chinese medicine
Jie Chen,Xiaohong Wei,Qian Zhang,Huan Xia,Guiyang Xia,Yuzhuo Wu,Sheng Lin,*,Hongcai Shang,*
1 Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing,Dongzhimen Hospital,Beijing University of Chinese Medicine,Beijing 100700,China;
2 Guangdong Provincial Key Laboratory of Chinese Medicine for Prevention and Treatment of Refractory Chronic Disease,Guangzhou,China.
Abstract Heart failure(HF)represents one of the leading causes of morbidity and mortality in the modern world,which threatened approximately 1% ~ 2% of adults’ lives.HF is the end stage of multiple cardiovascular diseases characterized by cardiac hypertrophy and myocardial remodeling,and the pathophysiological processes of which include oxidative stress,endoplasmic reticulum homeostasis,apoptosis,autophagy,energy metabolism disorder,etc.The regulation of protein homeostasis intrinsically interrelates the above pathophysiological processes.Therefore,it is imperative to elucidate the molecular mechanism from the perspective of protein homeostasis to find new therapeutic targets for HF treatment.The dynamic regulation and post-translational modification of protein synthesis and degradation play a vital role in response of living organisms to physiological changes.The ubiquitin-proteasome system(UPS),which degrades 70-90% of endogenous proteins,plays an integral part in the pathophysiological processes of HF.The UPS can regulate oxidative stress,endoplasmic reticulum homeostasis,apoptosis and autophagy of cardiomyocytes(CMs),energy metabolism,targeting degradation signals and structural proteins,thus modulating cardiac hypertrophy,fibrosis and remodeling,finally contributing to the occurrence and progression of HF.Thus,regulating UPS is a promising effective strategy to treat HF.Increasing evidence indicates that traditional Chinese medicine(TCM)targeting the UPS is potential to ameliorate HF.This review will summarize the current knowledge focusing on the underlying mechanism and the important research advances related to UPS in treating HF,and the traditional Chinese medicine targeting UPS.
Keywords:Heart failure,the ubiquitin-proteasome system,Myocardial remodeling,Traditional Chinese medicine
Background
Heart failure(HF)is a kind of syndrome in which various cardiac structural or functional diseases lead to ventricular filling and ejection deficits,cardiac output could not meet the needs of body tissues,and congestion of pulmonary and systemic circulation,and insufficient blood perfusion of organs and tissues as clinical manifestations[1].It is the outcome of various heart diseases and can also be seen in the critical stages of other diseases.According to the time,speed,and severity of HF,it can be divided into chronic HF and acute HF.HF is one of the leading causes of morbidity and mortality worldwide.With the increase of the population aging,the incidence of HF is increasing year by year.The latest epidemiological data show that the incidence of HF in adults is 1% ~ 2%,while in people over 70 years old,the incidence of HF can reach 10%,and the 5-year mortality rate is about 50%,which severely threatens human life and health[2].Despite substantial advances in its diagnosis and treatment,the mortality rate of HF has not significantly improved.Therefore,it is crucial to explore the pathogenesis of HF and new therapeutic targets to change the status quo of HF research and prevention.
Myocardial remodeling has been confirmed to be the fundamental mechanism leading to HF because of a series of complex molecular and cellular mechanisms resulting in myocardial structure,function and phenotype changes.Studies have shown that multiple pathophysiological factors are involved in the process of myocardial remodeling,including oxidative stress,endoplasmic reticulum homeostasis,apoptosis,autophagy,and energy metabolism disorder[3].Oxidative stress is considered to be the triggering factor of the above pathogenesis.The overabundance of reactive oxide species(ROS)can lead to endoplasmic reticulum homeostasis imbalance,resulting in apoptosis autophagy and metabolic remodeling of cardiomyocytes(CMs),finally provoking myocardial remodeling and promoting HF occurrence[4,5].
Protein homeostasis,defined as the balance between protein stability and degradation,is essential for the pathogenesis of HF.The ubiquitin-proteasome system(UPS),a highly dynamic process composed of ubiquitination and deubiquitination,regulates protein homeostasis.Ubiquitination is identified as the process by which ubiquitins classify,target and modify intracellular proteins via a series of special enzymes including ubiquitin-activating enzyme(E1),ubiquitinconjugating enzyme(E2),and ubiquitin-ligase(E3)[6].While deubiquitination is mainly performed through the activity of the deubiquitination enzyme,which can not only remove ubiquitination,but also promote the ubiquitination process by recycling the ubiquitination molecule,revising the ubiquitination process,and decomposing the ubiquitination inhibitors[7].Animal and cellular models in vivo and in vitro can be used to evaluate the effect of UPS on HF and the causal relationship between UPS and disease phenotypes[8].The dysfunction of UPS can cause a series of cardiac diseases,including HF,myocardial ischemiareperfusion,atherosclerosis and so on.The imbalance of UPS initiates the degradation of several signaling proteins for myocardial fibrogenesis and differentiation,such as β-catenin and Calcineurin.Meanwhile,proteasomal damage can cause elevated levels of prohypertrophic and pro-apoptotic factors.Also,it promotes the non-adaptive remodeling of CMs by activating the Calcineurin-NFAT pathway[9].Recently,relevant experiments provide a solid theoretical basis for studying the specific mechanism of proteasome damage and identifying specific therapeutic targets in HF.Several proteasome-related deubiquitinase small molecule inhibitors targeting UPS,such as Auranofin,MG132,and IC86430,have been confirmed to regulate myocardial remodeling and alleviate HF[10-13].Thus,therapeutic interventions that target UPS will be promising strategies to treat HF.
More and more evidence indicated that traditional Chinese medicine(TCM)shows the potential and superiority in treating HF,with the advantages of significant efficacy and fewer side effects.The cardioprotective effects of TCM depend on the biological activities of its components,such as alkaloids,polysaccharides,glycosides,and flavonoids.These active compounds regulate the pathogenesis of HF by affecting protein homeostasis,thereby inhibiting the progression of HF.Nowadays,constituents from Chinese herbs,such as resveratrol,gambogic acid,magnolia,andrographolide,lignin,oxalone,and UNcarotene,have been verified to alleviate HF targeting UPS[14-19].In this review,we summarize the recent progress regarding the role of UPS in the pathogenesis of HF,and the therapeutic strategy targeting UPS,especially TCM.
Pathogenesis of HF
The activation of neuroendocrine and cytokine plays a vital role in the pathogenesis of HF[20].The reninangiotensin system(RAS)is a critical hormonal system in the body,of which overactivation is an essential determinant of the occurrence and development of HF.Angiotensin Ⅱ(Ang Ⅱ),as an effector molecule of RAS,regulates cell growth and apoptosis by activating downstream Ang Ⅱ type 1 receptor(AT1R)and affects the deposition of extracellular matrix(ECM).It has been confirmed that Ang Ⅱ affects the process of myocardial fibrosis and hypertrophy by regulating the expression of microRNA in cardiac fibroblasts[21].The transforming growth factor-β1(TGF-β1)/TGF-β activated kinase 1(TAK1)/Smads signaling pathway involves myocardial remodeling regulation.TGF-β1 can promote the phosphorylation of TAK1,activate downstream signal transduction molecules,and regulate the proliferation and differentiation of endothelial cells,vascular smooth muscle cells,myofibroblasts and other cells by binding to its receptor.Through the establishment of animal models,the researchers found that inhibiting the activation of TGFβ1-Smads2/3 or TGF-β1-TAK1 can reduce myocardial hypertrophy and fibrosis remodeling[22,23].
The direct effects of neuroendocrine and cytokines on CMs can promote various pathophysiological processes,including oxidative stress,endoplasmic reticulum homeostasis,apoptosis,autophagy,and energy metabolism abnormality[3].Oxidative stress is the product of the imbalance between the production and scavenging of ROS,which is currently recognized as the trigger factor in the pathogenesis of many cardiovascular diseases[4].Excessive ROS production can evoke endoplasmic reticulum stress(ERS)by inducing unfolded protein accumulation,and then ROS,together with ERS,causing cell death such as apoptosis and autophagy,which further leads to energy deficiency and maladaptive myocardial remodeling,finally promoting the occurrence and progression of HF[5].
Recent studies have found that repeated loss of CMs is an essential factor in the process of HF.Apoptosis,necrosis and autophagic death are the main manifestations of the decrease in the number of CMs,which play a role in HF.In a normal heart,apoptosis and autophagy are maintained at a shallow level,but apoptosis and autophagy levels will rise sharply under HF conditions.In HF caused by dilated cardiomyopathy,valvular cardiomyopathy,ischemic cardiomyopathy,and other unexplained cardiomyopathies,the significant aggregation of apoptosis and autophagy was detected[24-26].
There is compelling evidence that protein homeostasis is critical in the interconnection of HF pathogenesis and participants in the occurrence and progression of HF.UPS is the primary system of protein quality control(PQC)in all cells,which can clear damaged or misfolded proteins and regulate protein homeostasis[27].When each part of UPS functions normally,it can maintain the integrity of proteins necessary for sarcomere,mitochondria or cell membrane,so as to ensure normal cardiac function[28].The dysfunction of UPS is widely involved in the pathophysiology of HF.
Structure and Function of UPS
UPS,an ATP-dependent proteolysis system,composed of ubiquitin,ubiquitin-activating enzymes(E1),ubiquitin-conjugating enzymes(E2),ubiquitin ligases(E3),26S proteasome,and deubiquitinating enzymes(DUBs)(Figure 1).Among them,ubiquitin is a highly conserved polypeptide chain consisting of 76 amino acids.Its primary function is to label proteins that need to be degraded.E3 is a key regulatory enzyme in the process of ubiquitination.It can specifically recognize and bind target proteins,which determines the high specificity and selectivity of UPS.According to different structures,E3 can be divided into three categories:E3 with ring finger,E3 with U-box domain,and E3 with homeologous to E6-AP C-terminus(HECT)[29].26S proteasome contains one 20S core particle with proteasome catalytic activity and two 19S regulatory particles with regulatory function.The protein degradation process of UPS is as follows:firstly,E1 activates ubiquitin by adding a high-energy thioester bond to the C-terminal cysteine of ubiquitin,and the activated ubiquitin is transferred to E2 to form ubiquitin with E2.Then,E3 transports ubiquitin to the lysine residue of the target protein to form a single or polyubiquitin chain;finally,the ubiquitin-labeled protein substrate is discerned and degraded by 26S proteasome complex,and accessible and reusable ubiquitin molecules are released at the same time[30].UPS is not only involved in the PQC but also the transcription factors and lysosomes[31].UPS is the main way to activate the nuclear factor kappa-B(NFκB),and it takes part in the regulation of protein degradation-related genes in myocardium,such as muscle atrophy F-box(MAFbx/Atrogin-1),muscle ring finger 1(MuRF1)
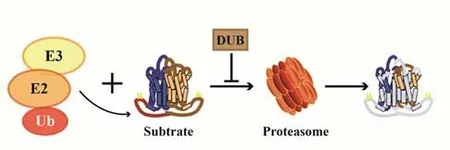
Figure 1.the Ubiquitin-Proteasome System(UPS)
UPS and HF
Molecular Mechanism of UPS Regulating HF
Regulates Oxidative StressOxidative stress increases cardiomyocyte apoptosis and decreases ventricular diastolic function through immoderate ERS,the reduction of nitric oxide and overexpression of oxidative stress-related molecules,such as ROS,iNOS,thus accelerating the progression of HF.Under the condition of oxidative stress,oxygen-derived free radicals accumulate excessively to stimulate mitochondria,resulting in the decrease of mitochondrial membrane potential,which makes the Cyt-C existing in mitochondria transferred into the cytosol[32].The Cyt-C in the cytoplasm can activate caspase-9,which is the initiation factor of caspase apoptosis and located in upstream of apoptosis.The activation of Caspase-3 can induce apoptosis.Carboxyl terminus of Hsp70-interacting protein(CHIP)that is highly expressed in heart and blood vessels can activate the ERK1/2 and STAT3 pathway,thus reducing oxidative stress and apoptosis,improving myocardial atrophy and dysfunction[33].Von Hippel-Lindau(VHL),an E3 ubiquitin ligase,regulates protein expression by polyubiquitination.The carbonyl cyamide m-chlorophenyl-hydrazone(CCCP)was used to mimic the HF condition in PLN-expressing HEK293 cells.PLN may be downregulated by VHL-mediated degradation through oxidative stress to attenuates HF[34].A crucial role of cyclindromatosis(CYLD),another deubiquitination enzyme,can significantly regulate cardiac function,and its mechanism is closely related to enhancing myocardial oxidative stress to cope with pressure overload.CYLD suppresses NF-E2 related factor-2(Nrf2)-operated anti-oxidative capacity through interrupting the ERK and p38/AP-1 and c-Myc pathways,thereby activating oxidative stress in the heart[35].
Regulates Endoplasmic Reticulum HomeostasisModerate ERS is an adaptive response of cells,conducive to the recovery of endoplasmic reticulum function and guarantees for cell survival.Severe or persistent ERS and protein misfolding will induce inflammation and apoptosis,causing an imbalance of ion channels among CMs,leading to myocardial electrical signal disorder,consequently result in various types of arrhythmias.Ubiquitin-fold modifier 1(Ufm1)is a novel 9.1-kDa ubiquitin-like protein highly conserved in multicellular organisms[36].RNA sequencing analysis and biochemical assays revealed that in cardiomyocyte-restricted Ufl1 knockout(Ufl1CKO)mice,the implication of prevalent ERS was revealed.During aging,a remarkably increased propensity to develop HF was shown in response to hemodynamic stress.The loss of function experiments in cultured CMs indicates that Ufl1 is required to initiates the adaptive ERS signaling.Lastly,pharmacological inhibition of ERS attenuated pressure overload-induced HF in Ufl1-deficient hearts[37].
Regulates AutophagyThe pathophysiological state of HF is that the heart unable to pump blood at a rate commensurate with the requirement of the metabolizing tissues.In this pathological state,myocardial cells are lost repeatedly and autophagic death is the leading cause.In these autophagic dead CMs,cell degeneration,the disintegration of nuclear ultrastructure,a large number of ubiquitin granules in autophagic vesicles,increased expression of ubiquitin and ubiquitin-protein conjugates in cytoplasm and nucleus were found,while the activities of E1 and E2 were not significantly different from those in standard CMs.CHIP,a ubiquitin E3 ligase,is a chaperone of HSP70/Hsp90,which is highly expressed in heart and blood vessels.CHIP suppresses AKT signaling and autophagy depended on autophagic flux in CMs and intact hearts to attenuate cardiac hypertrophy[38].HspB6/Hsp20(heat shock protein family B member 6)has emerged as a novel cardioprotective agent against stress-induced injury[39].Its mutant in mice hearts reduced interaction between mutant HspB6 and BECN1/Beclin 1,resulting in ubiquitination of BECN1 and its proteasomal degradation,inducing stimulated autophagy,causing myocardial remodeling and dysfunction,progressing to HF and early death[40].Regulates Apoptosis There is apoptosis of CMs in HF tissue,and apoptosis can reduce the number of working CMs and then lead to decreased myocardial contractility[41].Cardiomyocyte apoptosis in patients with end-stage HF is increased.Cardiomyocyte apoptosis in dilated cardiomyopathy is a persistent feature of end-stage HF and is related to the severity of the HF[42].The genes that regulate apoptosis in HF are mainly Caspase-8 and Bcl-2[43].UPS interacts with Bcl-2 to degrade pro-apoptotic or anti-apoptotic factors.Apoptosis suppressor genes can bind to caspase and mediate its degradation;however,in response to apoptosis stimulation,apoptosis suppressor genes will be ubiquitinated and eventually degraded.Hypoxiainduced apoptosis is relevant to the pathogenesis of HF.In H9c2 cells under hypoxic culture,USP7 expression was significantly upregulated while miR-409-5p expression was markedly downregulated.Augmentation of USP7 expression increased the release of the cytokines interleukin(IL)-1β,tumor necrosis factor(TNF)-α and IL-6,resulting in a dramatic increase of apoptosis of CMs.
Meanwhile,myocardial injury markers lactic dehydrogenase(LDH),troponin T(c-TnT)and creatine kinase MB(CK-MB)expressions also increased.The USP7 overexpression exacerbated left ventricular(LV)remodeling with decaying LV function.The miR-409-5p overexpression can reverse the effects of USP7 to protect the heart[44].UPS affects apoptosis by regulating the level of p53 in cells[45].Under normal physiological conditions,USP7 maintains the expression of p53 at a level;however,a high USP7 level can disrupt the balance between ubiquitination and deubiquitination of p53,leading to excessive apoptosis[46].In specimens of chronic primary mitral regurgitation worsening LV function,microarrays identified 718 differentially expressed genes,including genes relevant to the protein ubiquitination pathways,bone morphogenetic protein receptors,and eIF4 and p70S6K signaling regulation,compared to the preserved LV function.Furthermore,HL-1 cardiomyocyte cells transfected with ubiquitinactivating enzyme E1(UAE-E1)increased apoptosis,downregulated and ubiquitinated X-linked inhibitor of apoptosis protein(XIAP),and decreased cell viability[47].That upregulation of protein ubiquitinationrelated genes may exert adverse effects on LV through increased apoptosis and contractile protein degradation.
Regulates Cardiac Energy MetabolismThe heart produces ATP by oxidizing the substrates of carbohydrates,fatty acids(FA),amino acids,etc.The substrate selectivity of the heart depends on the material concentration and different stress states.The flexibility changes of substrate metabolic can make the cardiac quickly adapt to the material concentration in circulation to make the best choice of myocardial metabolism to provide adequate ATP[48].Energy metabolism is mainly divided into glucose metabolism and fatty acid metabolism.In HF,the expression of FA and glucose metabolism genes were inhibited,the CMs appeared "metabolic remodeling",the biological origin and function of mitochondria decreased,the metabolism of FA and glucose decreased,the energy metabolism of mitochondria decreased,the production of ATP decreased,and the myocardium was in a severe state of "energy starvation"[49].The peroxisome proliferator activating receptor(PPAR)transcription factors,including the most prominent isoform in the heart,PPARα plays diverse roles in regulating cardiac physiology and susceptibility to HF[50].MuRF3 can mono-ubiquitinate cardiac PPARα and activities in vivo via a post-translational modification to regulate fat and glucose storage to protect the heart[51].CD36 is a major protein involved in the uptake of sarcolemma FA into cardiomyocytes.The G protein-coupled receptor kinase 2(GRK2)-mediated ubiquitination of CD36 promotes CD36 degradation and reduces the intake of FA,thus exacerbating failing hearts[52].In patients with HFpEF and HFrEF,protein hydrolysis(MuRF-1 protein expression,ubiquitination,and proteasome activity)significantly increased with proteasome activity significantly correlated with the energy metabolism.And the energy metabolism of the two groups was disturbed.The malate dehydrogenase(MDH),mitochondrial complex-I,and complex-II decreased.An impaired CK activity in HFrEF patients but an up-regulation in HFpEF patients was found[53].The complex regulation of energy metabolism in different HF entities based on UPS needs further study.
UPS Regulates Pathological Manifestations of HF
Regulates Myocardial Hypertrophy and AtrophyAs stated above,myocardial remodeling is the basic pathogenesis of HF and myocardial hypertrophy is one of the manifestations of myocardial remodeling.Sustained cardiac hypertrophy often leads to HF.The right ventricular hypertrophy is a dangerous condition that ultimately results in right HF and death.Interacting with specific transcription factors,such as Rho-kinase and adrenergic signaling,MuRF1 regulates physiological and pathological hypertrophy and metabolism[54,55].Generally,it is reckoned that myocardial hypertrophy is an adaptive response to hemodynamic stress,which is reversible and compensatory under physiological stimuli such as physical activity or pregnancy[56,57].However,chronic and continuous biomechanical stress can result in pathological or maladaptive hypertrophy featured by cardiac remodeling,myocardial dysfunction,which may develop into end-stage HF[58].A Ca2+/Calcineurin-dependent transcriptional pathway is one of the crucial signaling pathways for cardiac hypertrophy.HectD3,an E3 ubiquitin ligase containing the HECT domain,which simultaneously attenuates cardiomyocyte hypertrophy driven by Calcineurin-NFAT and the pro-inflammatory effects of LPS/interferon-γ(IFN-γ)utilizing its cardiac substrates small ubiquitin-like modifier 1(SUMO1)and signal transducer and activator of transcription 1(STAT1),respectively[59].The miR-146a may be an upstream SUMO1-targeting microRNA in CMs.The miR-146a overexpression decreased the level of SUMO1,while the inhibition of miR-146a normalized the level of SUMO1,restored partly cardiac contractile function to alleviate hypertrophy[60].
The MuRF protein belongs to the TRIM family of ubiquitin ligases and is the most characterized E3 ligases in striated muscle.With the help of transgenic mice,we can understand the role of MuRF in myocardium.The absence of MuRF-2 or MuRF-3 led to protein aggregation and cardiac dysfunction,while MuRF-1 overexpression in HF models operated by a transverse aortic constriction(TAC)was injurious in genetic mice.Particularly,MuRF-1-deficient mice exposed to TAC developed substantial hypertrophy,while MuRF-2-deficient mice did not[61].Together,these studies favor that sarcomeric MuRFs are redundant to an extent,which is elemental for the structure and function of muscle but requires rigid regulation[62].Myocardial remodeling is also related to Atrogin-1/MAFbx.Atrogin-1/MAFbx is a ubiquitin E3 ligase specifically expressed in myocardium and skeletal muscle.The Atrogin-1/MAFbx expression increases with intraventricular pressure loading and adrenergic stimulation.Atrogin-1/MAFbx inhibits myocardial hypertrophy by ubiquitinating the k48 linkage and degradation of Calmodulin phosphatase,thereby preventing myocardial remodeling[63].Besides,Atrogin-1 can modulate aging-related cardiac fibrosis targeting FoxO1/3 activity[64].
Anthracycline chemotherapeutics,such as doxorubicin,are widely applied to treat numerous malignancies.Several years of exposure to anthracyclines atrophied the myocardium,and HF occurred.MuRF1-/-mice were safe against cardiac atrophy and discount in contractile function.MuRF1 may be a feasible target to prevent or reverse cardiac atrophy,thereby protecting the cardiac function of cancer patients receiving anthracyclines.Moreover,MuRF1 is also a vital effector molecule in the pathophysiological relationship between human aging and HF,regulating the function of CMs in which activin type II receptor is an effective upstream promoter[65].Even,MuRF1/MuRF2 is an effective treatment for diaphragm fiber atrophy and contractile dysfunction caused by HF[66].
UPS has long been considered a mechanism of myocardial atrophy because it can mediate protein degradation.With the deepening of research,the atrophy signaling pathway is considered to exert an effect to inhibit or reverse myocardial hypertrophy.Specific overexpression of USP18 in mouse CMs significantly blunted HF as evidenced by mitigated myocardial hypertrophy,fibrosis,ventricular dilation,and preserved ejection function,dependent on its modulation of the TGF-β1-p38/c-JNK1/2 signaling axis[67].While,the serum USP18 level is up to 10-fold higher in drug-naïve patients with early-stage systolic-diastolic arterial hypertension than in normotensive subjects and greater than in patients with isolated diastolic hypertension[67].Therefore,USP18 may indicate a counter-regulatory regenerative process to increase afterload,aiming at maintaining the proper cardiomyocyte structure and contractile function.
Regulates Myocardial FibrosisCardiac fibrosis is symbolized by excessive accumulation of ECM produced by the imbalance between the production and degradation of ECM,resulting in excessive accumulation[68,69].Excessive ECM deposition changes the myocardial structure,further alters cardiac phenotype and function[70].A research report suggests that[71]when the content of myocardial collagen fiber increases by 2-3 times,the ventricular filling can be changed,and the diastolic wall stiffness increases,which will promote diastolic HF;if the content of myocardial collagen fiber increases by four times or more,it will promote systolic HF.
ECM is mainly derived from fibroblasts and is composed of collagen,elastic fibers,glycosaminoglycans and glycoproteins.The proliferation of cardiac fibroblasts(CFs)and the collagen accumulation in cardiac interstitium are the leading causes and signs of cardiac fibrosis[72].In a cell model of cardiac fibroblasts activation induced by Ang II,the downregulation of USP2 inhibited the proliferation of CFs,collagen synthesis and cell cycle progression to delay cardiac fibrosis,which is owing much to deubiquitination and stabilization of β-catenin,and increased cyclin D1[73].USP2 may serve as a novel target to reverse the progression of myocardial fibrosis in HF.Furthermore,CYLD has also been found to participate in myocardial fibrosis.Knockout of CYLD enhanced survival rate in HF mice and attenuated cardiac hypertrophy and fibrosis[35].The small proline-rich protein 2b(Sprr2b)is a regulatory subunit of the USP7/MDM2-containing ubiquitination complex.It can stimulate the gather of MDM2 and the degradation of p53,which promotes the proliferation of pathological CFs,but this process can be reversed by insulin growth factor-1(IGF-1)signaling.And this result is also verified in human HF patients[74].TRIM32,also a TRIM family member,is concerned with the UPS-mediated degradation of thin filamentassociated proteins.TRIM32 was shown to ubiquitinate α-actinin,tropomyosin,myotilin,troponin I and the thin filament-associated proteins cTn T.
Meanwhile,it is a significant mediator of desmin turnover,and its knockdown blocked the degradation of desmin.TRIM32 seems to participate in an initial stage of muscle atrophy requiring the disassembly of desmin filaments[75].It is proved that TRIM32 has protective effects in the HF mice model since its overexpression holds back development into HF in mice exposed to TAC[76].The previous study found that Nogo-C protein ubiquitously expresses in the heart tissues and regulates cardiomyocyte apoptosis[77].Nogo-C inhibits ubiquitination of Sec61α protein to stabilize Sec61α and interacts with Sec61α on the endoplasmic reticulum to increase cytosolic Ca2+concentration and inhibit fibrotic responses in cardiac fibroblasts[78].
Treatment with Inhibitors Related to UPS
For the past few years,a few small molecule inhibitors of proteasome have been discerned,which can easily enter cells and screening to inhibit the proteolysis function of proteasome complex.Auranofin is a 19S proteasome-associated deubiquitinase inhibitor,which has been applied to treating rheumatic arthritis for decades.It can block the development of LVH induced by abdominal aortic constriction to partly reverse HF via inactivation of the NF-κB pathway[10].Nevertheless,in another study,the proteasome inhibitor MG132 was administered to the TAC rat model for four weeks.The result showed that MG132 significantly reduced fibrosis,which suppressed androgen receptors may achieve and there was no correlation between NF-κB expression levels and UPS function[11].Phosphodiesterase 1(PDE1)hydrolyzes cyclic nucleotides and is the main source of PDE activity in the human myocardium.PDE1 inhibitor(IC86430)improved myocardial 26S proteasome activity and proteolytic function of UPS in mice.IC86430 markedly attenuates HF with preserved ejection fraction,enhances mice survival rate,increases PKA-mediated proteasomal phosphorylation,and reduces misfolded myocardial CryAB[12].REGγ acts as 11S proteasome activator of 20S proteasome,promoting the protein degradation in a ubiquitin and ATP-independent manner.REGγ directly interacted with and targeted PP2Acα for degradation,resulting in increased levels of phosphorylation and nuclear export of Forkhead box protein O(FoxO)3a,which subsequently led to manganese superoxide dismutase(MnSOD/SOD2)decline,ROS accumulation,and cardiac hypertrophy.In vitro,the introduction of exogenous PP2Acα or SOD2 to human CMs markedly rescued the REGγ-mediated Ang II-stimulated ROS accumulation[13].It is suggested that regulating the REGγ-proteasome activity may be a practical therapeutic method in myocardial hypertrophy-relevant disorders.
Traditional Chinese Medicine(TCM)for HF
TCM Theory of HF
TCM theory holds that the etiology of HF is related to exogenous pathogenic factors such as wind cold dampness,rheumatic heat,pestilence and toxin,improper diet,emotional disorder,loss of work and leisure,old age and protracted illness,and abnormal endowment.Due to Qi,blood,Yin and Yang deficiency,and dysfunction of viscera,the heart loses nourishment and blood circulation,leading to Qi stagnation and blood stasis,phlegm stagnation,and retained fluid inhibiting Yang Qi of the heart,resulting in HF[79].Combined with modern medical research,organs and tissues function abnormally,sugar,lipids,proteins and other substances are immature and inactive.These substances cannot participate in normal material synthesis and catabolism[80].Neither can they be directly converted into energy surrogates,nor can they be synthesized into active proteins to perform their functions,affecting the progress of HF.The UPS is one of the main pathways mediating protein degradation.The misfolded intracellular proteins that need to be degraded in a specific time and space are labeled by ubiquitin molecules and are eventually recognized and degraded by the proteasome to maintain normal physiological functions[81].Once the UPS function is impaired,affecting protein unfolded and misfolded,resulting in accumulation of waste and toxins,causing HF.Unexpectedly,the two theories happen to have the same view.
hinese Herbs and Related Compounds Modulating UPS for HF
Chinese herbs contain various chemical components,among which alkaloids,polysaccharides,glycosides,flavonoids and enzymes may be active compounds with therapeutic value in HF.Chinese herbs(Figure 2)can enhance diuresis and myocardial contractility,dilate blood vessels,alleviate inflammation,oxidative stress and apoptosis,reduce fibrosis and ventricular remodeling[82],with the characteristics of multi-target comprehensive curative effect.The advantages of multi-channel and multi-target can avoid many compensatory adverse reactions caused by the single defect of western medicine and the weakening of pharmacological effects caused by drug metabolism.The oral administration of resveratrol alleviates cardiac hypertrophy and fibrosis and rescues diastolic cardiac function by downregulation of p300 via the UPS by deacetylation of lysine residues for ubiquitination,which indicates that resveratrol is conducive to suppressing the development of HF.[14].Gambogic acid,a major component of Gamboges,can be acted as a prodrug for proteasome inhibitors.Myocardial hypertrophy in HF increased 20S proteasome chymotrypsin(CT)-like enzyme activity.After gambogic acid treatment,the CT-like enzyme activity decreased significantly,which inhibited the activation of the NF-κB pathway to reverse myocardial hypertrophy in HF[83].Honokiol,a natural compound in the Magnolia plant,has been proved to retain antioxidative and anti-inflammatory activities.Honokiol resists CSE degradation via the UPS to strongly reduce CSE-Myc polyubiquitination levels,thus preventing vasoconstriction and endothelial dysfunction to alleviate failing hearts[15].Nrf2 specifically activates autophagy in CMs,attenuates oxidative stress,and inhibits myocardial hypertrophy,apoptosis and interstitial fibrosis,thereby improving cardiac pathological remodeling and cardiac dysfunction[84,85].KEAP1,a protein that together with CUL3 and RBX1 forms an E3 ubiquitin ligase that polyubiquitinates Nrf2 to degrade Nrf2.High concentrations of andrographolide can increase Nrf2 protein expression by inhibiting KEAP1 function.Andrographolide may be a potential drug for HF[17].Besides,Luteolin[86]and Huangqi(Radix Astragali Mongolici)-Danggui(Radix Angelicae Sinensis)[87]also can activate Nrf2 to regulate oxidative stress to treat HF to an extent,and the 3:2 ratio works best.Shionone can inhibit the activity of USP2 and delay the progression of HF,which acts as a new USP2 inhibitor from Chinese herbals[18].Compound Uncaria Tablets,mainly composed of Gouteng(Ramulus Uncariae Cum Uncis),Chuanxiong(Rhizoma Ligustici Chuanxiong)and Gouqi(Lycium Chinensis),can reduce LV hypertrophy by inhibiting the abnormal growth of CMs and cardiac fibrin;its mechanism involves ubiquitinrelated ribosomal protein based on iTRAQ technology[19].
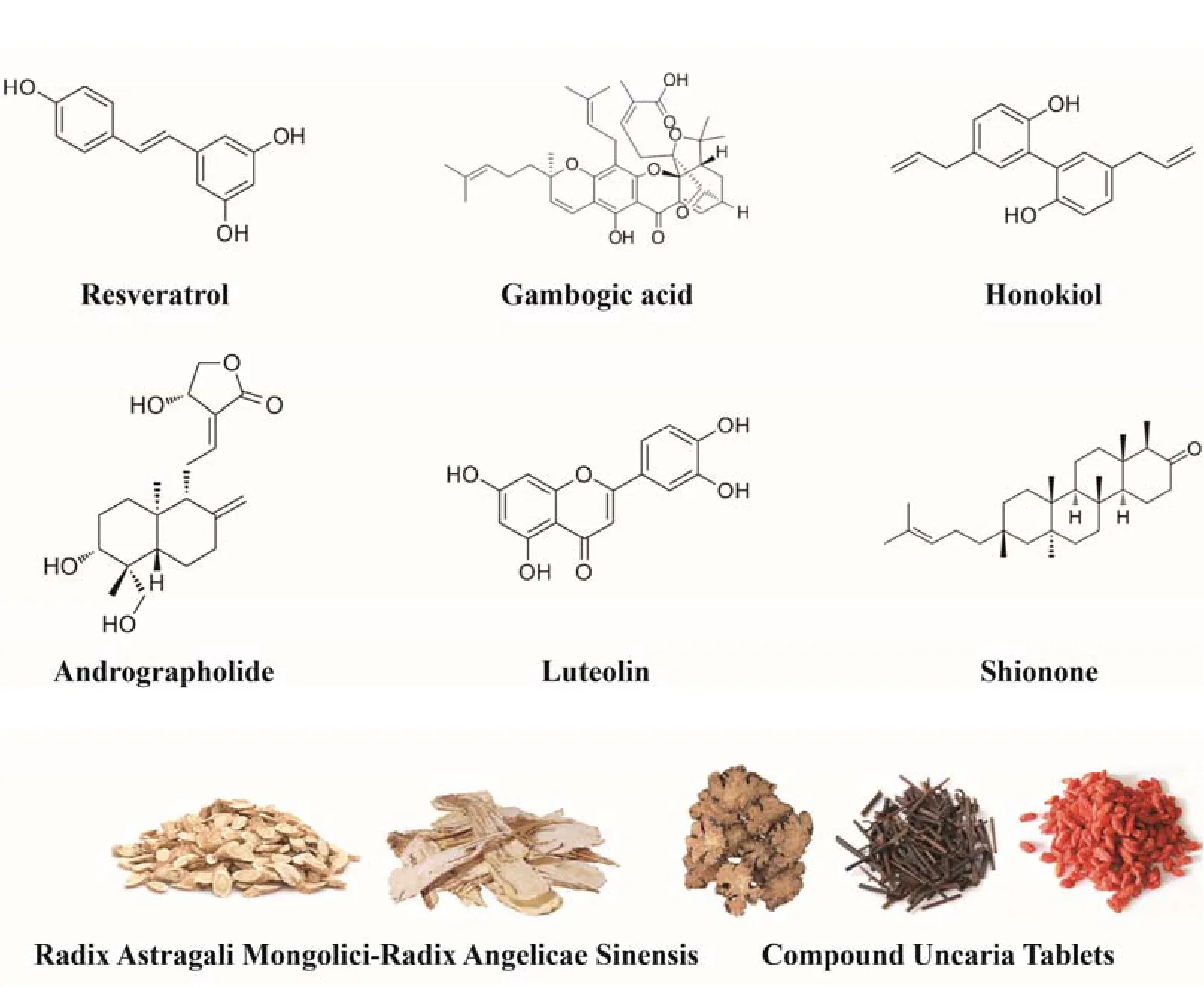
Figure 2.Chinese herbs and related compounds modulating UPS for HF
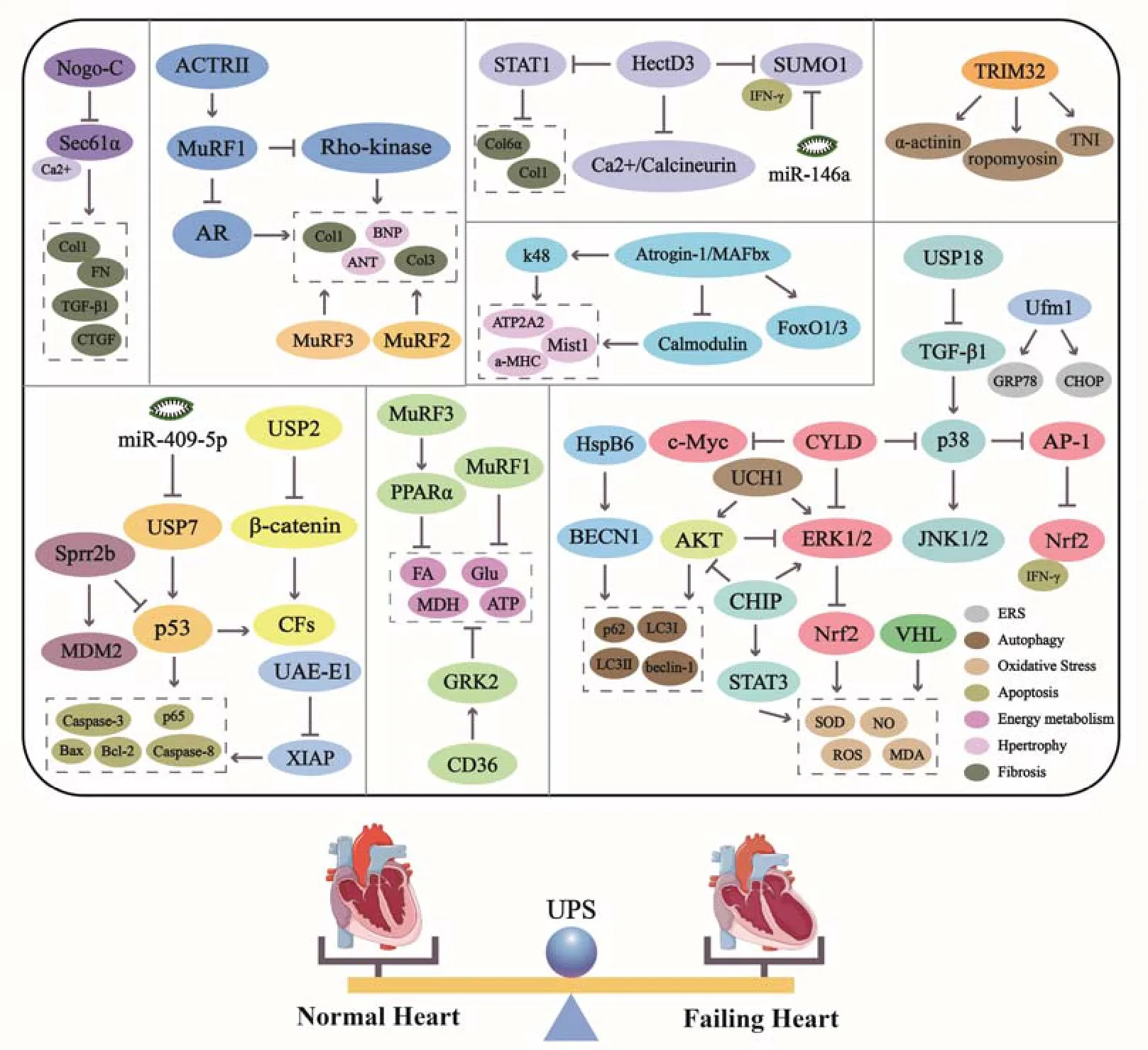
Figure 3.Pathways and molecules involved in UPS in HF
Discussion
HF is the ultimate end-stage outcome of cardiovascular diseases and the main cause of death.Myocarditis,myocardial infarction and hemodynamic overload are the initiating factors of HF.The decline of cardiac function leads to hemodynamic disorder,and the activation of neuroendocrine and the release of cytokines are the intermediate links.The circle of neuroendocrine activation and myocardial remodeling are the ultimate consequences of HF.Thus,reversing myocardial remodeling is the key to the treatment of HF.Long-term hemodynamic changes such as pressure and volume overload lead to myocardial remodeling by increasing myocardial wall tension,promoting the release of cytokines and signal peptides,neuroendocrine signaling mechanism or oxidative stress response.The clinical manifestations are enlargement of the ventricular cavity,hypertrophy of the ventricular wall and geometric changes of the ventricular cavity.However,hypertrophic myocardium is in the state of energy starvation,myocardial ischemia,cardiomyocyte apoptosis,LV progressive enlargement with dysfunction,and finally develops to irreversible myocardial damage.Myocardial hypertrophy and atrophy,fibroblast proliferation,apoptosis and autophagy,oxidative stress are involved in myocardial remodeling,which can cause,worsen or stop the process of ventricular remodeling.
UPS regulates fundamental cellular functions[88]containing mitosis,DNA replication and repair,cellular differentiation and transcriptional regulation,and receptor internalization,all of which make a vast difference in maintaining normal cardiac function.From the point of view of treatment,adjusting the function of UPS to a good state is essential for myocardial protection;it is also crucial to maintain the fundamental role of the UPS in PQC.UPS regulates different aspects of HF and runs through the whole pathophysiological process(Figure 3).Its regulation of HF is multi-channel and multi-target,consistent with the multi-target and multi-channel pharmacodynamic characteristics of traditional Chinese herbals[89-91].Just as ubiquitin C-terminal hydrolase 1(UCH1,also known as PARK5/PGP9.5),a subfamily of DUBs.Blockage of UCHL1 activity attenuates inflammation,oxidative stress,cardiac hypertrophy,and fibrosis.These positive actions possibly benefited from the inactivation of multiple signaling pathways,AKT,ERK1/2,STAT3,Calcineurin A,TGF-β/Smad2/3,and NF-κB pathways[92].In TCM theory,Qi stagnation and blood stasis,phlegm stagnation and retained fluid are considered to be pathological factors of HF.The nutrient substances such as sugar,fat,protein and others cannot be catabolized appropriately and those deteriorated into Qi stagnation and blood stasis,phlegm stagnation and retained fluid,thus induced diseases.Involvement in ubiquitination,deubiquitination or ubiquitin-mediated protein degradation is considered a promising therapeutic target.Animal experiments have verified that some UPS inhibitors in low doses partially reverse myocardial remodeling and significantly improve cardiac function.Studies presented in the review not only review the important molecules in UPS,but also the signaling molecules and pathways regulated by UPS in the pathogenesis of HF.Targeting these signaling molecules and pathways will make it possible to develop future therapeutic options.
 TMR Modern Herbal Medicine2021年3期
TMR Modern Herbal Medicine2021年3期
- TMR Modern Herbal Medicine的其它文章
- Effects and safety of Ding Kun Dan on lVF/lCSl-ET outcomes in patients with predicted poor ovarian response:A multicenter randomized clinical trial
- Combination of XianGui capsule and LCZ696 inhibits doxorubicininduced heart failure in mice
- Pharmaceutical care of a patient with antibiotic-associated encephalopathy
- Research of the clinical efficacy of Qiming Granule in treatment of diabetic nephropathy by meta-analysis
- Extraction and antibacterial potential of traditional medicinal plant Cypreus compressus
