Esophagogastric junction adenocarcinoma: Preoperative chemoradiation or perioperative chemotherapy?
Francisco Laxague, Francisco Schlottmann
Francisco Laxague, Francisco Schlottmann, Department of Surgery, Hospital Alemán of Buenos Aires, Buenos Aires 1118, Argentina
Francisco Schlottmann, Division of Esophageal and Gastric Surgery, Hospital Alemán of Buenos Aires, Buenos Aires 1118, Argentina
Abstract Multimodal treatment is currently the standard of care for locally advanced esophagogastric junction (EGJ) adenocarcinoma due to poor results after surgery alone. Neoadjuvant therapy is intended to shrink the tumor and eliminate potential circulating tumor cells. However, which neoadjuvant treatment is best for patients with EGJ tumors remains controversial. We aimed to compare outcomes of preoperative chemoradiation and perioperative chemotherapy for EGJ adenocarcinomas. For this purpose, we performed a thorough review of the literature describing neoadjuvant treatments for EGJ adenocarcinomas or comparing both therapies. Although some studies have shown better locoregional control and higher rates of complete pathologic response after chemoradiation,data suggest that both types of neoadjuvant therapy have similar survival benefits. As current data are heterogeneous and many studies have included significantly different types of patients in their analysis, future studies with better patient selection are still needed to define which neoadjuvant therapy should be chosen. In addition, targeted therapies and immunotherapy have promising results and should be further explored.
Key Words: Esophageal cancer; Esophagogastric junction tumor; Esophageal Adenocarcinoma; Chemotherapy; Chemoradiation; Neoadjuvant therapy
INTRODUCTION
Esophagogastric junction (EGJ) adenocarcinoma includes tumors originated from the gastric cardia and the distal esophagus, and is the most common pathological type of esophageal cancer in Western countries[1,2]. The prognosis of this entity is unfavorable in the great majority of patients due to its fast dissemination and advanced disease stages when diagnosed, with an overall 5-year survival of 27%-39%[3,4].
Surgical resection is the gold standard treatment modality for patients without distant disease. The esophagectomy consists of radical resection of the tumor along with the regional lymph nodes[5]. Nevertheless, the poor results after surgical treatment alone have motivated the adoption of neoadjuvant therapies to improve prognosis. Multiple studies have demonstrated that combined preoperative chemoradiotherapy or perioperative chemotherapy plus surgery provide a greater survival benefit than surgery alone[6-9].
Neoadjuvant therapy is intended to shrink the tumor and eliminate potential circulating tumor cells. However, which neoadjuvant treatment is best for patients with EGJ tumors remains controversial. We aimed to compare outcomes of preoperative chemoradiation and perioperative chemotherapy for EGJ adenocarcinomas. For this purpose, we performed a thorough review of the literature describing neoadjuvant treatments for EGJ adenocarcinomas or comparing both therapies.
NEOADJUVANT AND PERIOPERATIVE THERAPIES OVER TIME
Perioperative chemotherapy for EGJ adenocarcinomas has been explored over time.The first milestone was the British Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial of 2006, which compared patients with gastric and EGJ adenocarcinomas who underwent 3 cycles of epirubicin, cisplatin, and fluorouracil (ECF) before and after the surgery,vssurgery alone. Results showed a significant improvement in R0 resections and an overall survival benefit in patients receiving perioperative chemotherapy[9].
In 2011, a multicenter phase III trial was conducted (ACCORD-07) including patients with resectable adenocarcinomas of the stomach, EGJ, and distal esophagus.They compared surgery alonevsperioperative chemotherapy with cisplatin and fluorouracil plus surgery. In patients with resectable adenocarcinomas, perioperative chemotherapy significantly improved overall survival, disease-free survival, and curative resection rates[10].
Shortly after, the ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study (CROSS) Group, published the results of a large study that randomized patients with esophageal or EGJ tumors to surgery alone or preoperative chemoradiotherapy followed by surgery. Patients undergoing preoperative chemoradiotherapy with a 5 wk regimen of carboplatin and paclitaxel followed by concurrent radiotherapy,showed a significant improvement in pathological curative resections and overall survival with acceptable adverse events[7,11].
Finally, the German FLOT4 trial in 2019 compared perioperative ECFvsperioperative FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) for gastric and EGJ tumors. This trial was able to demonstrate that patients undergoing FLOT had higher rates of pathological remissions and R0 resections than patients undergoing the MAGIC regimen[12].
Several ongoing trials are currently investigating different neoadjuvant and perioperative therapies in patients with EGJ tumors (Figure 1).

Figure 1 Timeline of neoadjuvant and perioperative therapies in patients with esophagogastric junction cancer. MAGIC: The British Medical Research Council Adjuvant Gastric Infusional Chemotherapy; CROSS: ChemoRadiotherapy for Oesophageal cancer followed by Surgery Study.
RESULTS OF PREOPERATIVE CHEMORADIOTHERAPY
The CROSS trial included 366 patients [275 (75%) adenocarcinomas, 84 (23%)squamous-cell carcinomas, and 7 (2%) large-cell undifferentiated carcinomas]. The vast majority of patients had distal esophageal cancer, with only 22% of EGJ tumors.Patients were randomly assigned to surgery alone (n= 188) or chemoradiotherapy[intravenous carboplatin (AUC 2 mg/mL per min) and intravenous paclitaxel (50 mg/m2of body-surface area) for 23 d] with concurrent radiotherapy (41.4 Gy, given in 23 fractions of 1.8 Gy on 5 d/wk) followed by surgery (n= 178). The chemoradiotherapy group had significantly higher rates of R0 resections (curative resections) than the surgery alone group (92%vs69%;P< 0.001). Furthermore, the overall survival was significantly better in the experimental group (49.4 movs24 mo), with a 29% of complete pathological response in the neoadjuvant group. In addition, very few adverse events were reported in the chemoradiotherapy-surgery group (6%leukopenia, 5% anorexia, 3% fatigue, and 2% neutropenia)[7].
Long-term follow-up of the CROSS trial confirmed the benefits of neoadjuvant chemoradiotherapy followed by surgery in patients with EGJ and esophageal cancers.Interestingly, in the subgroup analysis by cancer type, patients with squamous cell carcinomas had a greater overall survival benefit than patients with adenocarcinomas[11].
The addition of radiotherapy to the chemotherapy treatment has shown to improved locoregional control by lymph node downstaging and higher rates of complete pathological response (R0 resections). However, this combination might not be highly effective for reducing the risk of distant metastases[2].
RESULTS OF PERIOPERATIVE CHEMOTHERAPY
The British MAGIC trial in 2006 introduced the first perioperative chemotherapy regimen for gastric and EGJ tumors, comparing patients who underwent 3 cycles of ECF before and after the surgery,vspatients who underwent surgery alone. The study showed a significant improvement in overall and progression-free survival, as well as higher rates of downsizing of the tumor in the chemotherapy group, with similar complications rates between groups (46%vs45%)[9]. It is worth mentioning that this trial included only 11% of patients with EGJ adenocarcinomas. In addition, few patients were able to complete the full perioperative treatment (91% completed the 3 preoperative cycles, 66% started the 3 postoperative cycles, and only 76% of these patients completed the three cycles), with only 42% of the patients completing the full 6-cycle regimen. Furthermore, no complete pathological response was observed[9].
The French Actions Concertées dans les cancers COloRectaux et Digestifs(ACCORD)-07 trial in 2011 compared patients receiving 2 or 3 cycles of cisplatin and fluorouracil before and after surgery with patients undergoing surgery alone. The authors observed better overall survival (38%vs24%), 5 year disease-free survival(34%vs19%), and higher rates of R0 resections in patients with perioperative chemotherapy[10]. In addition, patients receiving chemotherapy had similar morbidity rates than those undergoing surgery alone. In contrast with the MAGIC-trial, 64% of the patients included in the study had EGJ tumors. However, as well as the MAGICtrial, one of the main disadvantages was that most patients could not finish the complete regimen due to postoperative morbidity[10].
Based on the results of the MAGIC and ACCORD trials, perioperative chemotherapy was widely embraced for EGJ tumors. In 2019, the German FLOT-4 trial(fluorouracil, leucovorin, oxaliplatin, and docetaxel) analyzed the efficacy and safety of perioperative chemotherapy for locally advanced, resectable gastric and EGJ tumors. In this trial, 716 patients (56% with EGJ tumors) were randomly assigned to perioperative FLOT (n= 356) or ECF (n= 360) plus surgery. An overall survival benefit was observed in the FLOT group (50 movs35 mo) and serious adverse events,morbidity, and mortality rates were similar between both groups. These encouraging results have motivated most physicians to adopt FLOT as the standard chemotherapy regimen for patients with EGJ tumors. Remarkably, only 50% and 37% of the patients completed the entire perioperative FLOT or ECF treatment, respectively[12].Therefore, physicians should be aware that a considerable proportion of patients might not be able to receive the entire planned systemic treatment. Table 1 describes relevant characteristics of current available neoadjuvant and perioperative therapies.

Table 1 Relevant characteristics of current available neoadjuvant and perioperative therapies for esophagogastric junction tumors
PERIOPERATIVE CHEMOTHERAPY VS PREOPERATIVE CHEMORADIATION
To FLOT or to CROSS: that is the question. Regrettably, which is the most effective neoadjuvant therapy for locally advanced EGJ adenocarcinomas remains unclear. In fact, the most important guidelines recommend either perioperative chemotherapy or preoperative chemoradiotherapy for resectable and locally advanced EGJ tumors[13,14].
Unfortunately, scarce studies have compared both therapies. A recent meta-analysis of 13 randomized controlled trials with almost 5000 patients found no significant differences in overall survival between both regimens (FLOT reached a non-significant HR of 0.88 (95%CI: 0.46-1.62) compared to CROSS for overall survival in randomeffects models)[15]. Petrelliet al[2] conducted another large systematic review and meta-analysis including 22 studies comparing perioperative chemotherapy and preoperative chemoradiotherapy for GEJ adenocarcinomas, and showed that both therapies had similar overall survival rates. Interestingly, chemoradiotherapy was associated with better locoregional control and higher R0 resection rates but poorer distant metastases control[2].
A propensity score-matched analysis of patients with locally advanced esophageal and EGJ adenocarcinomas compared 40 patients receiving CROSS and 40 receiving FLOT. The study showed that patients undergoing preoperative chemoradiotherapy had higher rates of complete pathological response (97%vs85%;P= 0.049) and higher rates of negative lymph node metastases (68%vs40%;P= 0.014) than those receiving perioperative chemotherapy. Nevertheless, despite these benefits associated with the CROSS regimen, no difference in overall survival was found between groups[16].
Recently, a study group conducted a propensity score-matched analysis of 3300 patients (1650 for arm) undergoing preoperative chemoradiationvsperioperative chemotherapy for resectable lower esophageal and EGJ adenocarcinomas. The authors hypothesized that chemoradiation was superior to chemotherapy. They found that although patients undergoing chemoradiation achieved higher rates of complete pathological response (2.7 times), overall survival was similar in both groups[17].Similarly, a 2-center retrospective analysis, failed to demonstrate a greater benefit between different neoadjuvant therapies for resectable EGJ adenocarcinomas. They analyzed 85 patients (33 received neoadjuvant/perioperative chemotherapy and 52 neoadjuvant chemoradiotherapy). There was a significantly higher pathological complete response after chemoradiotherapy (30%vs12%;P= 0.01). However, these differences did not translate into a different disease-free or overall survival[18].
At our institution, neoadjuvant chemoradiation is mostly used for patients with distal squamous cell carcinoma (Siewert type I). This strategy is based on the subgroup analysis by cancer type of the CROSS trial, which showed that patients with squamous cell carcinomas had greater overall survival benefit than patients with adenocarcinomas.
In patients with EGJ adenocarcinoma, we try to avoid the morbidity of radiation and we usually offer perioperative chemotherapy based on the multiple trials showing good outcomes with this approach (MAGIC, ACCORD, and FLOT). Currently, we offer FLOT regimen due to the recent results of the FLOT trial. Radiation is usually added in patients with extensive loco-regional involvement (i.e.bulky tumors).
Overall, further studies are needed to clarify which is the best neoadjuvant treatment for EGJ tumors. Each neoadjuvant modality has advantages and disadvantages that should be considered in a case-by-case basis (Table 2).
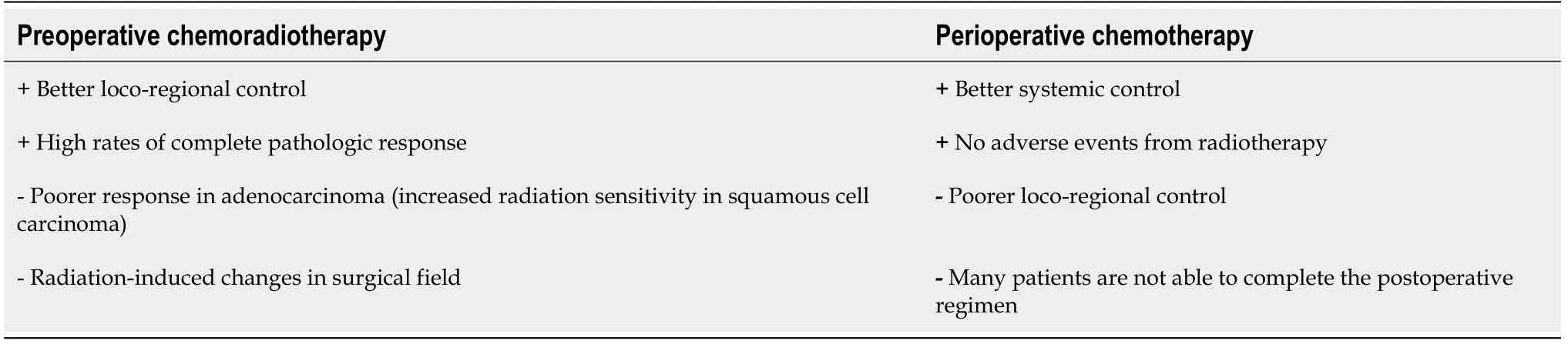
Table 2 Potential advantages (+) and disadvantages (-) of preoperative chemoradiation and perioperative chemotherapy
FUTURE DIRECTIONS
As no study could demonstrate greater benefit between chemoradiotherapy or perioperative chemotherapy for resectable EGJ adenocarcinomas, efforts to elucidate the best multimodal treatment are still needed.
The ongoing multicenter randomized controlled phase III ESOPEC-trial compares neoadjuvant CROSSvsFLOT in patients with resectable and potentially curative esophageal adenocarcinoma. The authors hypothesized that the FLOT regimen might improve overall survival and distant metastases disease control. The results of this trial will hopefully help to decide the most suitable neoadjuvant therapy for patients with EGJ adenocarcinoma[4].
Targeted therapies are designed to inhibit specific molecules overexpressed in patients' tumors and are also currently explored for the treatment of esophageal cancer. The human epidermal growth factor receptor 2 (HER2) is involved in diverse cellular functions such as cell growth, differentiation, and survival. Trastuzumab is a monoclonal antibody targeting the extracellular domain of HER2. The trastuzumab for gastric cancer (TOGA) trial evaluated patients with advanced gastroesophageal adenocarcinoma with overexpression of HER2, and found that the addition of trastuzumab to standard chemotherapy was associated with improved overall survival[19]. A recent trial, however, did not show a survival advantage with the addition of trastuzumab to neoadjuvant chemoradiation in patients with HER2 overexpressing esophageal adenocarcinoma[20]. Pertuzumab is another monoclonal antibody targeting HER2. The PETRARCA trial is currently evaluating the outcomes of perioperative trastuzumab and pertuzumab in combination with FLOTvsFLOT alone for patients with HER2-positive resectable esophagogastric adenocarcinoma[21].
The vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR)regulate angiogenesis and play a key role in tumor growth. Bevacizumab (monoclonal antibody against VEGF-A), ramucirumab (monoclonal antibody against VEGFR-2),and apatinib (molecule inhibitor selective for VEGF-2) are some of the drugs under investigation[22-25].
The advent of immunotherapy has also brought hope for the treatment of esophageal cancer. Immunotherapy utilizes monoclonal antibodies directed against immune checkpoint proteins such as program death 1 (PD-1) receptor, programmed death ligand 1 (PD-L1) or cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).Recent studies have demonstrated survival advantages with monoclonal antibodies targeting PD-1/PD-L1 (e.g., pembrolizumab, nivolumab) in patients with advanced gastric esophageal cancer[26].
Although targeted therapeutics and immunotherapies are indeed promising, further studies are needed to define the safety and efficacy of these drugs.
CONCLUSION
Although some studies have shown better locoregional control and higher rates of complete pathologic response after chemoradiation as compared to perioperative chemotherapy, current data suggest that both types of neoadjuvant therapy have similar survival benefits. Future studies comparing both treatment modalities and with better patient selection are still needed to define which neoadjuvant therapy should be chosen. Targeted therapeutics and immunotherapies have promising results and might also be part of the treatment armamentarium in the future.
 World Journal of Clinical Oncology2021年7期
World Journal of Clinical Oncology2021年7期
- World Journal of Clinical Oncology的其它文章
- BRCA mutations and gastrointestinal cancers: When to expect the unexpected?
- Mechanisms of acquired resistance of BRCA1/2-driven tumors to platinum compounds and PARP inhibitors
- Therapeutic potential of thymoquinone in combination therapy against cancer and cancer stem cells
- Proteoglycans and their functions in esophageal squamous cell carcinoma
