Research of the clinical efficacy of Qiming Granule in treatment of diabetic nephropathy by meta-analysis
Ning Liu,Tao He,Chuanxin Liu*,Jianmei Huang*
1 Beijing University of Chinese Medicine,School of Chinese Materia Medica,Beijing 100029,China.
Abstract Objective:To systematically evaluate the clinical efficacy of Qiming Granule combined with conventional therapy in improving diabetic nephropathy.Methods:The Chinese and English databases such as Wanfang,VIP,CNKI,PubMed,Cochrane library and Web of Science database were searched.The searching time was from the database was established to March 1,2021.The papers were screened strictly according to the inclusion and exclusion criteria.The inclusion risk assessment tool provided by the Cochrane Collaboration was used to evaluate the included literature,the data was analyzed using STATA 12.0 software.Results:Twelve papers were included,involving 1369 patients.The results of meta-analysis showed that Qiming Granule combined with conventional treatment had statistical significance in improving the clinical total effective rate[RR = 1.24,95% CI(1.12,1.36)],urinary albumin excretion rate[SMD = -2.68,95% CI(-3.86,-1.50)],creatinine[SMD = -1.46,95% CI(-1.84,-1.08)],urea nitrogen[SMD = -2.61,95% CI(-3.85,-1.36)],fasting blood glucose[SMD = -1.17,95% CI(-2.03,-0.31)]and cystatin C[SMD = -3.26,95% CI(-4.65,-1.87)]of patients with diabetic nephropathy.Conclusion:The combination of conventional therapy and Qiming Granule can improve the clinical effect for diabetic nephropathy.However,due to the low level of overall literature evidence,high risk and some kind of publication bias,it still needs more high-quality and large-scale clinical studies for further verification.
Keywords:Qiming Granule,Diabetic Nephropathy,Meta-Analysis,Clinical Effects,Randomized Controlled Trials
Introduction
Diabetic nephropathy(DN)is one of the most common chronic microvascular complications of diabetes mellitus and the main inducement of end-stage renal disease[1-2],with clinical manifestations including polyuria,edema and loss of appetite[3].Due to the absence of obvious features in the early stage,it is generally treated with routine hypoglycemic drugs,and is transferred to the department of nephrology when edema and proteinuria occur,which often causes patients to miss the optimal treatment period,affecting the efficacy and prognosis[4].At present,a lot of clinical treatment with western medicine is given priority to,can reduce the patients' blood glucose,blood pressure,improve renal function[5-10].But due to the complexity of the disease etiology,western medicine is given priority to with single target function again,can only delay the disease cannot effect a radical cure,at the same time a variety of drug combination may produce allergic,adverse reactions such as nausea,vomiting,also may produce resistance for a long time,cause the overall efficacy of poor[11].Traditional Chinese medicine has a variety of components,can act on multiple targets at the same time,play a variety of physiological effects,and has the advantages of mild action,small side effects,can significantly reduce the patient's urine protein,improve symptoms,reduce kidney injury,and improve the quality of life of patients[12-14].
Qiming Granule is composed of Huangqi(Radix Astragali Mongolici),Gegen(Puerariae LobataeRadix),Dihuang(Rehmanniae Radix),Gouqizi(LyciiFructus),Juemingzi(Cassiae Semen),Chongweizi(Leonuri Fructus),Puhuang(Typhae Pollen),Shuizhi(Hirudo)extract of Chinese medicine preparations(Zhejiang wanma pharmaceutical co.,LTD.,approved by Z20090036,went public in 2009).Its main chemical components are astragaloside IV,astragaloside polysaccharide,puerarin,Catalpol,hirudin,etc[15-16],has the functions of replenishing qi and body fluid,nourishing liver and kidney,etc.Previous studies have shown that Qiming Granule can effectively reduce blood glucose and lipid levels in patients with DN,significantly improve urinary microalbumin and delay the development of diabetic nephropathy[17],but there is not enough evidence-based medicine to support it.Based on the above research situation,this research takes the western medicine routine treatment as the control group,the Qiming Granule union routine treatment as the observation group,carries on the analysis to the Qiming Granule auxiliary treatment DN clinical curative effect,in order to provide the basis and the reference for the clinical application.
Information and methods
Inclusion criteria
The type of study is a randomized controlled trial(RCT),and there is no restriction on whether to use the blind method.The language is limited to Chinese and English.The subjects were patients with diabetic nephropathy,and the diagnostic criteria were in accordance with Chinese Nephrology[18]or Expert Consensus on Diabetic Nephropathy(2014 Edition)[19].The control group was given routine western medicine treatment.The observation group was given Qiming Granule on the basis of the control group treatment.The use of other hypoglycemic and antihypertensive drugs was consistent between the two groups.The total effective rate,urinary albumin excretion rate(UAER),creatinine(CR),urea nitrogen(BUN),fasting blood glucose(FBG)and cystatin C(Cys-C)were used as indexes for clinical evaluation.
Exclusion criteria
In addition to diabetic nephropathy,there were studies with other complications;animal studies;literature reviews;meta-analyses;Repeated published literature;and studies with incomplete or unextractable data.
Literature retrieval
"Diabetic Nephropathy" and "Qiming Granule" were used as the search terms,and the literature on the treatment of Diabetic Nephropathy with Qiming Granule was searched in Wanfang,VIP,CNKI,PubMed,Cochrane library and Web of Science database by the way of subject words combined with free words.The retrieval time is from the establishment of each database to February 2021.Take PubMed as an example to list the retrieval strategies,
#1 "Diabetic Nephropathy"[Mesh]
#2 "diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("nephropathies"[All Fields]AND "diabetic"[All Fields])OR "nephropathies diabetic"[All Fields]OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("nephropathy"[All Fields]AND "diabetic"[All Fields])OR "nephropathy diabetic"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("diabetic"[All Fields]AND "nephropathy"[All Fields])OR "diabetic nephropathy"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("diabetic"[All Fields]AND "kidney"[All Fields]AND"disease"[All Fields])OR "diabetic kidney disease"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("diabetic"[All Fields]AND "kidney"[All Fields]AND"diseases"[All Fields])OR "diabetic kidney diseases"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("kidney"[All Fields]AND "disease"[All Fields]AND "diabetic"[All Fields])OR "kidney disease diabetic"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("kidney"[All Fields]AND "diseases"[All Fields]AND "diabetic"[All Fields])OR "kidney diseases diabetic"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("diabetic"[All Fields]AND "glomerulosclerosis"[All Fields])OR "diabetic glomerulosclerosis"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("glomerulosclerosis"[All Fields]AND "diabetic"[All Fields])OR "glomerulosclerosis diabetic"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("intracapillary"[All Fields]AND"glomerulosclerosis"[All Fields])OR "intracapillary glomerulosclerosis"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("nodular"[All Fields]AND "glomerulosclerosis"[All Fields])OR "nodular glomerulosclerosis"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("glomerulosclerosis"[All Fields]AND "nodular"[All Fields])OR "glomerulosclerosis nodular"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR "diabetic nephropathies"[All Fields]OR("kimmelstiel"[All Fields]AND "wilson"[All Fields]AND "syndrome"[All Fields])OR "kimmelstiel wilson syndrome"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("kimmelstiel"[All Fields]AND "wilson"[All Fields]AND "syndrome"[All Fields])OR "kimmelstiel wilson syndrome"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("syndrome"[All Fields]AND "kimmelstiel"[All Fields]AND "wilson"[All Fields]))OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("kimmelstiel"[All Fields]AND "wilson"[All Fields]AND "disease"[All Fields])OR "kimmelstiel wilson disease"[All Fields])OR("diabetic nephropathies"[MeSH Terms]OR("diabetic"[All Fields]AND "nephropathies"[All Fields])OR"diabetic nephropathies"[All Fields]OR("kimmelstiel"[All Fields]AND "wilson"[All Fields]AND "disease"[All Fields])OR "kimmelstiel wilson disease"[All Fields])
#3 #1 OR #2
#4 "Qiming Granule"[Title/Abstract]
#5 #3 AND #4
Data extraction and quality assessment
Based on the 12 articles finally included above,two researchers independently extracted the data,mainly including the title of the article,the first author,publishing unit,publishing years,intervention measures and observation indicators,etc.If there were differences,they would communicate with corresponding authors to determine the final included data.The evaluation of literature quality refers to the"bias risk assessment tool" recommended by Cochrane Collaboration Network[20].
Statistical Analysis
STATA 12.0[21]software was used for meta-analysis of the literature.The specific analysis model was based on heterogeneity test.IfP< 0.1 or I2> 50%,random effect model was used.Otherwise,fixed effect model was used.Among the outcome indicators of this study,the total effective rate was categorical data,which was expressed by relative risk(RR).And the other indicators were measurement data,which was expressed by standard weighted mean difference(SMD).The results were described by 95% CI,and the point estimate was given.The Significant level was α =0.05.If there was significant heterogeneity in the literature,sensitivity analysis was further used to investigate the source of heterogeneity.
Results
Literature search results
There were 39 literatures retrieved from CNKI,29 literatures retrieved from Wanfang Science and Technology Database,23 literatures retrieved from VIP database,0 literatures retrieved from PubMed,0 literatures retrieved from Cochrane Library and 0 literatures retrieved from Web of Science.After gradual screening,12 articles were finally included[22-33],involving a total of 1369 patients.The specific process and results are shown in Figure 1.

Figure 1.The specific process of literature screening.A total of 91 articles were retrieved,and 12 were selected.
Basic characteristics of the included literature
Based on the above literature screening process,12 RCTs were finally included,involving a total of 1369 patients,all of whom had the same basic conditions.The specific information is shown in Table 1.

Table 1.Basic information included in the study
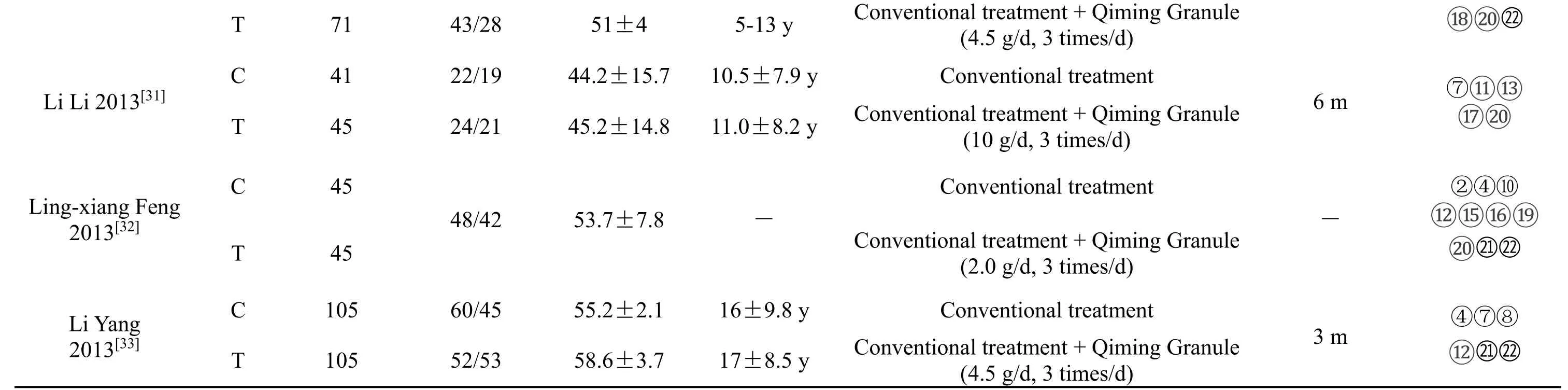
T.Observation group.C.Control group.—.Not mentioned.y:year.m:mouth.Routine treatment.diabetes education,proper exercise,diet control and drug therapy for hypoglycemia,blood pressure control and lipid regulation.① Total efficiency.②FBG.③ BUN.④ Total cholesterol.⑤Edema score.⑥ 24h urine output.⑦ UAER.⑧ HbA1c.⑨ ALB/Cr.⑩ Cys-C.⑪ Cr.⑫ Triglycerides.⑬ Urine α1 microglobulin.⑭ 2h PG.⑮ Apolipoprotein A1.⑯ Apolipoprotein B.⑰ Cr clearance.⑱ ALB.⑲ 24h Urinary protein quantification.⑳ β2 microglobulin.㉑ HDL.㉒LDL.
Quality evaluation of the included literature
We used the "bias risk assessment tool" recommended by the Cochrane Collaboration Network to evaluate the quality of the included literatures.If the specific randomization scheme was described,it was evaluated as "low risk",If not described,it was evaluated as"unclear risk".The included studies did not mention the allocation concealment and blind method,it was evaluated as "unclear risk".All literatures had clear outcome indicators and were evaluated as "low risk".All studies had no repeated or published bias and were evaluated as "low risk".Other biases were unknown and were evaluated as "unclear risk".The specific information is shown in Figure 2.
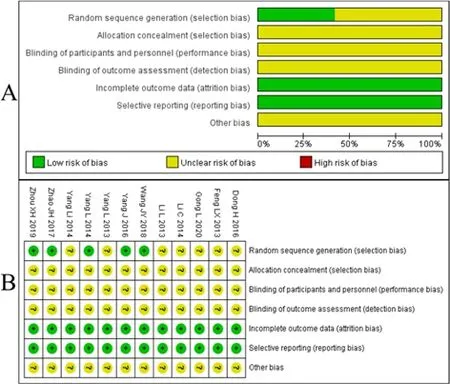
Figure2.Bias risk assessment of included studies.A,overall bias risk chart for included studies;B,Specific bias risk diagrams for included studies.
Meta-analysis
Total effective rateFour articles reported the effect of two treatment regimens on the total effective rate[21,24-25,27],including 440 patients.The results showed that there was homogeneity among the studies(P= 0.84,I2= 0%).The fixed effect model was used.meta-analysis results showed that the total clinical effective rate of the observation group was higher than that of the control group,with statistically significant difference[RR = 1.24,95% CI(1.12,1.36),P=0.000<0.05].As shown in Figure 3A.
UAERSeven studies have reported the effect of two treatment regimens on the UAER[23-25,27-28,31,33],including a total of 863 patients.The results showed that there was heterogeneity among the studies(P=0.000<0.05,I2= 97.8%).The random effect model was used.Meta-analysis results showed that UAER in the observation group was lower than that in the control group,the difference was statistically significant[SMD= -2.268,95% CI(-3.86,-1.50),P= 0.000<0.05].As shown in Figure 3B.
CrSix studies[22-23,26-27,29,31]showed changes in creatinine levels before and after treatment,involving 582 patients.The results showed that there was heterogeneity among the studies(P= 0.000<0.05,I2=76.6%).The random effect model was used.Metaanalysis results showed that the creatinine level of the observation group was lower than that of the control group,the difference was statistically significant[SMD= -1.46,95% CI(-1.84,-1.08),P= 0.000<0.05].As shown in Figure 3C.
BUNBUN levels have been reported in five studies[22-23,26-27,29]involving 496 patients.The results showed that there was heterogeneity among the studies(P= 0.000<0.05,I2= 96.5%).Meta-analysis using random effects model showed that there was a statistically significant difference in treatment effect between the two groups[SMD = -2.61,95% CI(-3.85,-1.36),P= 0.000<0.05].As shown in Figure 3D.

FBGChanges in fasting blood glucose before and after treatment were reported in nine literatures[22-28,30,32],and 870 patients were included.The results showed that there was heterogeneity among the studies(P<0.007,I2= 97.0%).Random-effect model was used for meta-analysis,and the results showed that compared with the control group,there was a statistical difference in fasting blood glucose in the observation group[SMD = -1.17,95% CI(-2.03,-0.31),P=0.000<0.05].As shown in Figure 3E.
Cys-C Cystatin C was reported in five literatures[23-25,28,32],involving a total of 723 patients.There was heterogeneity among the studies(P= 0.000<0.05,I2= 96.8%).A random-effect model was used for metaanalysis,and the results showed that compared with the control group,the difference in cystatin C in the observation group was statistically significant[SMD =-3.26,95% CI(-4.65,-1.87),P= 0.000<0.05].As shown in Figure 3F.
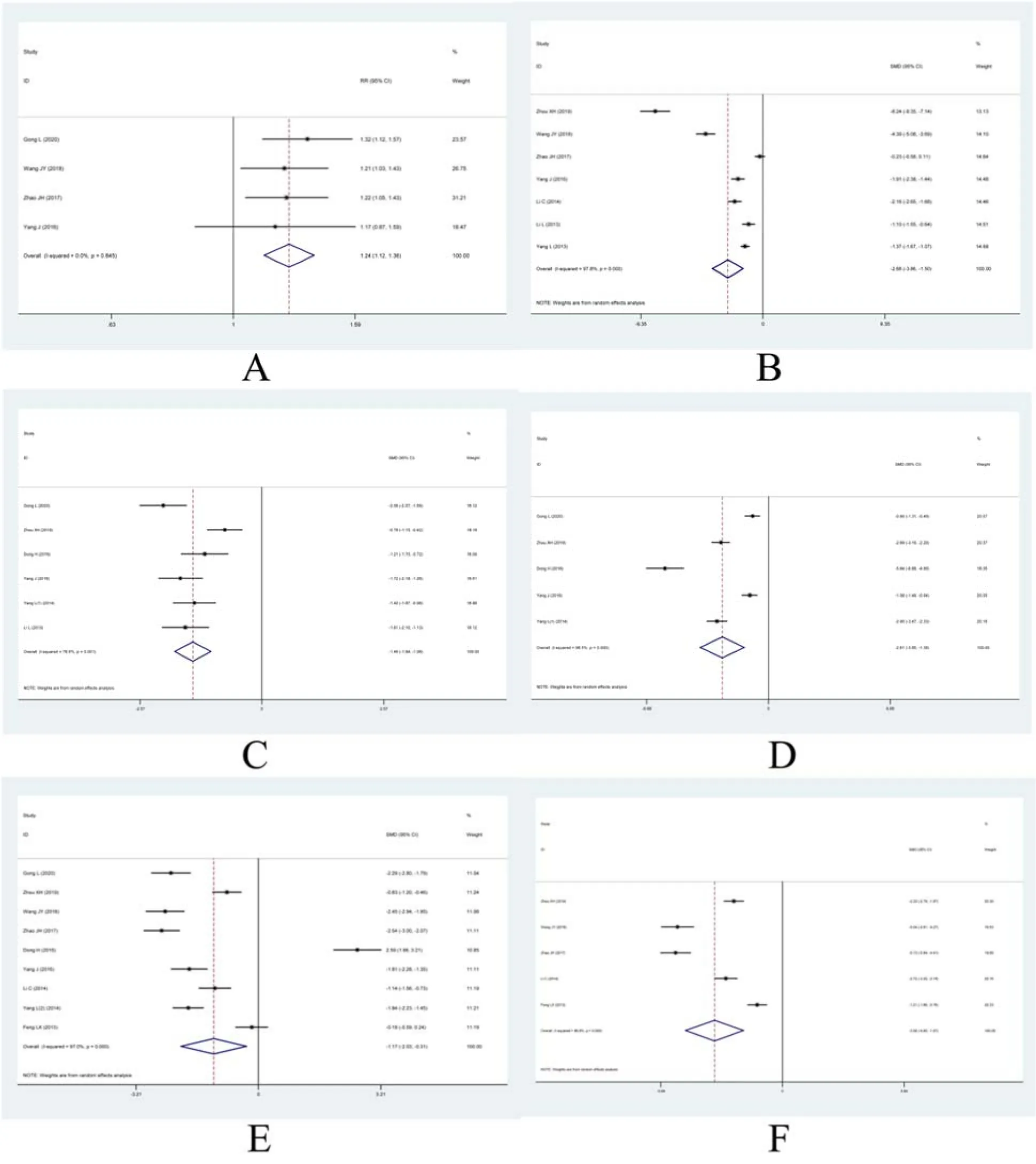
Figure 3.Meta-analysis of each index.A,Total effective rate;B,UAER;C,Cr;D,BUN;E,FBG;F,Cys-C.
Publication bias analysis
The funnel plot was made based on Cr.A total of six literatures were observed for their symmetry.The center line was taken as the axis of symmetry,with the upper and lower asymmetry,indicating that the literatures included in this study had certain publication bias.As shown in Figure 4A.
Sensitivity analysis
STATA software was used for sensitivity analysis[34],and the method of successive exclusion of single studies was adopted,that is,one study was excluded at a time,and the rest were re-performed for Meta analysis.Results showed that all the point estimates fell within the 95% CI of the combined effect size,indicating that the stability of the results of this study reached the standard.As shown in Figure 4B.

Figure 4.Publication bias analysis of clinical total effective rate(A)and sensitivity analysis(B)
Discussion
Clinical effect analysis
DN is a serious long-term disease with a high mortality rate and easy to induce other complications[35-37].At present,its pathogenesis is still uncertain,which may be related to abnormal glucose and lipid metabolism,hemodynamic changes,oxidative stress and other factors.Therefore,its development can be delayed by controlling blood glucose and lipid,adjusting diet,and carrying out antioxidant treatment[38-40].Qiming Granule is made of Astragalus,Pueraria,pollen Typhae,leech,rehmannia and other traditional Chinese medicine.It has the effects of Supplementing Qi,nourishing liver and kidney,activating blood circulation and dredging collaterals.Among them,Astragalus membranaceus and pollen Typhae have antioxidant effect;can enhance the body's immune function;Pueraria can improve hemorheology and inhibit protein glycosylation;rehmannia glutinosa has hypoglycemic effect;leech can significantly delay the kidney injury of diabetic rats[16,41],and play a role in the treatment of DN.
Twelve RCTs involving 1369 patients were included in this study.Among them,four reported the total clinical effective rate,seven reported UAER,six reported Cr,five reported BUN,nine reported FBG and five reported Cys-C.The results showed that Qiming Granule combined with conventional western medicine treatment can significantly increase the clinical total effective rate of patients with diabetic nephropathy,reduce UAER,and improve the levels of Cr,BUN,FBG and Cys-C.
Source analysis of heterogeneity
The results showed that,except for the homogeneity of the total effective rate,the heterogeneity of the other indicators was high,which may be caused by the following reasons:(1)the clinical stage and clinical manifestations of the included studies during the treatment were different,leading to the difference in the treatment results;(2)differences in intervention measures of some studies,such as different dosage,dosage form and course of treatment;(3)Patients in the literature involved had different course of disease and different pathological changes;(4)DN caused by type I or type II diabetes was not mentioned in the included literature,and the pathological mechanism was different;(5)the drugs and doses used in conventional treatment were not clear,which may cause the difference of treatment effect.
Implications for future research
This study has a certain reference value for the clinical treatment of DN.The results show that Qiming Granules adjuvant therapy has better effect than single treatment,suggesting that Qiming Granules can be used in combination in future treatment to enhance the therapeutic effect.However,the quality of the literature included in the study was low,the sample size was small,the dose and course of administration were different,and the adverse reactions and safety of the body after drug administration were not explained.Therefore,it is suggested to further improve the scientificity of clinical trials,strictly control the implementation standards,clarify the details of randomized grouping method and blind method,conduct more large sample RCT studies,and standardize the report writing of randomized controlled trials,so as to obtain more high-quality research reports;at the same time,it is suggested that clinical studies pay more attention to the safety of Qiming Granules and classify the adverse reactions To accumulate the data of adverse reactions/events,so as to further verify the advantages of Qiming Granule in the treatment of diabetic nephropathy,and provide reliable basis for more high-quality evidence-based research in the future.
Clinicians should ask the patient's medical history and medication history in detail when using Qiming Granules for treatment,and those who are allergic to the ingredients in this medicine should use it with caution.Moreover,one of its components,Cassiae seed,is slightly cold,and long-term use will cause diarrhea,so it should be used with caution in patients with diarrhea,hypotension and women in menstrual period and pregnancy[42].In addition,attention should be paid to the interaction between the components,as well as the adverse symptoms that may be caused when combined with large doses of traditional Chinese medicine to nourish Yin and kidney and dilate blood vessels.
Shortcomings and limitations of the research
Shortcomings of this study:(1)The included literatures had certain publication bias,which would affect the results to a certain extent;(2)This study included fewer literatures and small sample size,resulting in poor stability of conclusions;(3)The languages are limited to Chinese and English,and no other languages are involved,which may lead to incomplete retrieval;(4)The specific medication was not specified in the basic treatment,and the course of medication was not described in some literatures,so the subgroup analysis was not conducted in this study.
Limitations of this study:(1)The included literature did not mention adverse reactions and safety after medication,which may be caused by short marketing time and few clinical studies;(2)The quality of the 12 literatures included in this study was low,and some of them did not describe the method of random grouping,and none of them indicated whether the practice was blind.(3)The observation period was short,and the long-term efficacy of the drug could not be determined;(4)There is a large difference in the observed indexes among literatures,and there are few descriptions of the key indicators for DN evaluation,such as the ratio of urinary microalbumin/creatinine,urinary microalbumin level,etc.,which may affect the research results to a certain extent;(5)Some literature did not explain the severity of the disease;(6)The treatment plan of the control group is not the same,which may affect the research results;(7)The 12 articles are all single-center,small-sample studies,lack of multi-center,large-cohort RCT studies,and the conclusions are not stable.
Conclusion
Qiming Granule combined with western medicine conventional treatment was better than conventional treatment,the clinical total effective rate,urinary albumin excretion rate,creatinine,urea nitrogen,fasting blood glucose,Cystatin C were improved,the difference was statistically significant(P<0.05).However,due to the above deficiencies,more highquality,large-scale,double-blind clinical studies are needed to prove it.
 TMR Modern Herbal Medicine2021年3期
TMR Modern Herbal Medicine2021年3期
- TMR Modern Herbal Medicine的其它文章
- Effects and safety of Ding Kun Dan on lVF/lCSl-ET outcomes in patients with predicted poor ovarian response:A multicenter randomized clinical trial
- Combination of XianGui capsule and LCZ696 inhibits doxorubicininduced heart failure in mice
- Pharmaceutical care of a patient with antibiotic-associated encephalopathy
- Progress of ubiquitin-proteasome system in the pathophysiology of heart failure and the intervention of traditional Chinese medicine
- Extraction and antibacterial potential of traditional medicinal plant Cypreus compressus
