GOECP/SEOR radiotherapy guidelines for thymic epithelial tumours
Mikel Rico, Sonia Flamarique, Cristina Casares, Tamara García, Miriam López, Maribel Martínez, Javier Serrano, Manuel Blanco, Raúl Hernanz, Lourdes de Ingunza-Barón, Francisco José Marcos, Felipe Couñago
Mikel Rico, Maribel Martínez, Department of Radiation Oncology, Complejo Hospitalario de Navarra, Pamplona 31008, Navarra, Spain
Mikel Rico, Health Research Institute of Navarre (IdiSNA), Navarra Biomed, Pamplona 31008,Navarra, Spain
Sonia Flamarique, Department of Radiation Oncology, University Hospital Miguel Servet,Zaragoza 50009, Aragón, Spain
Cristina Casares, Francisco José Marcos, Department of Radiation Oncology, University Hospital of Caceres, Cáceres 10004, Extremadura, Spain
Tamara García, Department of Radiation Oncology, Hospital Universitario de Fuenlabrada,Fuenlabrada 28942, Madrid, Spain
Miriam López, Department of Radiation Oncology, Hospital Clínico Universitario Lozano Blesa,Zaragoza 50009, Aragón, Spain
Javier Serrano, Department of Radiation Oncology, Clínica Universidad de Navarra, Madrid 28027, Spain
Manuel Blanco, Department of Radiation Oncology, Hospital Universitario Torrecárdenas,Almería 04009, Andalucía, Spain
Raúl Hernanz, Department of Radiation Oncology, Hospital Universitario Ramón y Cajal,Madrid 28034, Spain
Lourdes de Ingunza-Barón, Department of Radiation Oncology, Hospital Universitario Puerta del Mar, Cádiz 11009, Andalucía, Spain
Felipe Couñago, Department of Radiation Oncology, Hospital Universitario Quirónsalud Madrid, Hospital La Luz, Universidad Europea de Madrid, Madrid 28223, Spain
Abstract Thymic epithelial tumours (TET) are rare, heterogeneous neoplasms that range from resectable indolent tumours to aggressive thymic carcinomas with a strong tendency to metastasize. The pathological diagnosis is complex, in part due to the existence of several different classification systems. The evidence base for the management of TETs is scant and mainly based on non-randomised studies and retrospective series. Consequently, the clinical management of TETs tends to be highly heterogenous, which makes it difficult to improve the evidence level. The role of technological advances in the field of radiotherapy and new systemic therapies in the treatment of TETs has received little attention to date. In the present clinical guidelines, developed by the GOECP/SEOR, we review recent developments in the diagnosis and classification of TETs. We also present a consensus-based therapeutic strategy for each disease stage that takes into consideration the best available evidence. These guidelines focus primarily on the role of radiotherapy, including recent advances, in the management of TETs. The main aim of this document is to promote the standardisation of clinical practice and lay the foundations for future studies to clarify the main unresolved questions related to the optimal management of TET.
Key Words: Targeted therapies; Thymic epithelial tumors; Radiotherapy; Reirradiation;Guidelines; Chemotherapy
INTRODUCTION
Thymic epithelial tumours (TET) are a group of rare, heterogeneous thoracic tumours originating in the thymus gland. They account for 20% of mediastinal tumours and up to 30% of all anterior mediastinal masses in adults. The thymus gland is composed of two identical lobes involved in the process and maturation of T lymphocytes. At birth,the thymus gland weighs approximately 15 g, reaching its maximum size during puberty (40-45 g). Later, the gland undergoes a physiological involution and atrophies in adulthood, where the gland consists mainly of adipose tissue with scattered lymphocytes, weighing only 5 g in older people. Macroscopic or microscopic thymic tissue remains have been described in other locations between the hyoid bone and the diaphragm. This could be due to variations in the migration of the embryonic endodermal epithelium from the third pharyngeal pouches[1].
The World Health Organization (WHO) classification divides TETs into three histological types: Thymomas, thymic carcinomas, and thymic neuroendocrine tumours[2]. Together, these three types of TETs represent less than 1% of all neoplasms.Thymomas are the most common type of TETs, with an annual incidence ranging from 0.13 to 0.32 casesper100000 people. By contrast, thymic carcinomas, with an annual incidence of 0.02-0.05per100000 people, account for only 10%-15% of TETs[3-5].
Thymomas are usually slow-growing, indolent tumours. The most common presentation is an incidental finding on imaging tests. They tend to be locally invasive.The most common route of dissemination is the pleura, pericardium, and diaphragm;extrathoracic metastases are very rare[6].
Thymic carcinomas are aggressive tumours with a worse prognosis than other TETs.Their behavior pattern is similar to other tumours that share the same histology. Most thymic carcinomas are diagnosed in advanced stages, presenting lymphatic and/or hematogenous metastases in 10% to 30% of cases[7]. Thymic neuroendocrine tumours should be considered a distinct clinical entity and its management is not covered in the present guidelines.
The incidence rate for TETs is similar in men and women. The peak incidence rate occurs in the 6thdecade of life, although the age of presentation may be earlier in patients with myasthenia gravis (MG)[4,8]. Genetic risk factors, such as multiple endocrine neoplasia 1, could influence the development of thymomas and thymic carcinomas[9]. Environmental or infectious factors have not been shown to play a role in the pathogenesis of TETs. However, there does seem to be an association between thymomas and systemic diseases such as MG (the most commonly associated illness),pure red-cell aplasia, hypogammaglobulinemia, and systemic lupus erythematosus.Some hematopoietic cancers (such as leukaemia or diffuse large B-cell lymphoma), as well as other solid tumours (colon, stomach, pancreas, and thyroid) occur more frequently in patients diagnosed with thymoma[10].
METHODOLOGY: LEVELS OF EVIDENCE
These guidelines have been prepared by radiation oncologists with extensive expertise in the management of thoracic tumours. All the physicians in this expert group are members of the GOECP of the SEOR.
These guidelines are based on a review of the most relevant studies published in high-impact, peer-reviewed, indexed journals, together with a review of the clinical guidelines and recommendations produced by the major scientific societies.
The Medline database was searched for relevant systematic reviews, clinical guidelines, and primary studies up to September 2020. Search terms included‘thymoma’, ‘thymic carcinoma’, ‘TET’, ‘thymic neoplasms’, ‘thymectomy’, ‘guidelines’,‘International Thymic Malignancy Interest Group (ITMIG)’, ‘staging’, ‘postoperative radiotherapy (PORT)’, ‘radiation therapy’, ‘chemotherapy’, ‘proton’, ‘recurrent’,‘reirradiation’, ‘targeted therapies’, ‘immunotherapy’.
To define the levels of evidence and degrees of recommendation, we used the classification of the Standard Operating Procedures for authors and templates for the European Society of Medical Oncology (ESMO) Clinical Practice Guidelines and ESMO-Magnitude of Clinical Benefit Scale Scores, adapted from the system of the American Society of Infectious Diseases[11,12].
The levels of evidence and grades of recommendation are defined in Table 1.
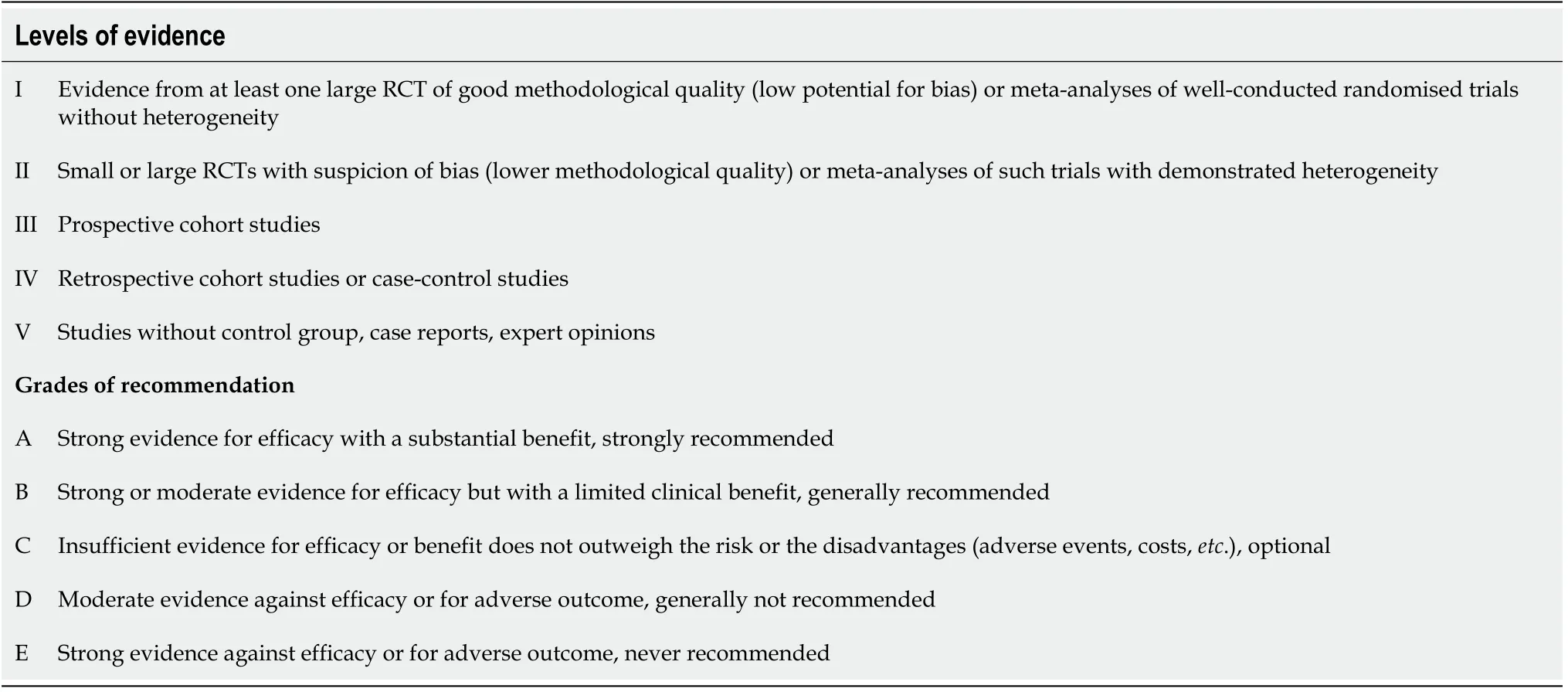
Table 1 Levels of evidence and grades of recommendation
DIAGNOSIS AND STAGING
Diagnosis
The diagnostic process requires an appropriate differential diagnosis to rule out other types of anterior mediastinal masses, including lymphomas, teratomas, seminomas and non-seminomatous germ cells tumours[13]. A complete, meticulous medical history should be performed, including a comprehensive physical examination (especially neurological). The appropriate complementary examinations (imaging studies and blood tests) should also be performed.
TETs can have a variable form of presentation. Approximately one-third of patients are asymptomatic at diagnosis[10]. The most common clinical manifestations include compressive thoracic symptoms (chest pain, cough, dyspnea, dysphagia, superior vena cava syndrome) and/or symptoms derived from related autoimmune disorders,mainly MG[4]. MG is more common in type AB, B1 and B2 thymomas and much less common in thymic carcinomas[14,15]. Approximately 25%-45% of patients diagnosed with thymoma have MG, although only 15%-20% of patients with MG develop thymoma[14,16,17].
The imaging test of choice is chest computed tomography (CT) with intravenous contrast, which enables a complete evaluation of the mediastinum and pleura from the apex to the costodiaphragmatic recesses (IVA)[18]. CT imaging is comparable or superior to magnetic resonance imaging (MRI) for the diagnosis of masses in the anterior mediastinum, except for cystic lesions (IVB)[13,19].
In terms of laboratory tests, a complete blood analysis is advisable, including hemogram and biochemistry, as well as the assessment of biomarkers (beta-human chorionic gonadotropin and alpha-fetoprotein) for the differential diagnosis with germ cell tumours[13].
The use of 18F-fluorodeoxyglucose (FDG) positron-emission tomography-computed tomography (PET/CT) is not recommended for use in routine diagnostic imaging.However, this imaging modality may be useful to complete the imaging study to assess distant disease if dissemination is suspected. 18F-FDG PET-CT may be especially valuable during follow-up if there are doubts about the persistence or recurrence of the disease. A recent meta-analysis highlighted an association between types B3 and C(thymic carcinoma) and increased uptake on 18F-FDG PET-CT, although it should be noted that thymic hyperplasia can also present elevated FDG uptake[20].
Table 2 Lists the most common clinical manifestations of TET, as well as the most characteristic clinical criteria and signs to establish the differential diagnosis with other anterior mediastinal masses[4,10,13,14,16-18,20].

Table 2 Characteristic clinical manifestation of thymomas and differential diagnosis with other causes of mediastinal masses
If the diagnostic tests and imaging studies suggest a high probability of a clinical diagnosis of thymoma, and if the lesion is resectable (no break in continuity with the thyroid gland), then no preoperative biopsy is necessary (IVE). In all other cases, a histological sample must be obtained, preferably by CT-guided core needle biopsy or surgical incisional biopsy by mediastinotomy or thoracoscopy (IVD). Whenever possible, transpleural access should be avoided due to the risk of pleural dissemination[21].
Classification
Thymomas are the most common TET subtype. Pathologically, they are classified into types A, A/B, B1, B2, and B3, in addition to several newer, less frequent subtypes. Use of the term “benign thymoma” is discouraged, as aggressive behaviour can never be completely ruled out. In some cases, it can be highly challenging to accurately determine the pathological type. In the 4thedition of the WHO classification system,updated in 2015, the histological criteria were refined, and parameters based on immunohistochemical characteristics introduced. These changes led to a more reproducible and robust classification, thus facilitating diagnosis of thymomas with an uncertain histology (Table 3)[2,5,22].
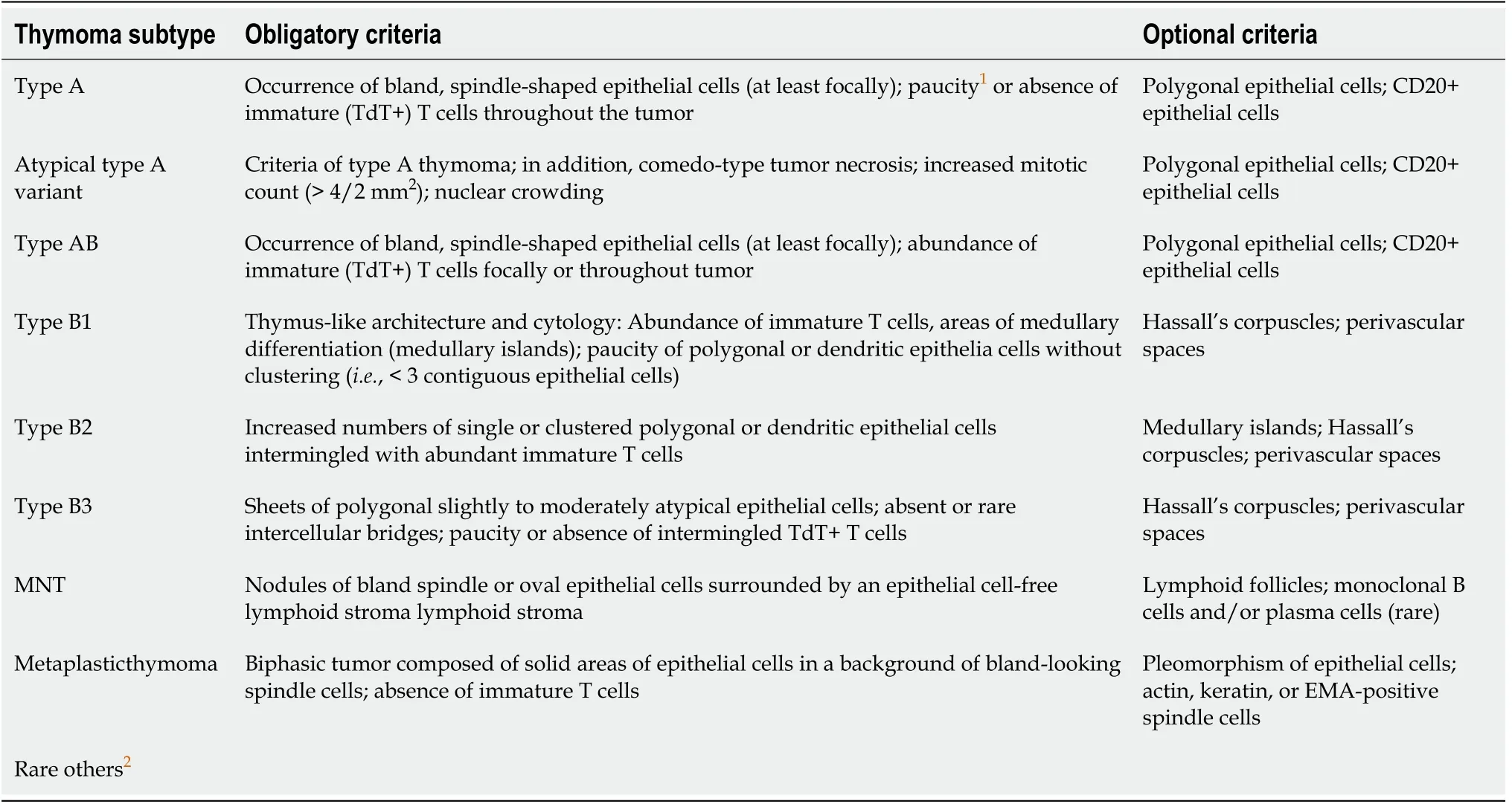
Table 3 Diagnostic criteria of thymomas in the 2015 World Health Organization classification of thymic tumors
Thymic carcinomas have several histological subtypes, as follows: Squamous carcinoma (the most common), basaloid carcinoma, mucoepidermoid carcinoma,lymphoepithelioma-like carcinoma, clear cell carcinoma, sarcomatoid carcinoma,adenocarcinoma, “NUT” carcinoma, and undifferentiated carcinoma[2].
Neuroendocrine tumours include small-cell neuroendocrine carcinomas, large-cell neuroendocrine carcinomas, and carcinoids with neuroendocrine differentiation.Neuroendocrine markers include: Chromogranin-A, synaptophysin, CD-56, and neuronal-specific enolase[2].
Staging
The classification of TETs by clinical or tumour stage is based on the pathological and surgical criteria first proposed in 1978 and published by Masaokaet al[23]in 1981 and modified by Koga in 1994 (Table 4) (IIIA). The Masaoka-Koga classification system is the most widely used as it correlates pathologic and surgical findings with overall survival (OS)[23,24].

Table 4 Clinical stage of thymic epithelial tumours according to Masaoka-Koga
The International Association for the Study of Lung Cancer (IASLC), together with the ITMIG, jointly developed a tumor-node-metastasis (TNM) classification system based on an international retrospective analysis of more than 10000 cases (Table 5)[25].This classification system emphasises the degree of invasion of adjacent structures to determine levels of involvement. This system is highly useful to assess tumour resectability. Levels T1-3 indicate invasion of structures susceptible to surgical resection, while level T4 indicates the involvement of unresectable structures. In addition, this classification system deemphasises features not considered clinically relevant, such as whether the tumour is encapsulated or not[26].

Table 5 Clinical stage of thymic epithelial tumours: International Association for the Study of Lung Cancer/International Thymic Malignancy Interest Group tumor-node-metastasis
The IASLC-ITMIG system has been incorporated into the 8thedition of the American Joint Committee on Cancer-TNM classification as the official classification system for TETs, offering a universal, standardised system with a consistent nomenclature. As this system becomes more widely used, it should further improve our understanding of this disease[26-30].
TREATMENT OF THYMOMAS
The management of TETs is complex and not without controversy, mainly due to the rarity and heterogeneous nature of these tumours. In addition, the recent introduction of the new staging system (the aforementioned TNM developed by the IASLC/ITMIG)may generate confusion when comparing studies that use different staging criteria[29,30]. The Masaoka-Koga stage classification system remains the most widelyused system and it has been employed for this guidelines[23,24,26].
Numerous clinical guidelines and recommendations from expert working groups are available. However, the recommendations in these documents are based mainly on data from non-randomised trials and small, retrospective case series[18,31-33]. Due to the lack of high-level scientific evidence, many recommendations are based exclusively on expert opinion. In this context, all cases should be referred to a multidisciplinary tumour board for clinical decision-making.
Surgery
In early-stage disease, complete surgical resection is achieved in nearly all cases, with negligible mortality rates. Consequently, surgery is the treatment of choice for stages I,IIa, and IIb (IVA).
The aim of surgical treatment should be complete resection of the tumour, the residual thymus, and the perithymic fat (IVC). In the case of thymomas, unlike thymic carcinomas, thymectomy should not include systematic lymphadenectomy due to the minimal risk of nodal metastasis. Nodal sampling could be considered in more locallyadvanced stages or less favourable histologies, although there is a lack of clearevidence to support this decision.
The standard surgical approach is median sternotomy (IVA). Minimally-invasive techniques, such as video-assisted thoracoscopy or robotic surgery, are not usually recommended due to the lack of mature data in TETs[18,31]. However, in the hands of an expert surgeon, these techniques could be considered in stages I and II provided that complete oncological resection can be assured[34-36].
In stage III disease, the use of minimally-invasive surgery is discouraged due to the lack of long-term data and the difficulty of achieving complete resection in cases that require resection of the pericardium, phrenic nerve, pleura, lung parenchyma, and even vascular structures. During thymectomy, the pleural surface must be carefully checked for the presence of metastatic implants, which, if present, should be resected if possible[18,31].
In the case of extensive tumours with a high risk of close surgical margins or residual tumour, the placement of surgical clips to guide PORT is highly recommended.
Radiotherapy
Adjuvant PORT is not indicated in completely resected early-stage tumours (IIE). A key trial randomised patients with stage I disease (n= 29) to surgery or surgery plus adjuvant PORT, with significantly better 10-year survival rates in the group treated with surgery alone (92%vs88%)[37].
An analysis by the Surveillance, Epidemiology, and End Results group evaluated the role of adjuvant radiotherapy in 901 patients with thymomas treated with surgical resection between the years 1973 and 2005. The results of that analysis showed that PORT did not provide any clinical benefit in stage I disease: The cause-specific survival rate at 5 years was 98% with surgery alonevs91% in the group that received adjuvant PORT (P= 0.03)[38].
In stage II TETs, there is some controversy between clinical guidelines and the recommendations of expert working groups. The ESMO guidelines recommend considering radiotherapy in patients with unfavourable histologies (e.g., B3), but that recommendation is not included in other guidelines such as the Italian collaborative group for thymic malignancies (TYME) or the recommendations of a Canadian group by Falksonet al[33], who consider that there is insufficient evidence to recommend PORT in stage II disease due to the toxicity risk. Falkson and colleagues recommend discussing the advantages and disadvantages of this option with the patient. However,some groups still recommend considering PORT after complete resection in cases with unfavourable histologies (IVC)[18,32,33].
Several studies have shown that adjuvant radiotherapy provides some survival benefits in stage IIB and III disease, increasing 5-year survival rates from 66% to 76% (P= 0.01)[38]. The value of adjuvant radiotherapy in patients with higher tumour stages may be that higher stages have lower complete resection (R0) rates, with R0 rates of 47% in stage III disease and 26% in stage IV disease[39-41].
Adjuvant radiotherapy is indicated in most patients who undergo resection for stage IIB, III, and IV thymoma. However, this indication is not absolute as the evidence to support this approach is limited and the reported results are sometimes contradictory[38,42-47].
Therapeutic recommendations
Below we provide treatment recommendations for thymomas according to disease stage using the Masaoka-Koga classification shown in Figures 1 and 2.

Figure 1 Therapeutic algorithm for resectable thymomas. PORT: Postoperative radiotherapy; ADJ ChT: Adjuvant Chemotherapy.

Figure 2 Therapeutic algorithm for unresectable thymomas. ChT: Chemotherapy; RT: Radiotherapy; CRT: Chemoradiotherapy. PORT: Postoperative radiotherapy.
Stage I:Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): No adjuvant treatment (IIE)[42,43].
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage IIA:Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): A-B2: No adjuvant treatment (IVC); B3: Consider PORT(45-50 Gy) (IVC).
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage IIB:Biopsy not indicated (IVE).
The treatment of choice is surgical resection (IVA).
Complete resection (R0): A-B1: No adjuvant treatment (IVC); B2-B3/pericardium+:Consider PORT (45-50 Gy) (IVC).
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage III-IVA:Resectable tumour. The treatment of choice is surgical resection (IVA).After surgery, administer PORT (IVB). The recommended dose is 45-50 Gy for R0,with a boost to 54 Gy for R1 or 60-70 Gy for R2[38,48-51].
Unresectable tumour. A biopsy should be performed to confirm the diagnosis of thymoma and to rule out thymic carcinoma. Initiate anthracycline/cisplatin-based induction chemotherapy (ChT). After ChT, re-evaluate tumour resectability with imaging tests[48-51].
If the tumour is resectable after ChT: Surgical resection (IIIA) + PORT (IVB). The recommended dose is 45-50 Gy for R0; 54 Gy for R1; 60-70 Gy for R2. In cases of R2,assess concomitant platinum/etoposide-based chemoradiotherapy (CRT) (IVB).
If the tumour is unresectable after ChT: Radical radiotherapy (60-70 Gy) with or without concomitant platinum/etoposide-based ChT (IVB)[18].
Stage IVB:The treatment of choice is chemotherapy (IIIA).
If the patient is in good general condition [performance status (PS), 0-1], the use of anthracycline-based ChT regimens is recommended.
The most common regimen is capecitabine (cyclophosphamide + doxorubicin +cisplatin) for a maximum of 6 cycles (IIIB).
Imaging re-evaluation (using the same imaging tests used for diagnosis) is recommended mid-treatment (VA)[52].
In allergic patients or patients with poor PS, carboplatin-paclitaxel ChT could be considered[53].
If the tumour is resectable after ChT, assess surgery and PORT, or radical radiotherapy (IVC).
Debulking surgery is not recommended[32].
In advanced stages, palliative radiotherapy for pain relief or decompressive radiotherapy could be considered in cases with severe vascular involvement.
TREATMENT OF THYMIC CARCINOMA
Thymic carcinoma is a very rare but aggressive tumour commonly diagnosed in advanced stages. These tumours have the capacity to metastasize to the lymph nodes and even to extrathoracic regions[54,55]. Thymic carcinoma has a worse prognosis than thymoma, mainly related to tumour resectability and stage. The 5-year survival rates are 90% in stage I-II disease and 28% in stages III-IV[56].
Thymic carcinoma differs from thymoma in terms of the histologic, immunohistochemical and genetic characteristics. The new WHO classification clearly identifies thymic carcinoma as a distinct entity. Determination of the immunohistochemical parameters is often required for the differential diagnosis[5]. It is important to differentiate thymic carcinomas from primary squamous cell lung cancers that have metastasized to the thymus, since these tumours sometimes have histological characteristics that are similar to those of thymic carcinoma[57].
As with thymomas, clinical staging of thymic carcinoma is essential to determine the appropriate treatment[33]. However, given the rarity of this tumour and the lack of data from prospective, randomised studies to define the optimal strategy, there is no consensus regarding the ideal therapeutic approach. Given the aggressiveness of these tumours, multimodal treatment involving multidisciplinary teams is common.
Complete surgical resection is an independent prognostic factor and a key treatment recommendation (IVA)[58]. ITMIG/IASLC guidelines define complete resection as complete resection of the tumour, residual thymus, the surrounding fat, and lymphadenectomy[59,60].
Therapeutic recommendations
Below we provide treatment recommendations for thymic carcinoma according to disease stage using the Masaoka-Koga classification (Figures 3 and 4).
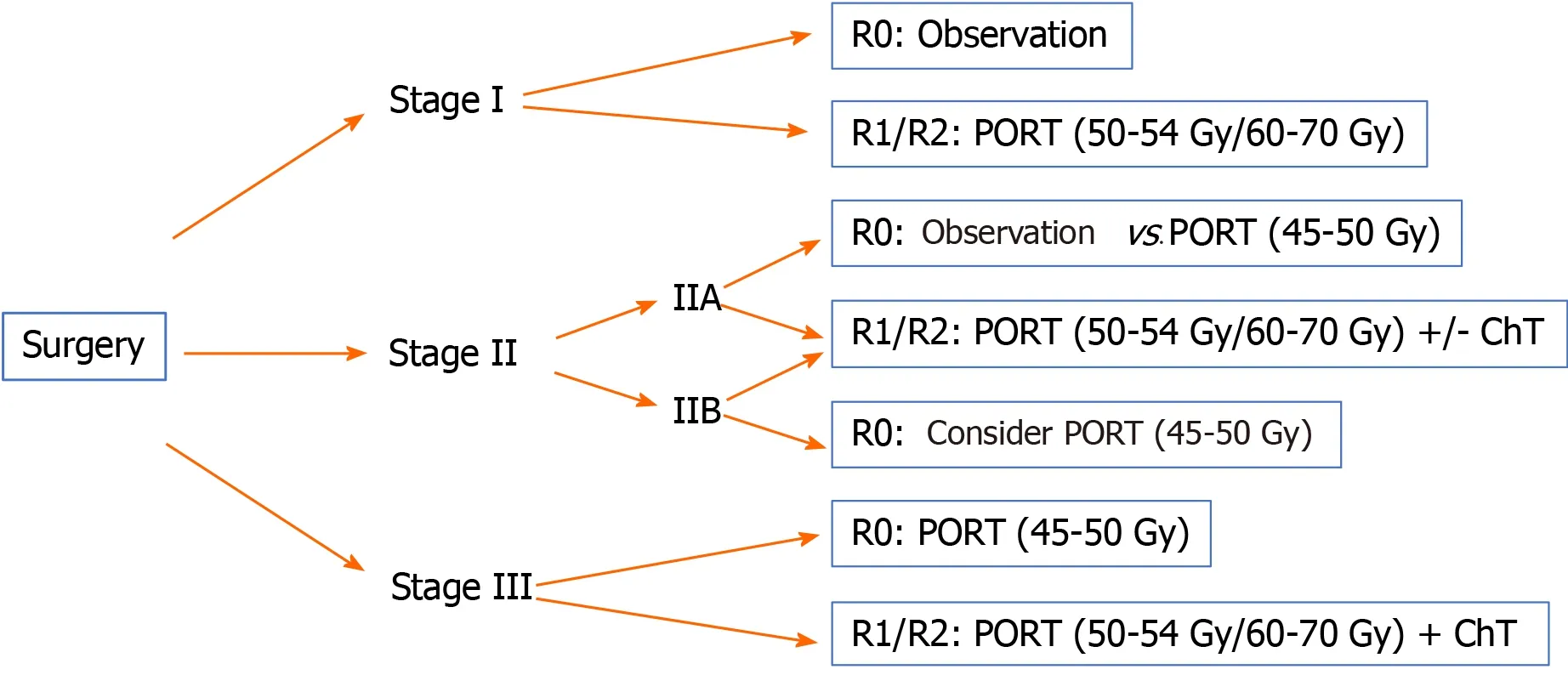
Figure 3 Therapeutic algorithm for resectable thymic carcinomas. PORT: Postoperative radiotherapy; ChT: Chemotherapy.

Figure 4 Therapeutic algorithm for unresectable thymic carcinomas. PORT: Postoperative radiotherapy; ChT: Chemotherapy; RT: Radiotherapy.
Stage I:Surgical resection is the treatment of choice (IVA).
Complete resection (R0): No additional treatment required.
Incomplete resection (R1): PORT (50-54 Gy) (IVB).
Stage II:Surgical resection is the treatment of choice (IVA).
Complete resection (R0): Consider PORT (45-50 Gy), especially for stage IIB (IVB).
Incomplete resection (R1): PORT (50-54 Gy) (IVB). Postoperative ChT should be considered.
Stage III-IVA:Resectable tumour. Surgical resection (IVA) followed by PORT (IB).
The recommended doses are 45-50 Gy, 54 Gy, and 60-70 Gy for R0, R1 and R2 resections, respectively.
In R0 resections, postoperative ChT is optional; in R1-R2 cases, postoperative ChT is recommended[61].
Unresectable tumour. The diagnosis of thymic carcinoma should be confirmed by biopsy. 2-4 cycles of induction ChT followed by imaging studies to reassess tumour resectability are recommended[48-51]. Various ChT schemes are available based on cisplatin (IIIA) combined with other agents such as etoposide (IIIB), doxorubicin, or cyclophosphamide[18,34].
If the tumour is resectable after ChT: Surgery (IIIA) followed by PORT (in R0, 45-50 Gy; in R1, 54 Gy) (IVB). Consider postoperative ChT.
If the tumour is unresectable after ChT or the resection is classified as R2, the treatment indication is radiotherapy (60-70 Gy) with or without concomitant ChT(IVB)[18].
Stage IVB:The treatment of choice is cisplatin-based ChT (IIIA). Various ChT regimens are available[18]. Table 6 shows the most common schemes.

Table 6 Most commonly used chemotherapy regimens for thymic carcinoma
Due to advances in the molecular characterisation of TETs in recent years, our understanding of the tumour response to targeted agents has improved substantially,especially in thymic carcinomas. Several signalling pathways have been evaluated as potential targets, including KIT, vascular endothelial growth factor receptor (VEGFR),and mammalian target of rapamycin (mTOR).
In chemotherapy-refractory thymic carcinoma, the targeted agent imatinib may be a treatment option, although this drug is not recommended in patients without a KIT mutation (IVB)[62]. Sunitinib may also be an alternative in refractory tumours,regardless of KIT status (IIIA)[63]. Everolimus can also be considered in treatmentrefractory cases (IIIB)[64].
RADIOTHERAPEUTIC TREATMENT OF TET
Standardisation of the treatment approach is important to enable the comparison of results among different centres, which is why it is important to reach a consensus with regard to target volumes, techniques, and treatment schemes. In turn, this will allow us to develop clinical-therapeutic guidelines for TETs.
In terms of the radiotherapeutic treatment of TETs, various different approaches are used, ranging from preoperative radiotherapy alone, or radiotherapy combined with ChT, and PORT (the most common approach), in which the surgical margin status (R0,R1 or R2) is a crucial factor to select the radiation dose or the need for radical treatment, in which radiotherapy will be administered alone or concomitantly with ChT as the only treatment for the thymic tumour.
Patient positioning
The patients should preferably be immobilized in the supine position with the neck extended and the arms above the head. A contrast-enhanced CT should be performed to help identify neighbouring structures and to assess postsurgical changes (CT slice thickness: 3-5 mm).
If four-dimensional (4D)-CT is available, acquisition of the full respiratory cycle will improve volume delimitation by determining tumour motion with respiration(internal margin), thus allowing for smaller margins than conventional CT[65]. PET/CT fusion (18F-FDG PET/CT) may be useful in certain circumstances to improve volume definition[66].
Volume definition
Gross tumour volume (GTV): The GTV, if present, is the identifiable macroscopic disease.
Clinical target volume (CTV): Defined as the GTV plus microscopic disease. The incorporation of preoperative imaging tests, as well as pathological findings for the surgical margins, will help define these limits.
Internal target volume (ITV): This is obtained by adding the internal motion of the lesion (internal margin) to the CTV.
Planning target volume (PTV): Defined as the ITV plus systematic uncertainties for each treatment unit (set-up margin).
Depending on the planning technique, daily verification, patient imaging control(IGRT), tumour location, and differences in routine clinical practice, the margins may vary, as follows[67]:
GTV to CTV: 0.5 to 1 cm.
CTV to PTV (conventional CT): 1 to 1.5 cm.
ITV to PTV (4D-CT without IGRT): 0.5 to 1 cm.
ITV to PTV (4D-CT and IGRT): 0.5 cm.
Organs at risk and dose limits
Given the thoracic localization of TETs, it is essential to pay careful attention to the radiation doses to the surrounding organs at risks (OARs), particularly the lungs,esophagus, heart, spinal cord, trachea, and great vessels. The proposed dose limits for the main OARs are shown in Table 7[68].

Table 7 Constraints to the main organs
Irradiation techniques
When evaluating the role of radiotherapy in TETs, it is important to consider that most of the available evidence is based on large retrospective series and multicentre databases. A common limitation of these studies is high variability in diagnostic and treatment standards — including radiotherapy — which makes comparative analyses difficult. In addition, rapid technological progress in the field of radiotherapy has significantly improved treatment precision while simultaneously reducing the associated toxicity. However, the clinical impact of these advances in the treatment of TETs has received scant attention to date[69]. Nevertheless, given the mediastinal location of these tumours, experience and lessons learned from treating other thoracic cancers, such as lung cancer or malignant pleural mesothelioma, can likely be extrapolated to TETs.
Intensity-modulated radiation therapy (IMRT) is the standard radiotherapy modality today due to improved dose conformity to the target volume and lower doses to healthy tissues. If IMRT is unavailable, 3D conformal radiotherapy (3D-CRT)is an acceptable alternative[70]. A retrospective study compared IMRT/3D-CRT to conventional RT found that better survival outcomes in patients treated with IMRT/3D-CRT due to the smaller radiation field, increased precision, and lower doses to healthy organs[71].
The combination of IMRT and 4D-CT simulation could reduce radiation-induced toxicity, with better local control and OS[72,73]. Given the location of these tumours in the thorax, coordinated breathing control techniques could be beneficial in selected patients[65].
Due to the proximity of critical organs (spinal cord, heart, lungs, and esophagus),the risk of toxicity secondary to treatment — especially cardiovascular toxicity — must be carefully considered. One study assessed a consecutive cohort of 74 patients with thymoma treated with surgery plus adjuvant radiotherapy, finding that cardiovascular disease (CVD) was the primary cause of non-cancer mortality, accounting for 50% of deaths. The median time from treatment to CVD diagnosis was 101 mo. In that study,the mean heart dose was an independent risk factor for CVD[74].
It is important to underscore the increased risk of second malignant tumours(particularly esophageal and lung cancer, non-Hodgkin's lymphoma, and soft tissuesarcomas) in patients with thymoma. These second tumours may be related to the combination of immune dysregulation, and genetic or environmental factors[75-77]. This association has important implications, as irradiation of healthy tissues can promote the development of second tumours due to genetic damage; in addition, if chest reirradiation is required, there is an increased risk of toxicity due to overlapping fields.
IMRT improves dose conformity and distribution in the target volume; however,due to the physical properties of photons, it may not be possible to adequately reduce the dose to the OARs. This has led some authors to propose proton therapy as an alternative to limit the radiation dose to healthy tissues; predictive models suggest that this approach would reduce the risk of acute and late toxicity and second neoplasms[78-81].
A study published in 2018 performed a dosimetric comparison for advanced radiotherapy techniques in 10 surgically-treated patients with thymoma. Dosimetric plans were prepared for 3D-CRT, volumetric modulated arc therapy (VMAT),tomotherapy, proton therapy, and carbon ion therapy. The results showed that heavy particle radiotherapy significantly reduced radiation doses to the OARs compared to photon radiotherapy. In terms of target coverage, the best results were achieved with tomotherapy, although this also resulted in higher low doses to the OARs. The dosimetric parameters for VMAT were superior to those obtained with 3D-CRT[82].Another study, published in 2019, compared the dosimetric characteristics of VMAT to proton therapy in 20 cases. While the two techniques yielded similar results in terms of coverage, doses to healthy organs were lower with proton therapy[81].
Despite the potential advantages of proton radiotherapy, the clinical evidence to support this approach in TETs is scant. However, one prospective series of 27 patients treated with proton therapy reported a local control rate of 100% and a 3-year OS rate of 94% at a median follow-up of 2 years. Toxicity was mild to moderate with no grade 3 or higher events[83]. Therefore, although proton beam radiation therapy has a clear dosimetric benefit, and theoretical models support a secondary clinical benefit, longterm prospective studies are still needed to compare this radiotherapy modality to other advanced radiotherapy techniques, such as tomotherapy and VMAT.
RECURRENT DISEASE
Disease recurrence is not uncommon in TETs, ranging from 7.5% to 36% of cases.However, in cases with complete surgical resection (R0), the recurrence rate is less than 15%[45,84]. In stage I and II disease, the relapse rate is only 5%-10%; by contrast, in more advanced stages, the recurrence rate is approximately 30% and some cases series have reported relapse rates > 50%[45,85].
Histology is a prognostic factor for recurrence, which can be as high as 28.6% in type B3. Thymic carcinoma is more aggressive than thymoma, with relapse rates greater than 50%[61]. Tumour size is another possible prognostic factor[86]. The mean time to recurrence is 5.5 years (range, 2 to 16)[84].
Locoregional recurrence is the most common pattern of recurrence after complete surgical resection, although thymic carcinomas tend to progress distantly[84,85,87]. There is some evidence suggesting that some thymomas can become more aggressive subtypes when they recur[87].
Treatment of recurrent disease
The management of recurrent disease in TETs should be individualized based on the resectability of the recurrent lesion(s), prior treatments received, and time interval since treatment. These patients should be included in clinical trials whenever possible.
Recurrences should be managed according to the same strategy used to treat the primary tumour (IVA). Thus, surgical salvage with complete resection is the treatment of choice for both local and regional recurrences (isolated pleural metastases)[33,88]. The available data indicate that surgery is superior to non-surgical treatment in resectable recurrences, with 10-year survival rates of around 65% after a second resection[85,87]. In patients with multiple pleural metastases, neoadjuvant ChT followed by surgical resection is worth considering when feasible[33].
In patients not previously treated with radiotherapy, the indication is the same as in newly-diagnosed tumours: PORT in patients with subtotal resections or stage III/IVA disease with complete resection (consider in stage II). Radiotherapy can be administered as definitive treatment for unresectable tumours or inoperable cases,potentially with concomitant ChT (IIIA)[18].
Historically, thoracic reirradiation has been limited to palliative doses due to the potential for toxicity related to the proximity of OARs. Cardiac toxicity merits special attention given the tumour localization, patient age, and the frequent use of cardiotoxic chemotherapy agents. However, reirradiation is increasingly being considered due to continuous technological advances in radiotherapy, which allows for increasingly conformal radiation dose delivery.
When considering reirradiation, it is essential to evaluate the patient’s clinical status, prior radiotherapy treatments (total dose, fractionation, treatment volumes,technique used, time elapsed since the initial radiation therapy) and other therapeutic alternatives. The risks and benefits of reirradiation are often equally balanced due to the potential toxicity of the dose and treatment volumes.
The available scientific evidence on reirradiation in thymic cancer is scant, although some studies have reported acceptable tolerance[89,90]. Most of the studies involving chest reirradiation have been performed in patients with lung cancer treated with 3DCRT, IMRT, proton therapy, or stereotactic body radiation therapy. In general, radical doses achieve better disease control than palliative doses, and the best results are obtained in patients with good PS, small reirradiation volumes, recurrence outside the original radiotherapy field, and a longer interval between the two radiotherapeutic treatments[91-95]. By extrapolating these experiences to thymic tumours (in the absence of specific studies in TETs), it appears that reirradiation is safe and feasible in wellselected patients, as long as the appropriate technology is available, and the teams are experienced (IVB).
SYSTEMIC TREATMENT OF ADVANCED DISEASE
Chemotherapy
ChT is typically used to treat recurrent disease that is not suitable for surgical salvage,or to treat advanced metastatic disease. Generally, anthracycline/cisplatin-based chemotherapy is the regimen of choice. However, other first-line regimens include vincristine or cyclophosphamide. If the first-line treatment is effective, it can be readministered in patients who develop disease progression (IVB), although patients should be closely monitored for cardiac toxicity due to the accumulated dose of anthracyclines[96]. In thymic carcinomas, carboplatin-paclitaxel ChT is the most widelyaccepted first-line chemotherapy approach[31].
The use of successive lines of treatment in patients with progressive disease can prolong progression-free survival. Multiple ChT regimens are accepted, although the most common are carboplatin-paclitaxel, platinum-etoposide, capecitabinegemcitabine, pemetrexed-etoposide, and 5-FU-leucovorin.
Patients not considered candidates for ChT but who have a positive octreoscan may benefit from treatment with octreotide, with or without prednisone (IIB)[18].
Targeted therapies
In recent years, targeted therapies have been incorporated into the therapeutic arsenal(IIIB)[97]. The identification of molecular alterations in various pathways—KIT signalling, VEGFR, and mTOR—has opened opportunities for the off-label use of targeted therapies in treatment-refractory thymic tumours.
The c-KIT receptor is overexpressed in 80% of thymic carcinomas, although it is mutated in only 9%. The efficacy of tyrosine inhibitors (TKIs) such as imatinib,sunitinib, and sorafenib has been evaluated in numerous studies. Some phase II trials involving imatinib in patients with non-mutated B3 thymoma and thymic carcinoma have found no response (IIIE)[62]. However, other studies have shown that treatment with TKIs in patients with mutated c-KIT can achieve disease control[98].
Sunitinib and sorafenib both act on the VEGFR receptor, with activity in tumour angiogenesis, and both have demonstrated efficacy in the treatment of TETs,regardless of the c-KIT receptor status[99]. Sunitinib has been evaluated in patients with TETs in two phase II trials. In one trial, which included patients with thymic carcinoma or thymoma, response rates were 26% and 6%, respectively[63]. The second trial (KOSMIC) included only patients with thymic carcinoma, achieving response rates of 22%, although disease stabilization was achieved in 70% (IIIA)[100].
Lenvatinib is a second-line TKI with activity on the VEGFR, fibroblast growth factor and platelet-derived growth factors receptors. In the phase II REMORA trial,lenvatinib significantly improved the response rate (up to 38%). At present, lenvatinib is only administered within clinical trials[101]. Other antiangiogenic agents (anti-VEGFR) such as bevacizumab or aflibercept have demonstrated efficacy in thymic tumours[18].
Zucaliet al[64]conducted a phase II trial in 51 patients to evaluate everolimus, an mTOR inhibitor, reporting a disease control rate of 88%, although this was mainly disease stabilization rather than objective response. However, everolimus has been associated with the emergence of potentially severe pneumonitis. Consequently, more studies are needed to better define the indication for everolimus (IIIB)[64].
Several targeted therapies have shown promising results in patients with thymic tumours, but more studies are needed to better determine the most appropriate treatment type and sequence according to individual pathological and molecular characteristics[18]. Targeted therapies are currently used in clinical trials or as off-label drugs, mainly sunitinib or other TKI/anti-VEGFR agents and everolimus (IIB).
Immunotherapy
Several studies have evaluated the efficacy of anti-PD-1 antibodies, such as pembrolizumab. Giacconeet al[102]conducted a phase II trial involving 41 patients with thymic carcinoma, reporting a response rate of 23%, mainly disease stabilization (53%).However, these treatments induce a strong immune system response and were associated with non-negligible toxicity and potentially serious adverse effects (e.g.,myocarditis, polymyositis, hepatitis, among others) in up to 15% of patients[102].
A study conducted in Korea in patients diagnosed with thymic carcinoma or thymoma reported results that were similar to those described by Giaccone and colleagues. Disease stabilization was achieved in 72% and 54% of patients with thymoma and thymic carcinoma, respectively; however, a high percentage of patients experienced autoimmune side effects[103].
The efficacy of nivolumab was assessed in phase II trial in Japan in 15 patients with thymic carcinoma. Of these, 11 achieved disease stabilization. However, none of the patients showed an objective response, which led to the premature termination of study recruitment[104].
FOLLOW-UP
At present, there are no prospective data on which to establish recommendations regarding the optimal post-treatment follow-up protocols for thymic tumours.European guidelines—based on the expert consensus statements and published data on the cumulative incidence of recurrences and the pattern of recurrence—recommend chest CT as the imaging technique of choice for follow-up purposes. The first CT image should be obtained 3-4 mo after surgery (VC)[18].
In patients with complete resection of a stage I/II thymoma, an annual CT scan is recommended during the first five years of follow-up and then every two years thereafter. In stage III/IV thymomas, thymic carcinoma, or after an incomplete (R1-2)resection, CT scans should be performed every 4-6 mo during the first two years and annually thereafter. Follow-up CT scans should be performed for 10-15 years after treatment.
The most recent version of the American guidelines recommend follow-up with contrast-enhanced CT every 6-12 mo for the first 2 years followed by annual CT scans for 5 (thymic carcinoma) or 10 years (thymoma). However, these guidelines do not indicate the recommended duration of follow-up. In some cases, the guidelines suggest that MRI may be a useful alternative to CT scans[33].
The emergence of second tumours and autoimmune diseases has been described during follow-up. Patients with clinical MG (or only anti-acetylcholine receptor antibody positivity) have a higher risk of myasthenic crisis in stressful situations or when certain drugs are administered (VA)[18].
CONCLUSION
TET are rare, heterogeneous tumours. The evidence base to support the diagnosis and therapeutic management of this disease is limited. Some recommendations are based mainly on data from retrospective or non-randomised studies, or even expert opinions(due to the lack of evidence). As a result, disease management is often highly heterogeneous. In recent years, several scientific societies and working groups have sought to standardise the management of TETs. The recent development of a universal, standardised classification system with a consistent nomenclature (IASLCITMIG) is expected to reduce variability in the management of these tumours. In addition, new guidelines have been published to provide a clear, schematic approach to treatment. Although these guidelines differ on certain points due to the lack of definitive evidence (e.g., the role of radiotherapy in stage II disease), the overall trend is towards the convergence of recommendations, which should facilitate the performance of multicentre studies and, in turn, raise the level of evidence for the management of TETs.
The present document was developed by the GOECP/SEOR to help unify the management of TET in Spain and to lay the foundations for the creation of a stable working group. We believe that it is crucial to prioritise closer collaboration between centres and the relevant scientific societies in order to carry out prospective studies and randomised clinical trials, particularly to resolve the most controversial aspects of this disease management.
 World Journal of Clinical Oncology2021年4期
World Journal of Clinical Oncology2021年4期
- World Journal of Clinical Oncology的其它文章
- Tongue swelling as a manifestation of tongue metastasis from pulmonary sarcomatoid carcinoma: A case report
- Lenvatinib-induced multiorgan adverse events in Hurthle cell thyroid cancer: A case report
- Hepatocellular carcinoma with biliary and neuroendocrine differentiation: A case report
- Positron emission tomography complete metabolic response as a favorable prog-nostic predictor in esophageal cancer following neoadjuvant chemotherapy with docetaxel/cis-platin/5-fluorouracil
- Cytotoxic CD8+ T cells and tissue resident memory cells in colorectal cancer based on microsatellite instability and BRAF status
- Oncogenic driver mutations in non-small cell lung cancer: Past,present and future
