柑橘果实萜类色素和挥发物生物合成研究进展
李春秀 李勋兰 孙海艳 梁国鲁 向素琼 韩国辉
摘要:柑橘果实的色泽和香气品质主要由果实中的萜类色素和挥发物的种类和含量决定。2-C-甲基-D-赤藓醇-4-磷酸(MEP)和甲羟戊酸(MVA)途径合成共同前体异戊烯焦磷酸和二甲基丙烯焦磷酸,经八氢番茄红素合成酶和萜类合成酶的作用分别进入萜类色素和挥发物的合成代谢。本文介绍了柑橘果实萜类色素的组成及呈色特点、萜类挥发物的组成及香气特点,综述了萜类色素和挥发物的生物合成途径及关键基因、主要的转录调控因子和环境调控因子的研究现状,对该领域未来的研究方向提出了展望,为改善柑橘果实色泽和香气品质提供了参考。
关键词:柑橘;果实;类胡萝卜素;萜类挥发物;萜类色素
中图分类号:S666.01 文献标志码: A
文章编号:1002-1302(2021)12-0020-09
收稿日期:2020-10-17
基金项目:重庆市技术创新与应用发展专项(编号:cstc2019jscx-msxmX0371);重庆市农业发展专项(NKY-2019AB006、NKY-2020AC006);重庆市基础与前沿创新专项(编号:cstc2016jcyjA0046);重庆市特色水果产业技术体系项目[编号:2020(3)03]。
作者简介:李春秀(1996—),女,重庆人,硕士研究生,主要从事果树遗传育种与生物技术研究。E-mail:li1378602852@163.com。
通信作者:韩国辉,男,博士,副研究员,主要从事果树遗传育种与生物技术研究,E-mail:hghui2007@126.com;向素琼,女,博士,研究员,主要从事果树遗传育种与生物技术研究,E-mail:xiangsq@swu.edu.cn。
柑橘包括宽皮柑橘(Citrus reticulata)、甜橙(C. sinensis)、柠檬(C. limon)、柚(C. grandis)和葡萄柚(C. paradisi)等主要栽培类型[1]。柑橘是世界第一大水果,截至2018年,全世界柑橘栽培面积已经达到1 114.39万 hm2,产量达到15 244.88万t,我国柑橘栽培面积达274.96万hm2,产量达到4 190.55万t,均居世界第1位。色泽和香气是决定消费者偏好的重要果实品质因素,柑橘果实具有独特的色香味,色泽由果实中的色素决定,香味取决于大量挥发物质的互作[2]。柑橘果实中的色素有水溶性的酚类色素和脂溶性的萜类色素,酚类色素中除花青素外大多为无色,而花青素仅存在于血橙和紫柚果实中,萜类色素是柑橘果实中主要的呈色色素,使柑橘果实产生从淡黄、橙、粉到深红的颜色特征变化[3-6]。柑橘果实中的挥发物质有萜、醇、醛、酸、酯和烷烃类等,其中萜类挥发物种类和含量最多,是柑橘果实香气的主要贡献者[7-11]。随着物质提取分析技术、基因组学和转录组学的发展,柑橘果实萜类色素和挥发物在生化与分子方面取得较多进展。本文对柑橘果实中2种物质的组成、合成途径及关键基因、主要调控因素进行综述,以期为柑橘果实色泽和香气品质相关研究提供参考。
1 柑橘果实萜类色素和挥发物
柑橘果实中的萜类色素主要为类胡萝卜素,可以分为胡萝卜素和叶黄素2类,胡萝卜素为碳氢化合物,叶黄素中含有多以羟基或环氧基形式存在的氧原子,柑橘果实中胡萝卜素主要有八氢番茄红素、ζ-胡萝卜素、番茄红素、α-胡萝卜素和β-胡萝卜素,叶黄素主要有叶黄质、β-隐黄质、玉米黄素、β-柠乌素和紫黄质[12-14]。除β-柠乌素为C30分子外,其余类胡萝卜素均为8个异戊二烯单位聚合而成的C40分子。在幼果中,类胡萝卜素的颜色被叶绿素掩盖,果实发育后期类胡萝卜素的颜色才开始显现[15]。柑橘成熟果实中积累的类胡萝卜素总量和不同颜色类胡萝卜素成分比例差异使柑橘果实呈现不同色泽[16]。类胡萝卜素的颜色是由于分子中共轭双键系统的出现,其共轭双键数目越多,主要吸光波长越长,共轭双键数多于7时才可呈现颜色,共轭双键的顺反异构也影响类胡萝卜素的颜色,全-反式类胡萝卜素颜色最深,增加顺式双键数量使颜色逐渐变浅,光、热和酸是使反式双键转换为顺式双键的因素[17]。
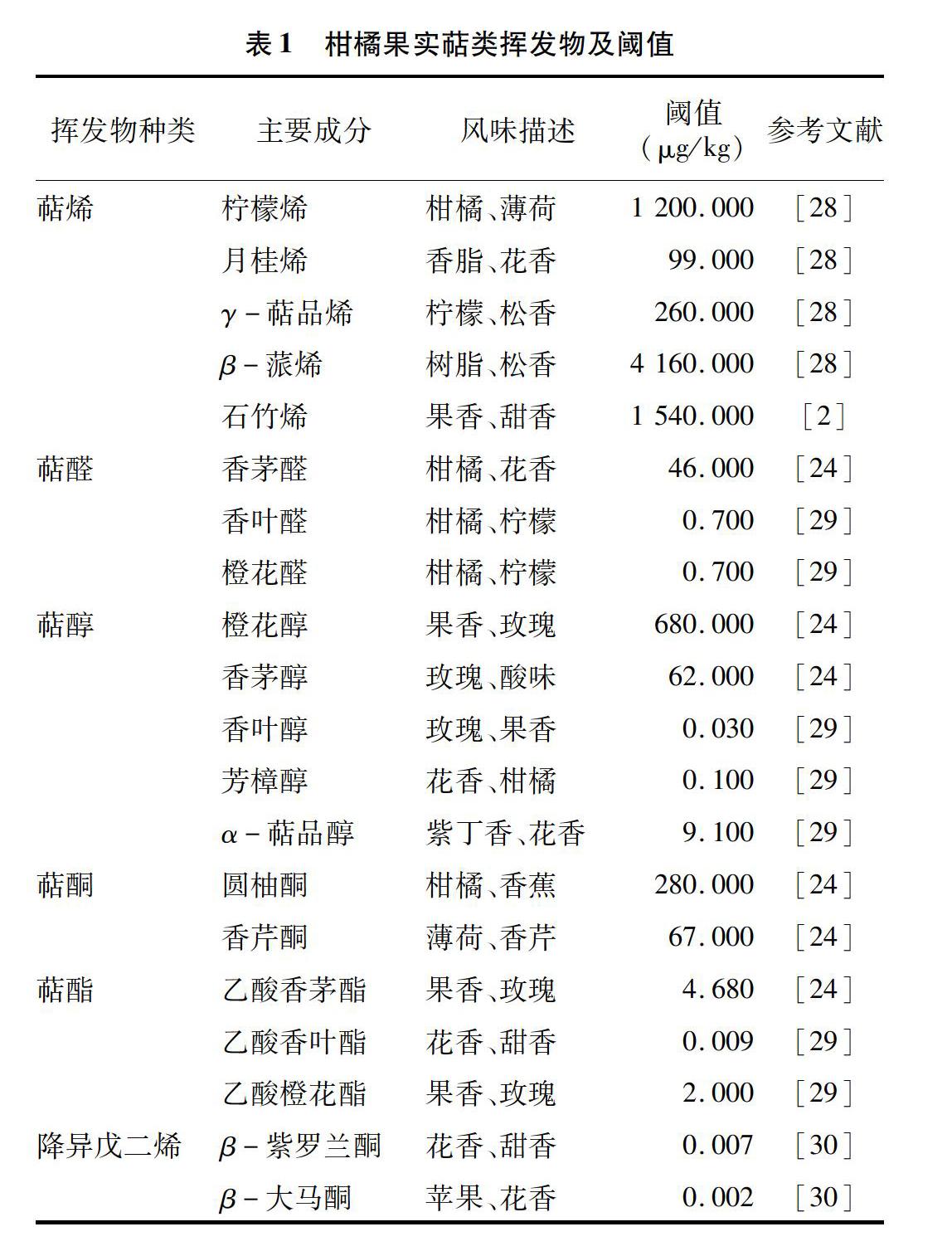
柑橘果实中的萜类挥发物主要包括单萜烯、倍半萜烯及其含氧衍生物(醛、醇、酮、酯)[18]。柠檬烯、月桂烯、γ-萜品烯和β-蒎烯是主要的單萜烯,石竹烯、大根香叶烯、荜澄茄烯、β-榄香烯和瓦伦烯是主要的倍半萜烯,萜烯类约占柑橘果实挥发物总量的90%[11,19]。萜烯类含量虽多,但阈值较高,对柑橘果实香气的直接影响较小,通常只起背景香气的作用,对柑橘果实香气起关键作用的主要是萜类的含氧衍生物[20-22]。含氧衍生物中,萜醛主要是香叶醛和橙花醛,具有柑橘和柠檬香味,是柑橘风味的主要形成物质[23-24];萜醇主要包括芳樟醇、萜品醇、橙花醇和香叶醇,萜酮主要包括香芹酮和圆柚酮,萜醇和萜酮可促进柑橘产生花香、果香和薄荷香味[9];萜酯主要是乙酸橙花酯和乙酸香叶酯,对柑橘的果香和玫瑰香味贡献较大[25]。降异戊二烯是由类胡萝卜素降解的C9~C13的挥发物,也属于萜类,包括β-紫罗兰酮、β-大马酮、香叶基丙酮和β-环柠檬醛等,这些物质浓度通常很低,但阈值也极低,对果实整体香气的感知有强烈的影响(表1)[26-27]。
2 柑橘果实萜类色素和挥发物的合成
2.1 柑橘果实萜类色素和挥发物的合成途径
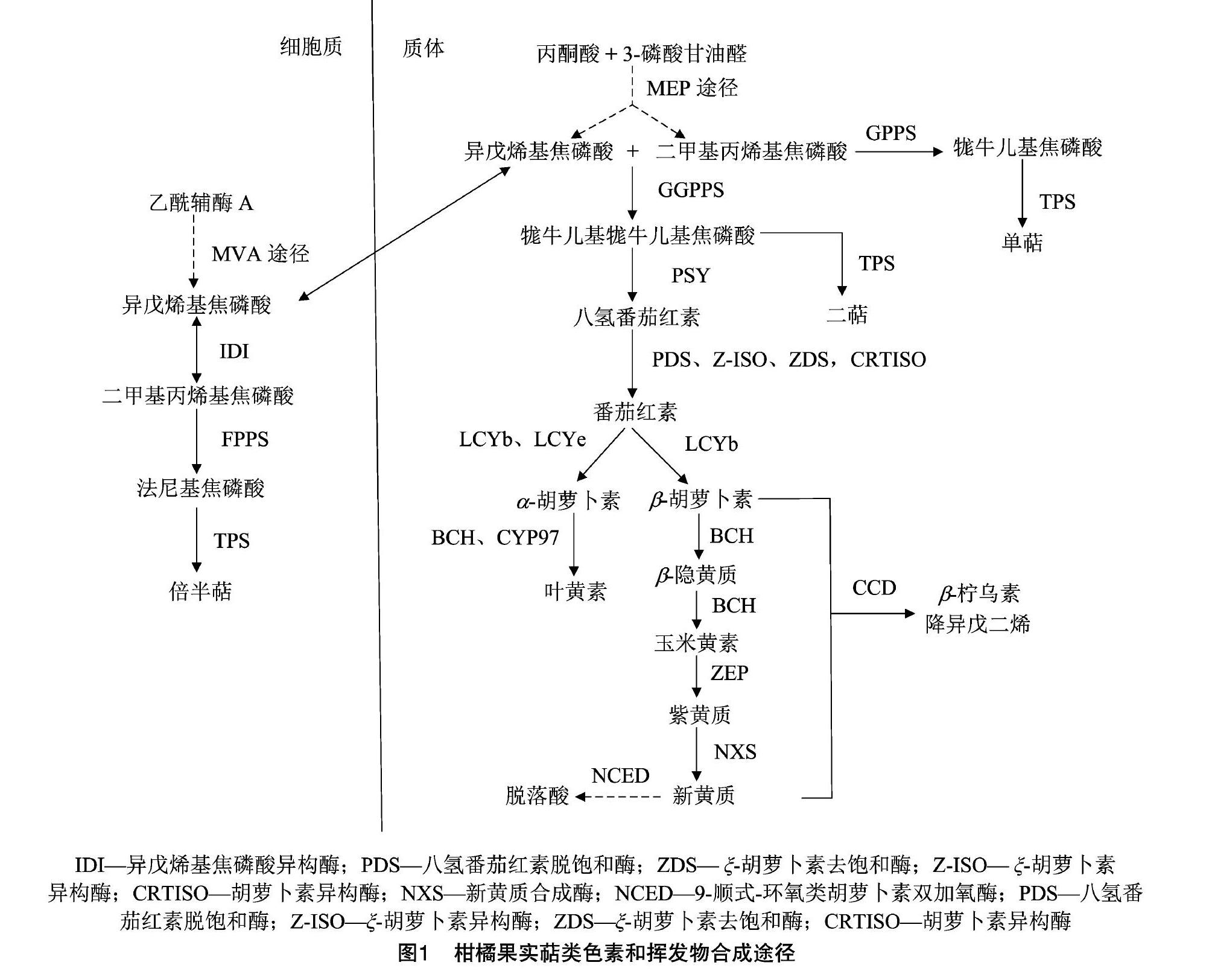
类胡萝卜素和萜类挥发物合成的前体物质均为异戊烯焦磷酸(IPP)及其烯丙基异构体二甲基丙烯焦磷酸(DMAPP),IPP和DMAPP可由2个独立的途径合成,即细胞质内的甲羟戊酸(MVA)途径和质体内的2-C-甲基-D-赤藓醇-4-磷酸(MEP)途径[31-32]。由图1可知,在细胞质中IPP由乙酰辅酶A经MVA途径合成,在质体中IPP由丙酮酸和3-磷酸甘油醛经MEP途径合成,2个途径合成的IPP可以交换,主要由质体向细胞质转移[27,32-34]。
类胡萝卜素的合成是利用质体中的IPP和DMAPP,IPP和DMAPP经牻牛儿基牻牛儿基焦磷酸合酶(GGPPS)的缩合作用产生牻牛儿基牻牛儿基焦磷酸(GGPP),2分子GGPP和八氢番茄红素合成酶(PSY)作用产生第1个类胡萝卜素产物八氢番茄红素,后经去饱和酶和异构酶(PDS、ZDS、Z-ISO、CRTISO)的催化发生一系列去饱和异构反应产生番茄红素,番茄红素被番茄红素ε-环化酶(LCYe)或β-环化酶(LCYb)环化产生α-胡萝卜素和β-胡萝卜素,分别代表α和β分支途径,α-胡萝卜素和β-胡萝卜素在非血红素羟化酶(BCH)和血红素羟化酶(CYP97A、CYP97C)的作用下在α分支产生叶黄素,在β分支产生玉米黄素[33]。玉米黄素经玉米黄素环氧化酶(ZEP)的连续2次作用,2个β环发生环氧化产生紫黄质,紫黄质在新黄质合酶的作用下产生新黄质[34]。类胡萝卜素在类胡萝卜素裂解双氧合酶(CCD)作用下降解产生脱辅基类胡萝卜素包括β-柠乌素、植物激素和降异戊二烯。
萜类挥发物中,单萜和二萜的合成在质体中进行,倍半萜的合成在细胞质中进行,在质体内1分子IPP 和1分子DMAPP在牻牛儿基焦磷酸合酶(GPPS)的作用下缩合产生单萜的前体牻牛儿基焦磷酸(GPP),3分子IPP和1分子DMAPP在GGPPS 的作用下缩合产生二萜的前体GGPP,在细胞质内2分子IPP 和1分子DMAPP在法尼基焦磷酸合酶(FPPS)的作用下缩合产生倍半萜的前体法尼基焦磷酸(FPP)[35]。GPP、GGPP、FPP随后在萜类合成酶(TPS)的作用下分别产生单萜、二萜、倍半萜。TPS是导致萜类挥发物质种类多样的重要原因,首先TPS成员众多,如甜橙TPS基因家族成员有95个[36],其次1种TPS不仅可以1种底物产生多种萜类,而且可以识别多个底物合成多种萜类,1种 TPS可以产生的产物最多可达20种[31]。
2.2 柑橘果实萜类色素和挥发物合成关键基因
2.2.1 类胡萝卜素合成关键基因
PSY是类胡萝卜素生物合成途径的第1个酶,甜瓜中有1个PSY基因,葡萄中有2个PSY基因,番茄和柑橘中有3个PSY基因[37],CitPSY1在柑橘果实发育早期表达水平较低,随果实成熟逐渐积累,和类胡萝卜素的积累量平行变化,表明CitPSY1在柑橘类胡萝卜素积累中起着重要作用,而CitPSY2和CitPSY3的表达水平较低,其在类胡萝卜素积累中的作用有待进一步研究[38-39]。PDS和ZDS催化八氢番茄红素的脱氢经Z-ISO和CRTISO异构化后形成番茄红素,PDS和ZDS是结合氧化还原活性辅因子的膜结合电子受体,醌类是PDS和ZDS去饱和反应的电子接受体,植物PDS突变体通常产生白化表型[34]。PDS基因首先从大豆中被分离鉴定[40],随后辣椒[41]、番茄[42]、玉米[43]中也分离到PDS基因。Kita 等从温州蜜柑中分离到首个柑橘PDS基因[44]。柠檬果实发育后期,CitPDS转录水平降低导致柠檬果实中积累大量无色的八氢番茄红素,同时β,β-叶黄素的积累受到限制[12]。Cara Cara(卡拉卡拉)是脐橙的1个红色果肉芽变,果实发育早期Cara Cara果肉PDS表达较脐橙增强,番茄红素在发育早期大量积累[45]。Z-ISO 催化9,15,9′-tri-cis-ζ-胡萝卜素转化为9,9′-di-cis-ζ-胡萝卜素,Z-ISO突变导致类胡萝卜素合成途径受阻,使果实中上游胡萝卜素和β,β-叶黄素含量失衡,突变脐橙果实变为黄色[46]。
LCYb和LCYe使番茄红素末端环化形成有2个β环的β-胡萝卜素及有1个β环和1个ε环的α-胡萝卜素。在玉米[47]和水稻[48]中,LCYb由单基因编码,而在番茄[49]、猕猴桃[50]、番木瓜[51]和枇杷[52]中有2个LCYb基因。在柑橘中,Alquézar等从甜橙中分离到2个LCYb基因,果实成熟过程中CitLCYb1表达水平恒定且较低,而CitLCYb2在有色体中特异表达并随果实成熟增加,在红葡萄柚果实成熟过程中CitLCYb2表达较甜橙大量减少,且从红葡萄柚中分离到CitLCYb2的2个等位基因,b基因编码无环化活性的酶,红葡萄柚主要表达无功能的CitLCY2b[53]。在植物中LCYe通常为单拷贝,与LCYb有37%~40%的同源性[54]。CitLCYe与CitLCYb1或CitLCYb2共表達时均可以产生α-胡萝卜素[55],在柑橘果实成熟过程中CitLCYe的转录水平逐渐下降直至消失[56]。
BCH和CYP97催化α-胡萝卜素和β-胡萝卜素的末端环羟基化形成叶黄素,BCH定位于质体被膜和类囊体膜上,而CYP97仅定位于叶绿体被膜上[57]。Ma等研究了4个胡萝卜素羟化酶基因在柑橘果实叶黄素形成中的作用,发现CitBCH表达随果实成熟逐渐增加,与β分支叶黄素积累一致,CitCYP97A和CitCYP97C表达在9月达到峰值,与α分支叶黄素积累一致,CitCYP97B表达水平较低;功能分析表明,CitBCH催化α-胡萝卜素和β-胡萝卜素β环的羟化反应,而CitCYP97C为ε环羟化酶,与CitBCH共同表达时可催化α-胡萝卜素形成叶黄素[58]。ZEP催化叶黄素环氧化产生紫黄质,目前柑橘果实中仅鉴定表达出1个ZEP基因,在汁囊大量积累β-隐黄质的温州蜜柑中CitBCH和CitZEP的表达水平远低于以紫黄质积累为主的甜橙[12]。由此可以看出类胡萝卜素合成途径基因对果实类胡萝卜素积累起着关键作用,其合成途径基因表达量改变和基因突变导致类胡萝卜素积累改变,另外果实类胡萝卜素积累量还受类胡萝卜素降解的影响,合成与降解的平衡决定了果实类胡萝卜素最终积累量。
2.2.2 脱辅基类胡萝卜素合成的关键基因
CCD催化类胡萝卜素裂解产生脱辅基类胡萝卜素,CCD对底物的选择性较混杂,但对裂解的双键具有特异性[59]。首个CCD基因从玉米中克隆得到[60],根据裂解位点不同植物CCD可分为5个亚家族,即NCED、CCD1、CCD4、CCD7和CCD8[61]。NCED、CCD7和CCD8裂解类胡萝卜素通常产生植物激素前体[34]。NCED影响柑橘果实类胡萝卜素组成,Kato等发现在9-cis-紫黄质含量低的柠檬果皮、汁囊和温州蜜柑汁囊中CitNCED2基因高度表达,而在9-cis-紫黄质含量高的甜橙果皮、汁囊和温州蜜柑果皮中CitNCED2基因表达水平较低[62]。CCD1裂解类胡萝卜素产生降异戊二烯,但在不同果实中CCD1对类胡萝卜素含量的调节作用不同。在柑橘果实中CCD1可以裂解β-隐黄质、玉米黄质和紫黄质产生降异戊二烯,CCD1的表达不影响类胡萝卜素的积累[62],同样在番茄果实中降低CCD1的表达,β-紫罗兰酮和香叶基丙酮的含量显著降低,类胡萝卜素的含量并不改变[63];然而在草莓和杏果实中,随着CCD1表达的增加,脱辅基类胡萝卜素类香气物质含量显著增加,但类胡萝卜素积累显著减少[64-65]。CCD4裂解类胡萝卜素的产物因果实种类而不同,在苹果和桃果实中CCD4和类胡萝卜素起源的香气挥发物的产生有关[66-67];而在柑橘果实中CCD4是β-柠乌素生物合成的关键基因,可以裂解β-隐黄质和玉米黄质产生β-柠乌素,在积累β-柠乌素的温州蜜柑成熟过程中大量表达,而在缺少β-柠乌素的温州蜜柑中表达水平极低[68]。Zheng等发现红色柑橘品种CCD4b基因启动子区域含有微小反向重复转座子的2个单核苷酸多态性的G碱基(SNP2G),顺式调节CCD4b表达量增强,促使红色C30脱辅基类胡萝卜素的合成增加[68]。因此,CCD是控制果实色泽和香气的关键基因,在CCD的作用下类胡萝卜素可转化为脱辅基类胡萝卜素类挥发物质,除作为脱辅基类胡萝卜素类挥发物质合成的前体外,类胡萝卜素还与单萜和倍半萜挥发物具有相同的初始前体。
2.2.3 萜类挥发物合成的关键基因
TPS是单萜和倍半萜类挥发物合成的关键酶。植物TPS基因大多成簇排列在染色体上,数量通常为19~152个[36,69]。根据序列差异植物TPS基因可分为a~h 8类,其中b、g类TPS基因包含大多数单萜合成酶编码基因,a类TPS基因包含大多数倍半萜合成酶编码基因[70]。柑橘TPS是最大的被子植物TPS家族之一,但目前克隆到的柑橘TPS基因仅占少数[36]。在柑橘单萜合成酶基因方面,Lücker等从柠檬幼果中分离到4个TPS基因,将其在大肠杆菌(E.coli)中表达产生相应的酶蛋白,加入GGP(牻牛儿基焦磷酸)为底物时其主要产物分别为柠檬烯合成酶1、柠檬烯合成酶2、β-蒎烯合成酶和γ-松油烯[71]。隨后Shimada等从温州蜜柑果实中鉴定到CitMTSL1和CitMTSL4,分别编码1,8-桉树脑合酶和E-β-罗勒烯合酶,2个基因均在果实发育早期表达,随果实发育逐渐降低,和单萜的生物合成一致[72]。Shimada等从温州蜜柑中鉴定到3个芳樟醇合酶基因,CuSTS3-1和CuSTS3-2仅以GPP为底物合成芳樟醇,而CuSTS4以GPP和FPP为底物分别合成芳樟醇和橙花叔醇,CuSTS3-1、CuSTS3-2和CuSTS4分别在幼果、花和叶中大量表达[73]。Li等发现甜橙CitTPS16在体外可催化E-香叶醇的合成,体内过表达CitTPS16导致果实和叶片中积累 E- 香叶醇[74]。
在柑橘倍半萜类合成基因研究方面,Sharon-Asa等从甜橙中分离到瓦伦烯合酶基因Cstps1,Cstps1的重组蛋白可以FPP为底物合成瓦伦烯,从果实发育到成熟过程中Cstps1的转录水平逐渐增加,和果实中瓦伦烯的变化一致,采后乙烯处理可以增加果实中Cstps1的表达和瓦伦烯的含量[75]。与Temple杂柑相比,Murcott杂柑果实发育过程中Cstps1大量减少,Murcott果实中缺少瓦伦烯,脱辅基类胡萝卜素类挥发物质也减少,但类胡萝卜素积累量增加[76]。随后研究发现Murcott杂柑Cstps1启动子区域12个核苷酸的缺失是导致其果实不能合成瓦伦烯的原因[77]。
3 柑橘果实萜类色素和挥发物生物合成调控
3.1 转录因子调控
尽管柑橘果实类胡萝卜素积累差异大多是由类胡萝卜素合成途径基因差异表达导致,但目前调控类胡萝卜素合成基因表达的转录因子报道较少,在柑橘中仅鉴定到MYB和MADS转录因子对类胡萝卜素合成基因起调控作用。甜橙CsMADS6随着果实发育和转色协调表达,CsMADS6多靶点调控类胡萝卜素代谢,CsMADS6不仅可与类胡萝卜素合成途径基因LCYb1、PSY和PDS的启动子序列直接结合,并激活其表达,而且可直接结合CCD1的启动子使其表达上调,在柑橘愈伤组织中过表达CsMADS6可增加类胡萝卜素的含量[78]。青瓯柑CrMYB68可与BCH2和NCED5的启动子结合并抑制其表达,延缓α-胡萝卜素、β-胡萝卜素向叶黄素转化,导致其成熟期果实依然保持绿色[79]。
对萜类挥发物合成关键基因TPS起调控作用的仅鉴定到AP2/ERF转录因子。甜橙CitAP2.10可激活瓦伦烯合酶基因CsTPS1的表达,在甜橙中瞬时过表达CitAP2.10可启动果实瓦伦烯生物合成[80]。甜橙CitERF71可与萜类合酶基因CitTPS16的启动子ACCCGCC和GGCGGG序列直接结合,从而激活CitTPS16基因的启动子,调控CitTPS16转录和果实中香叶醇的产生[81]。
3.2 环境因素调控
3.2.1 温度
温度对柑橘果实类胡萝卜素的积累有显著影响。在Navelina、Valencia、Satsuma、Ponkan、Palmer Navel、Lisbon等多个柑橘品种中研究发现,10~15 ℃是果实类胡萝卜素积累的适宜温度[82-84],在10~15 ℃下类胡萝卜素合成基因CitPSY、CitPDS、CitZDS、CitLCYe、CitHYb、CitLCYb1、CitLCYb2、CitBCH、CitZEP及β-柠乌素合成基因 CitCCD4b1表达上调,类胡萝卜素分解代谢基因CitNCED2 和CitNCED3表达下调,类胡萝卜素积累总量增加[85-86]。但在一些红葡萄柚品种中,30 ℃以上高温可促进果实积累更多番茄红素,与 30 ℃ 抑制CitLCYb的表达有关[18]。Cara Cara橙果实中主要含有线性胡萝卜素[87],Lu等研究了不同产区Cara Cara橙类胡萝卜素含量变化,发现Cara Cara橙果实类胡萝卜素含量差异和温度直接相关,高温能显著增加类胡萝卜素的积累[88]。关于温度对柑橘果实萜类挥发物影响的报道还较少,Lado等研究表明,葡萄柚果实采后12 ℃与2 ℃贮藏相比果皮颜色和类胡萝卜素含量增加,而挥发物总量和单萜类挥发物(柠檬烯、芳樟醇、α-萜品醇)释放减少[89]。
3.2.2 光照
光是影响柑橘果实类胡萝卜素生物合成的关键环境因素。光照度对果实类胡萝卜素积累的影响取决于柑橘种类。在甜橙和宽皮柑橘中,遮光会降低果实类胡萝卜素的积累[3,18],遮光后CitPSY、CitPDS、CitZDS、CitLCY2a、CitLCY2b、CitHYb基因的表达下调[90]。但在红葡萄柚和柚中遮光有利于类胡萝卜素积累,Lado等报道套袋后完全遮光的红葡萄柚果实番茄红素较正常光照增加49倍,遮光果实中类胡萝卜素合成基因下调,有色体分化相关基因上调[91]。姜启航也报道套袋能增加马家柚果实番茄红素的积累,同时套袋降低了果实萜类挥发性物质含量,套袋后马家柚果实中诺卡酮、D-柠檬烯、α-蒎烯、β-月桂烯和β-水芹烯含量显著减少[92]。光质对柑橘果皮和果肉类胡萝卜素积累具有不同影响,Ma等发现红光诱导柑橘果皮类胡萝卜素积累和类胡萝卜素合成途径基因CitPSY、CitPDS、CitZDS、CitLCYb1、CitLCYb2、CitHYb、CitZEP表达,而蓝光则无诱导作用,红光处理后温州蜜柑果皮β-隐黄质、全反紫黄质、9-顺-紫黄质、叶黄素含量和类胡萝卜素积累总量增加[93-94]。与果皮相反,Zhang等发现蓝光诱导柑橘果实汁囊类胡萝卜素的积累,而红光不影响类胡萝卜素积累,CitPSY基因的表达受蓝光上调,而不受红光影响[95]。光质对柑橘果皮和果肉萜类挥发物的影响还未见报道。
4 展望
近年來,柑橘果实中类胡萝卜素和萜类挥发物的组成被广泛研究,关于类胡萝卜素合成关键基因的研究也取得较多进展,但是,目前报道已验证功能的柑橘TPS基因还相对较少。柑橘果实中萜类挥发物种类繁多,TPS基因家族成员众多,对柑橘TPS基因进行克隆、鉴定和功能验证是今后的研究重点。此外,结构基因表达受转录因子调控,转录因子对柑橘果实类胡萝卜素和萜类挥发物的合成关键基因调控的报道还较少,有待深入研究。
果实中类胡萝卜素和萜类挥发物的生物合成途径相互联系,二者都以IPP和DMAPP作为合成前体,且一些类胡萝卜素在CCD的作用下可以产生脱辅基类胡萝卜素类挥发物质。在其他果实中已经证实TPS基因的表达能同时影响类胡萝卜素和萜类挥发物的含量,如表达1个单萜合酶基因的转基因番茄果实单萜积累增加,香气和风味更浓,但番茄红素的积累减少,果实颜色变淡[96],在柑橘果实中TPS基因表达对类胡萝卜素的影响还未知,有待进一步验证。在番茄和柑橘中研究表明,CCD1的表达不影响果实类胡萝卜素的积累,说明在柑橘中可以通过增加CCD1的表达或类胡萝卜素的积累,在不降低果实类胡萝卜素含量的同时提高脱辅基类胡萝卜素挥发物的含量,即获得兼具色泽和香气的品种,但类胡萝卜素合成途径基因表达对萜类挥发物的影响未知,须要进一步研究。
柑橘果实天然色泽突变体较多,最近一些研究发现柑橘果实类胡萝卜素发生变化的色泽突变体中,萜类挥发物也发生改变。Li 等报道Niurouhong(NRH,果肉牛肉红色)是Zhuhongju (ZHJ,果肉橙红色)的天然色泽突变体,具有与ZHJ不同的风味,NRH的色泽变化是由于β-隐黄素和β-胡萝卜素的过量积累,而NRH的风味变化是由于萜类挥发物较ZHJ增加1.27倍[74]。rohde red valencia(RRV)是valencia(VAL)的深橙色突变,RRV中β-隐黄质和α-柠檬醛、β-柠檬醛、1,4-萜品醇、α-紫罗兰酮和β-紫罗兰酮含量高于VAL[22]。Liu等发现突变型红肉琯溪蜜柚和红暗柳橙果实类胡萝卜素总量和单萜挥发物含量较对应野生型琯溪蜜柚和暗柳橙高[97],须要进一步对这些色泽突变体的突变机制开展深入研究,以便更好地解析柑橘果实色泽、香气变化规律和控制因子,为培育色香味更佳的柑橘优新品种提供理论和技术支持。
参考文献:
[1]丁晓波,张 华,刘世尧,等. 柑橘果品营养学研究现状[J]. 园艺学报,2012,39(9):1687-1702.
[2]Zheng H W,Zhang Q W,Quan J P,et al. Determination of sugars,organic acids,aroma components,and carotenoids in grapefruit pulps[J]. Food Chemistry,2016,205:112-121.
[3]Rodrigo M J,Alquézar B,Alós E,et al. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit[J]. Scientia Horticulturae,2013,163:46-62.
[4]Alquezar B,Rodrigo M J,Lado J,et al. A comparative physiological and transcriptional study of carotenoid biosynthesis in white and red grapefruit (Citrus paradisi Macf.)[J]. Tree Genetics & Genomes,2013,9(5):1257-1269.
[5]Chen C X. Pigments in fruits and vegetables[M]. New York:Springer,2015.
[6]Wei X,Hu H,Tong H R,et al. Profiles of gene family members related to carotenoid accumulation in Citrus genus[J]. Journal of Plant Biology,2017,60(1):1-10.
[7]Sawamura M,Kuriyama T. Quantitative determination of volatile constituents in the pummelo (Citrus grandis Osbeck forma Tosa-buntan)[J]. Journal of Agricultural and Food Chemistry,1988,36(3):567-569.
[8]Lota M L,Serra D D,Tomi F,et al. Volatile components of peel and leaf oils of lemon and lime species[J]. Journal of Agricultural and Food Chemistry,2002,50(4):796-805.
[9]Hgnadóttir A,Rouseff R L. Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry[J]. Journal of Chromatography A,2003,998(1/2):201-211.
[10]Miyazaki T,Plotto A,Baldwin E A,et al. Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation[J]. Journal of the Science of Food and Agriculture,2012,92(4):727-735.
[11]Zhang H P,Xie Y X,Liu C H,et al. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species[J]. Food Chemistry,2017,230:316-326.
[12]Kato M,Ikoma Y,Matsumoto H,et al. Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit[J]. Plant Physiology,2004,134(2):824-837.
[13]Fanciullino A L,Dhuique-Mayer C,Luro F,et al. Carotenoid diversity in cultivated citrus is highly influenced by genetic factors[J]. Journal of Agricultural and Food Chemistry,2006,54(12):4397-4406.
[14]Agócs A,Nagy V,Szabó Z,et al. Comparative study on the carotenoid composition of the peel and the pulp of different citrus species[J]. Innovative Food Science & Emerging Technologies,2007,8(3):390-394.
[15]Bartley G,Scolnik P A. Plant carotenoids:pigments for photoprotection,visual attraction,and human health[J]. The Plant Cell,1995,7(7):1027-1038.
[16]陶 俊. 柑橘果實类胡萝卜素形成及调控的生理机制研究[D]. 杭州:浙江大学,2002.
[17]Finley J W,de Man J M,Lee C Y. Color and food colorants[M]//Principles of Food Chemistry. Berlin:Springer,2018.
[18]Lado J,Gambetta G,Zacarias L. Key determinants of citrus fruit quality:metabolites and main changes during maturation[J]. Scientia Horticulturae,2018,233:238-248.
[19]Allegrone G,Belliardo F,Cabella P. Comparison of volatile concentrations in hand-squeezed juices of four different lemon varieties[J]. Journal of Agricultural and Food Chemistry,2006,54(5):1844-1848.
[20]Rouseff R L,Ruiz Perez-Cacho P,Jabalpurwala F. Historical review of citrus flavor research during the past 100 years[J]. Journal of Agricultural and Food Chemistry,2009,57(18):8115-8124.
[21]Liu Y Q,Heying E,Tanumihardjo S A. History,global distribution,and nutritional importance of citrus fruits[J]. Comprehensive Reviews in Food Science and Food Safety,2012,11(6):530-545.
[22]Wei X,Song M,Chen C X,et al. Juice volatile composition differences between Valencia orange and its mutant Rohde Red Valencia are associated with carotenoid profile differences[J]. Food Chemistry,2018,245:223-232.
[23]Perez-Cacho P R,Rouseff R. Processing and storage effects on orange juice aroma:a review[J]. Journal of Agricultural and Food Chemistry,2008,56(21):9785-9796.
[24]Cheong M W,Liu S Q,Yeo J,et al. Identification of aroma-active compounds in Malaysian pomelo[Citrus grandis (L.) Osbeck]peel by gas chromatography-olfactometry[J]. Journal of Essential Oil Research,2011,23(6):34-42.
[25]何朝飛,冉 玥,曾林芳,等. 柠檬果皮香气成分的GC-MS分析[J]. 食品科学,2013,34(6):175-179.
[26]Mahattanatawee K,Rouseff R,Valim M F,et al. Identification and aroma impact of norisoprenoids in orange juice[J]. Journal of Agricultural and Food Chemistry,2005,53(2):393-397.
[27]El H A,Zhang F J,Wu F F,et al. Advances in fruit aroma volatile research[J]. Molecules,2013,18(7):8200-8229.
[28]Liu C H,Cheng Y J,Zhang H Y,et al. Volatile constituents of wild citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil[J]. Journal of Agricultural and Food Chemistry,2012,60(10):2617-2628.
[29]Zhang W L,Chen T T,Tang J M,et al. Tracing the production area of citrus fruits using aroma-active compounds and their quality evaluation models[J]. Journal of the Science of Food and Agriculture,2020,100(2):517-526.
[30]Goff S,Klee H J. Plant volatile compounds:sensory cues for health and nutritional value?[J]. Science,2006,311(5762):815-819.
[31]Baldwin I T. Plant volatiles[J]. Current Biology,2010,20(9):392-397.
[32]Pulido P,Perello C,Rodriguez-Concepcion M. New insights into plant isoprenoid metabolism[J]. Molecular Plant,2012,5(5):964-967.
[33]Sun T H,Yuan H,Cao H B,et al. Carotenoid metabolism in plants:the role of plastids[J]. Molecular Plant,2018,11(1):58-74.
[34]Nisar N,Li L,Lu S,et al. Carotenoid metabolism in plants[J]. Molecular Plant,2015,8(1):68-82.
[35]Qualley A,Dudareva N. Plant volatiles[EB/OL]. (2010-09-15)[2020-09-17]. https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0000910.pub2.
[36]Alquézar B,Rodríguez A,de la Pea M,et al. Genomic analysis of terpene synthase family and functional characterization of seven sesquiterpene synthases from Citrus sinensis[J]. Frontiers in Plant Science,2017,8:1481.
[37]Young P R,Lashbrooke J G,Alexandersson E,et al. The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L.[J]. BMC Genomics,2012,13(1):1-17.
[38]Ikoma Y,Komatsu A,Kita M,et al. Expression of a phytoene synthase gene and characteristic carotenoid accumulation during citrus fruit development[J]. Physiologia Plantarum,2001,111(2):232-238.
[39]Peng G,Wang C Y,Song S,et al. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation[J]. Plant Physiology and Biochemistry,2013,71:67-76.
[40]Bartley G,Viitanen P V,Pecker I,et al. Molecular cloning and expression in photosynthetic bacteria of a soybean cDNA coding for phytoenedesaturase,an enzyme of the carotenoid biosynthesis pathway[J]. Proceedings of the National Academy of Sciences of the United States of America,1991,88(15):6532-6536.
[41]Hugueney P,Rmer S,Kuntz M,et al. Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and ζ-carotene in Capsicum chromoplasts[J]. European Journal of Biochemistry,1992,209(1):399-407.
[42]Giuliano G,Bartley G,Scolnik P A. Regulation of carotenoid biosynthesis during tomato development[J]. The Plant Cell,1993,5(4):379-387.
[43]Li Z H,Matthews P D,Burr B,et al. Cloning and characterization of a maize cDNA encoding phytoenedesaturase,an enzyme of the carotenoid biosynthetic pathway[J]. Plant Molecular Biology,1996,30(2):269-279.
[44]Kita M,Komatsu A,Omura M,et al. Cloning and expression of CitPDS1,a gene encoding phytoenedesaturase in citrus[J]. Bioscience Biotechnology and Biochemistry,2001,65(6):1424-1428.
[45]Alquezar B,Rodrigo M J,Zacarías L. Regulation of carotenoid biosynthesis during fruit maturation in the red-fleshed orange mutant Cara Cara[J]. Phytochemistry,2008,69(10):1997-2007.
[46]Rodrigo M J,Lado J,Alós E,et al. A mutant allele of ζ-carotene isomerase (Z-ISO) is associated with the yellow pigmentation of the “Pinalate” sweet orange mutant and reveals new insights into its role in fruit carotenogenesis[J]. BMC Plant Biology,2019,19(1):1-16.
[47]Bai L,Kim E H,Dellapenna D,et al. Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis[J]. The Plant Journal,2009,59(4):588-599.
[48]Chaudhary N,Nijhawan A,Khurana J P,et al. Carotenoid biosynthesis genes in rice:structural analysis,genome-wide expression profiling and phylogenetic analysis[J]. Molecular Genetics and Genomics,2010,283(1):13-33.
[49]Bramley P M. Regulation of carotenoid formation during tomato fruit ripening and development[J]. Journal of Experimental Botany,2002,53(377):2107-2113.
[50]Ampomah-Dwamena C,Mcghie T,Wibisono R,et al. The kiwifruit lycopene β-cyclase plays a significant role in carotenoid accumulation in fruit[J]. Journal of Experimental Botany,2009,60(13):3765-3779.
[51]Blas A L,Ming R,Liu Z,et al. Cloning of the papaya chromoplast-specific lycopene β-cyclase,CpCYC-b,controlling fruit flesh color reveals conserved microsynteny and a recombination hot spot[J]. Plant Physiology,2010,152(4):2013-2022.
[52]Fu X M,Kong W B,Peng G,et al. Plastid structure and carotenogenic gene expression in red-and white-fleshed loquat (Eriobotrya japonica) fruits[J]. Journal of Experimental Botany,2012,63(1):341-354.
[53]Alquézar B,Zacarías L,Rodrigo M J. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from citrus and its relation to lycopene accumulation[J]. Journal of Experimental Botany,2009,60(6):1783-1797.
[54]徐昌杰,張上隆. 柑橘类胡萝卜素合成关键基因研究进展[J]. 园艺学报,2002,29(增刊1):619-623.
[55]Zhang L C,Ma G,Shirai Y,et al. Expression and functional analysis of two lycopene β-cyclases from citrus fruits[J]. Planta,2012,236(4):1315-1325.
[56]Wei X,Chen C X,Yu Q B,et al. Novel expression patterns of carotenoid pathway-related genes in citrus leaves and maturing fruits[J]. Tree Genetics & Genomes,2014,10(3):439-448.
[57]Joyard J,Ferro M,Masselon C,et al. Chloroplast proteomics and the compartmentation of plastidialisoprenoid biosynthetic pathways[J]. Molecular Plant,2009,2(6):1154-1180.
[58]Ma G,Zhang L C,Yungyuen W,et al. Expression and functional analysis of citrus carotene hydroxylases:unravelling the xanthophyll biosynthesis in citrus fruits[J]. BMC Plant Biology,2016,16(1):1-12.
[59]Auldridge M E,Mccarty D R,Klee H J. Plant carotenoid cleavage oxygenases and their apocarotenoid products[J]. Current Opinion in Plant Biology,2006,9(3):315-321.
[60]Schwartz S H,Tan B C,Da G G,et al. Specific oxidative cleavage of carotenoids by VP14 of maize[J]. Science,1997,276(5320):1872-1874.
[61]Rodrigo M J,Alquézar B,Alós E,et al. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments[J]. Journal of Experimental Botany,2013,64(14):4461-4478.
[62]Kato M,Matsumoto H,Ikoma Y,et al. The role of carotenoid cleavage dioxygenases in the regulation of carotenoid profiles during maturation in citrus fruit[J]. Journal of Experimental Botany,2006,57(10):2153-2164.
[63]Simkin A J,Schwartz S H,Auldridge M,et al. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone,pseudoionone,and geranylacetone[J]. The Plant Journal,2004,40(6):882-892.
[64]García-Limones C,Schnbele K,Blanco-Portales R,et al. Functional characterization of FaCCD1:a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening[J]. Journal of Agricultural and Food Chemistry,2008,56(19):9277-9285.
[65]劉盛雨,章世奎,卢娟芳,等. 杏香气形成特异基因PaCCD1的克隆与功能分析[J]. 园艺学报,2018,45(3):471-481.
[66]Huang F C,Molnár P,Schwab W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes[J]. Journal of Experimental Botany,2009,60(11):3011-3022.
[67]Ma G,Zhang L C,Matsuta A,et al. Enzymatic formation of β-citraurin from β-cryptoxanthin and Zeaxanthin by carotenoid cleavage dioxygenase4 in the flavedo of citrus fruit[J]. Plant Physiology,2013,163(2):682-695.
[68]Zheng X J,Zhu K J,Sun Q,et al. Natural variation in CCD4 promoter underpins species-specific evolution of red coloration in citrus peel[J]. Molecular Plant,2019,12(9):1294-1307.
[69]Nieuwenhuizen N J,Green S A,Chen X,et al. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple[J]. Plant Physiology,2013,161(2):787-804.
[70]Falara V,Akhtar T A,Nguyen T T,et al. The tomato terpene synthase gene family[J]. Plant Physiology,2011,157(2):770-789.
[71]Lücker J,El Tamer M K,Schwab W,et al. Monoterpene biosynthesis in lemon (Citrus limon) cDNA isolation and functional analysis of four monoterpene synthases[J]. European Journal of Biochemistry,2002,269(13):3160-3171.
[72]Shimada T,Endo T,Fujii H,et al. Isolation and characterization of (E)-β-ocimene and 1,8 cineole synthases in Citrus unshiu Marc[J]. Plant Science,2005,168(4):987-995.
[73]Shimada T,Endo T,Fujii H,et al. Characterization of three linalool synthase genes from Citrus unshiu Marc. and analysis of linalool-mediated resistance against Xanthomonas citri subsp. citri and Peniciliumitalicum in citrus leaves and fruits[J]. Plant Science,2014,229:154-166.
[74]Li W Y,Liu C H,He M,et al. Largely different contents of terpenoids in beef red-flesh tangerine and its wild type[J]. BMC Plant Biology,2017,17(1):1-12.
[75]Sharon-Asa L,Shalit M,Frydman A,et al. Citrus fruit flavor and aroma biosynthesis:isolation,functional characterization,and developmental regulation of Cstps1,a key gene in the production of the sesquiterpene aroma compound valencene[J]. The Plant Journal,2003,36(5):664-674.
[76]Yu Q B,Plotto A,Baldwin E A,et al. Proteomic and metabolomic analyses provide insight into production of volatile and non-volatile flavor components in mandarin hybrid fruit[J]. BMC Plant Biology,2015,15(1):76.
[77]Yu Q B,Huang M,Jia H G,et al. Deficiency of valencene in mandarin hybrids is associated with a deletion in the promoter region of the valencene synthase gene[J]. BMC Plant Biology,2019,19(1):101.
[78]Lu S,Zhang Y,Zhu K,et al. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes[J]. Plant Physiology,2018,176(4):2657-2676.
[79]Zhu F,Luo T,Liu C Y,et al. An R2R3-MYB transcription factor represses the transformation of α-and β-branch carotenoids by negatively regulating expression of CrBCH2 and CrNCED5 in flavedo of Citrus reticulate[J]. The New Phytologist,2017,216(1):178-192.
[80]Shen S L,Yin X R,Zhang B,et al. CitAP2.10 activation of the terpene synthase CsTPS1 is associated with the synthesis of (+)-valencene in ‘Newhall orange[J]. Journal of Experimental Botany,2016,67(14):4105-4115.
[81]Li X,Xu Y Y,Shen S L,et al. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit[J]. Journal of Experimental Botany,2017,68(17):4929-4938.
[82]van Wyk A A,Huysamer M,Barry G H. Extended low-temperature shipping adversely affects rind colour of ‘Palmer Navelsweet orange[Citrus sinensis (L.) Osb.]due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature[J]. Postharvest Biology and Technology,2009,53(3):109-116.
[83]Zhou J Y,Sun C D,Zhang L L,et al. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon[J]. Scientia Horticulturae,2010,126(2):229-235.
[84]Carmona L,Zacarías L,Rodrigo M J. Stimulation of coloration and carotenoid biosynthesis during postharvest storage of ‘Navelina orange fruit at 12 ℃[J]. Postharvest Biology and Technology,2012,74:108-117.
[85]Yungyuen W,Ma G,Zhang L C,et al. Regulation of carotenoid metabolism in response to different temperatures in Citrus juice sacs in vitro[J]. Scientia Horticulturae,2018,238:384-390.
[86]Luan Y T,Wang S S,Wang R Q,et al. Accumulation of red apocarotenoid β-citraurin in peel of a spontaneous mutant of huyou (Citrus changshanensis) and the effects of storage temperature and ethylene application[J]. Food Chemistry,2020,309:125705.
[87]Tao N G,Wang C F,Xu J,et al. Carotenoid accumulation in postharvest “Cara Cara” navel orange (Citrus sinensis Osbeck) fruits stored at different temperatures was transcriptionally regulated in a tissue-dependent manner[J]. Plant Cell Reports,2012,31(9):1667-1676.
[88]Lu Q,Huang X J,Lv S Y,et al. Carotenoid profiling of red navel orange “Cara Cara” harvested from five regions in China[J]. Food Chemistry,2017,232:788-798.
[89]Lado J,Gurrea A,Zacarías L,et al. Influence of the storage temperature on volatile emission,carotenoid content and chilling injury development in Star Ruby red grapefruit[J]. Food Chemistry,2019,295:72-81.
[90]Lado J,Alós E,Manzi M,et al. Light regulation of carotenoid biosynthesis in the peel of Mandarin and sweet orange fruits[J]. Frontiers in Plant Science,2019,10:1288.
[91]Lado J,Cronje P,Alquézar B,et al. Fruit shading enhances peel color,carotenes accumulation and chromoplast differentiation in red grapefruit[J]. Physiologia Plantarum,2015,154(4):469-484.
[92]姜啟航. 套袋对柚果实类胡萝卜素代谢和品质的影响[D]. 武汉:华中农业大学,2019.
[93]Ma G,Zhang L C,Kato M,et al. Effect of blue and red LED light irradiation on β-cryptoxanthin accumulation in the flavedo of citrus fruits[J]. Journal of Agricultural and Food Chemistry,2012,60(1):197-201.
[94]Ma G,Zhang L C,Kato M,et al. Effect of the combination of ethylene and red LED light irradiation on carotenoid accumulation and carotenogenic gene expression in the flavedo of citrus fruit[J]. Postharvest Biology and Technology,2015,99:99-104.
[95]Zhang L C,Ma G,Kato M,et al. Regulation of carotenoid accumulation and the expression of carotenoid metabolic genes in citrus juice sacs in vitro[J]. Journal of Experimental Botany,2012,63(2):871-886.
[96]Davidovich-Rikanati R,Sitrit Y,Tadmor Y,et al. Enrichment of tomato flavor by diversion of the early plastidial terpenoid pathway[J]. Nature Biotechnology,2007,25(8):899-901.
[97]Liu C H,He M,Wang Z,et al. Integrative analysis of terpenoid profiles and hormones from fruits of red-flesh citrus mutants and their wild types[J]. Molecules,2019,24(19):3456.

