Proteoglycans and their functions in esophageal squamous cell carcinoma
Yun Zhu, Annie Lai Man Cheung
Yun Zhu, Annie Lai Man Cheung, School of Biomedical Sciences, The University of Hong Kong,Hong Kong, China
Abstract Esophageal squamous cell carcinoma (ESCC) is a highly malignant disease that has a poor prognosis. Its high lethality is mainly due to the lack of symptoms at early stages, which culminates in diagnosis at a late stage when the tumor has already metastasized. Unfortunately, the common cancer biomarkers have low sensitivity and specificity in esophageal cancer. Therefore, a better understanding of the molecular mechanisms underlying ESCC progression is needed to identify novel diagnostic markers and therapeutic targets for intervention. The invasion of cancer cells into the surrounding tissue is a crucial step for metastasis. During metastasis, tumor cells can interact with extracellular components and secrete proteolytic enzymes to remodel the surrounding tumor microenvironment.Proteoglycans are one of the major components of extracellular matrix. They are involved in multiple processes of cancer cell invasion and metastasis by interacting with soluble bioactive molecules, surrounding matrix, cell surface receptors, and enzymes. Apart from having diverse functions in tumor cells and their surrounding microenvironment, proteoglycans also have diagnostic and prognostic significance in cancer patients. However, the functional significance and underlying mechanisms of proteoglycans in ESCC are not well understood. This review summarizes the proteoglycans that have been studied in ESCC in order to provide a comprehensive view of the role of proteoglycans in the progression of this cancer type. A long term goal would be to exploit these molecules to provide new strategies for therapeutic intervention.
Key Words: Esophageal squamous cell carcinoma; Proteoglycan; Glycosaminoglycan;Serglycin; Extracellular matrix; Biomarker
INTRODUCTION
Esophageal cancer is the 7thmost common cancer in the world and the sixth highest ranking cancer in terms of mortality rate[1]. The two major subtypes of esophageal cancer are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). People who develop esophageal cancer usually have no specific symptoms at the early stage. The onset of symptoms is often accompanied by difficulty and pain in swallowing (dysphagia), loss of body weight, and heartburn, by which time the tumor is likely already in the advanced stage. Late diagnosis of esophageal cancer is the primary reason for its high lethality. Tobacco use and alcohol consumption are the two main risk factors of ESCC. In addition, genetic susceptibility also plays a role in ESCC. It has been reported thatTP53has the highest frequency of mutation in ESCC patients[2]. Other frequently mutated genes in ESCC areRB1,CDKN2A,PIK3CA,CCND1,ZNF750,NOTCH1,NFE2L2,FAT1, andFAT2[3-6]. It is widely accepted that tumor progression not only depends on accumulation of genetic alterations but also on changes within the surrounding microenvironment[7]. The contribution of tumor microenvironment and extracellular matrix (ECM) components to cancer development and progression is increasingly being recognized[7-9]. The ECM in the tumor microenvironment consists of proteoglycans, collagens, fibronectin,and laminins[10]. Interaction of these molecules with growth factors, chemokines,cytokines, and matrix metalloproteinases facilitate tumor cell survival, invasion, and metastasis[11,12]. It is a highly dynamic and complex interaction network. In this review, the characteristics of proteoglycans and their functions in ESCC are summarized.
STRUCTURE AND CLASSIFICATION OF PROTEOGLYCANS
Proteoglycans typically consist of polysaccharide chains termed glycosaminoglycan(GAG) and a core protein. The GAGs are covalently attached to the serine residues on the core protein. Proteoglycans differ from glycoproteins in several aspects. For example, the carbohydrate content in proteoglycans is 50%-60%, which is much higher than that in glycoproteins. The GAGs in proteoglycans are linear, negatively-charged long chains, whereas the oligosaccharides in glycoproteins are branched short chains that may or may not be negatively-charged. The GAGs of proteoglycans are composed of repeating disaccharide units of hexuronic acid and hexosamine. They may be modified with sulfate groups at various positions to achieve multiple biological functions[13]. The major categories of GAGs are chondroitin sulfate (CS), dermatan sulfate (DS), keratan sulfate (KS), heparan sulfate (HS), heparin, and hyaluronan (HA).
CSs consist of repeating disaccharide units of N-acetylgalactosamine (GalNAc) and D-glucuronic acid (GlcA), and can be sulfated at C4 and/or C6 position of the GalNAc unit. The CS chains that contain sulfate group modification at both C4 and C6 positions of the GalNAc unit are named CS-E, whereas those modified exclusively at C4 position of GalNAc are known as CS-A. CS-C contains sulfate group only at C6 position of the GalNAc. CS-D chains have sulfate groups at C6 position of the GalNAc and C2 position of the GlcA. DS chains, also named CS-B, are derived from CS, of which the GlcA residues are epimerized into L-iduronic acid (IdoA). DS chains are sulfated at C2 position of IdoA and C4/C6 position of the GalNAc. KS chains contain repeating disaccharide units of galactose (Gal) and N-acetylglucosamine (GlcNAc). HS chains consist of GlcA and GlcNAc repeats, while heparins consist of repeating disaccharide units of IdoA and GlcNAc. The sulfate modifications in HS and heparins are in clusters. HA, a linear, protein-free, non-sulfated GAG consists of GlcNAc and GlcA repeating units. The structures of GAG chains are shown in Figure 1.

Figure 1 Structure and components of glycosaminoglycan chains of proteoglycans. The disaccharide unit of glycosaminoglycan consists of epimers of uronic acid (GlcA, IdoA) or galactose and hexosamine (GlcNAc, GalNAc). The repeating disaccharide units in different types of glycosaminoglycan are shown with the potential sulfation positions. The different disaccharides types (Δdi) of chondroitin sulfate family have abbreviated names, which are based on their different sulfation positions.
Proteoglycans can be classified according to their predominant GAG types into CS proteoglycans and HS proteoglycans or according to their cellular and subcellular location, overall gene/protein homology, and the presence of specific protein modules within their respective protein cores into intracellular, cell-surface, pericellular, and extracellular proteoglycans[14]. In this review, the functions of proteoglycans in ESCC will be discussed according to this classification. These proteoglycans are classified and summarized in Table 1.
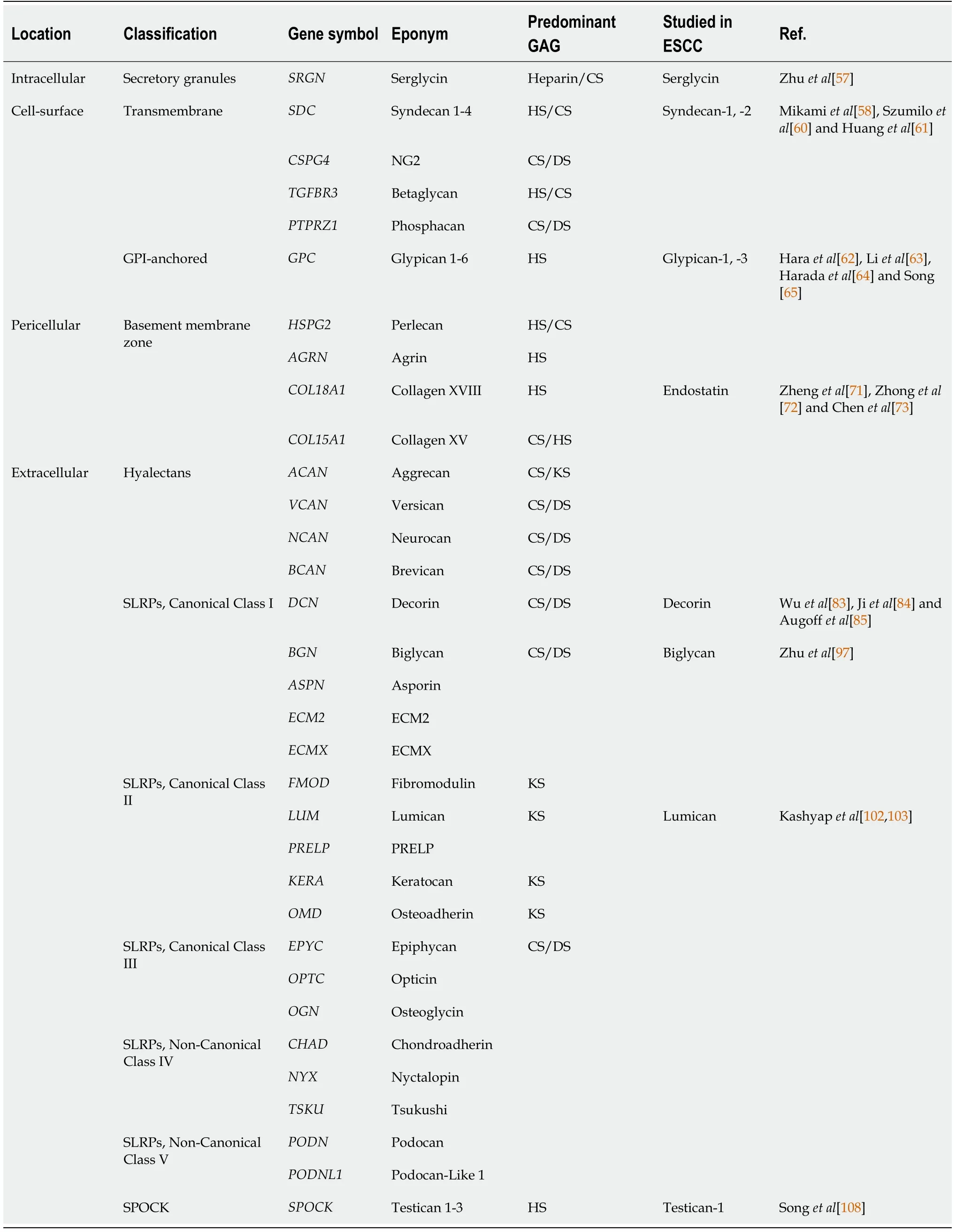
Table 1 Classification of proteoglycans[14] with clinicopathological and/or functional significance in esophageal squamous cell carcinoma
INTRACELLULAR PROTEOGLYCANS IN ESCC
Serglycin is the only known intracellular proteoglycan. It was first isolated from rat yolk carcinoma cell line L2 (named as pPG1) in 1985[15]. HumanSRGN(serglycin)was isolated from promyelocytic leukemia HL-60 cells in 1990[16] and from hematopoietic cells in 1992[17]. Its amino acid sequence was later found to be identical to that of human platelet proteoglycan protein, which was isolated and characterized in 1986[18], and the complete amino acid sequence determined in 1988[19,20].
The humanSRGNgene is located on chromosome 10 and spans about 16.7 kb with 7% of the gene comprising of exons[16,17,21]. Nicodemuset al[16] showed that humanSRGNgene contains three exons, with exon 1 encoding signal peptide (amino acids 1-27), exon 2 encoding amino acids 28-77, and exon 3 encoding amino acids 77-158,which includes the serine/glycine repeat region (amino acids 94-111). The serine/glycine repeat region is the GAG attachment region that allows the clustering of GAG chains close to the center of the core protein. This structure is unique to serglycin[22].
Serglycin was initially characterized as a hematopoietic proteoglycan[23] and was subsequently found to be present in many other non-hematopoietic cells such as endothelial cells[24], immune cells[25], chondrocytes[26-28], and cancer cells[29,30].The type and size of the GAG chains of serglycin vary among different cell types and can affect the functions of serglycin. Serglycin synthesized by rat serosal mast cells is mainly modified with heparin or HS, while CS chains are predominant in rat mucosallike mast cells[31]. HS/CS hybrid GAG chains are found in mouse mastocytoma cells and human erythroleukemia cells[32]. In human umbilical vein endothelial cells,serglycin is modified with CS-GAG chains and has smaller GAG chains than that expressed in platelets[24,33]. In human platelets, the predominant type of GAG chain is CS-4[18].
The enzymes involved in the synthesis of GAG chains have different functions in the particular physiological process of cells. Two enzymes that synthesize CS, namely chondroitin 4-sulfotransferase-1 and GalNAc(4S)-6-O-sulfotansferase, and N-deacetylase/N-sulfotransferase-2, which is essential for heparin synthesis, are positively associated with mast cell maturation, whereas chondroitin 6-sulfotransferase is negatively correlated with mast cell maturation[34]. Serglycin core protein, chondroitin 4-sulfotransferase-1, and GalNAc(4S)-6-O-sulfotansferase are upregulated during mast cell activation and accompanied by downregulation of N-deacetylase/Nsulfotransferase-2[34]. A recent study showed that hyaluronidase-4 can cleave the CS chains of serglycin in human mast cells[35]. These reports suggest that enzymes responsible for GAG synthesis are important in determining the functions of serglycin.
In the past two decades, an ever-increasing number of studies have shown that serglycin plays a significant role in human cancers. It has been reported that serglycin is increased in many human cancers including breast cancer[36-40], multiple myeloma[41-44], acute myeloid leukemia[45], nasopharyngeal carcinoma[46-48], hepatocellular carcinoma (HCC)[49-51], colon cancer[52,53], non-small cell lung cancer[54,55], and glioblastoma[56]. The reported functions of serglycin in promoting cancer progression include regulation of cell adhesion, promoting migration/invasion, inducing angiogenesis, and avoiding immune destruction (Figure 2).

Figure 2 Overview of serglycin functions in human cancers. The various binding partners of serglycin contribute to the multiple functions of serglycin in human cancers. Serglycin, with or without binding partner, can bind to receptors on cancer cells and activate the downstream signaling pathways, including β-catenin,Nanog, phosphatidylinositol-3-kinase (PI3K) and transforming growth factor (TGF) β pathways. MMP: Matrix metalloproteinase; NF-κβ: Nuclear factor-kappa B.
Our recent study showed for the first time that serum serglycin could be a potential non-invasive biomarker with prognostic significance in ESCC[57]. We found that serglycin and its binding partners (including matrix metalloproteinases) could promote ESCC cell invasion, migration, and metastasis. We also identified midkine as a novel GAG-dependent binding partner of serglycin, which contributes to ESCCprogression by upregulating mitogen-activated protein kinase/extracellular signalregulated protein kinase signaling and c-Myc expression in an autocrine manner[57].This study also highlighted the significance of CS-GAGs of serglycin in ESCC cell invasion. Strategies that target serglycin, its binding partners, or its GAG chains where most of the protein interactions take place may be a new direction in ESCC therapy.
CELL-SURFACE PROTEOGLYCANS IN ESCC
Syndecan
The syndecan family is a group of transmembrane proteoglycans consisting of syndecan-1, -2, -3, and -4. They generally carry HS-GAG chains. In normal esophageal epithelium, syndecan-1 is strongly expressed in cell membrane, and the expression of syndecan-1 core protein and HS-GAG chains are significantly decreased in T2 and T3 stage specimens compared with lower grade specimens[58]. Although the study by Conejoet al[59] found that syndecan-1 messenger RNA level in esophageal malignancies was not significantly different from that in the corresponding normal samples, a histochemical study by Szumiloet al[60] showed that well- and moderately-differentiated carcinomas are more frequently syndecan-1-positive compared with poorly differentiated carcinomas. Loss of syndecan-1 was found to be associated with the incidence of lymphatic invasion, and malignant phenotype of ESCC[58]. Notably, the reduction of HS-GAGs on syndecan-1 was more important than that of core protein for
tumor cell invasion; other pathological parameters such as nodal and distant organ metastasis were negatively correlated with HS-GAG expression but not with syndecan-1 core protein expression[58]. Unlike syndecan-1, which acts as a tumor suppressor in ESCC, syndecan-2 is positively correlated with tumor size in ESCC[61].Multivariate analysis showed that syndecan-2 is an independent prognostic factor for survival rate of ESCC patients after surgery[61].
Glypican
Glypican family includes six members, which are glypican-1 to -6. They also carry HSGAG chains. Glypican-1 was found to be upregulated in ESCC cell lines compared with normal epithelial cells, and its expression in ESCC tissue is negatively correlated with survival rate of patients[62]. Another study confirmed that ESCC tumor samples have higher expression of glypican-1 than that in para-tumor tissues[63]. Functionally,glypican-1 promotes cell motility and induces epithelial-mesenchymal transition(EMT) in ESCC, possibly through activation of AKT/β-catenin pathway[63]. Knockdown of glypican-1 (GPC1) significantly inhibited ESCC cell growth by inhibiting epidermal growth factor receptor pathway and inducing cell apoptosis[64]. Systemic administration of anti-GPC1 antibody significantly inhibited growth of tumor xenografts and tumor angiogenesis[64]. Based on these findings, glypican-1 was described as a promising therapeutic target in ESCC[65].
Unlike glypican-1, glypican-3 did not show significant correlation with histological type, tumor stage, tumor grade, or patient survival in ESCC[66,67], although it was reported to be a diagnostic molecule and a therapeutic target in HCC[68,69].
PERICELLULAR PROTEOGLYCANS
Pericellular proteoglycans include perlecan, agrin, collagen XVIII, and collagen XV(Table 1). The functions of perlecan, agrin, and collagen XV in ESCC have not been elucidated. Endostatin, which is a 20 kDa C-terminal fragment of collagen XVIII, was found to have anti-angiogenic activity[70]. It was later shown to have inhibitory effect on formation of ESCC-related lymphatic vessels[71]. The application of recombinant endostatin protein combined with chemoradiotherapy in ESCC treatment increased the overall survival rate of patients[72]. Recombinant endostatin combined with radiotherapy could significantly inhibit proliferation and migration/invasion of ESCC cells as well as reduce angiogenesis, but there was no effect on cell apoptosis[73].
EXTRACELLULAR PROTEOGLYCANS IN ESCC
Decorin
Decorin, also called PG40, belongs to the small leucine-rich proteoglycan (SLRP)family[74]. The core protein of decorin is about 42 kDa. There is a single GAG chain attached to the N-terminus of the core protein[75]. Proteoglycans belonging to SLRP family contain a region with leucine-rich tandem repeats (LRR). The LRR region is modified by N-glycosylation. N-glycosylation and the O-linked GAG side chains are crucial for the interactions of decorin with other molecules. Studies have shown that the DS-GAGs are essential in fibrillar network formation through bridging collagen fibers[76,77]. The GAG chains and LRR region of SLRPs are both involved in ECM assembly.
Decorin was found to be necessary for appropriate fibrillogenesis due to its ability to bind to collagen[74]. The significance of decorin, as well as other SLRPs, in ECM assembly has been intensively investigated and reviewed[78]. Of note, same classes of SLRPs have the same function of binding to collagen and therefore compete with each other. For example, two other class I SLRPs, namely biglycan and asporin, are able to compensate for the loss of decorin[79]. Asporin can compensate for both decorin and biglycan loss[80]. Lumican, a class II SLRP, can compete with fibromodulin, which belongs to the same class[81,82].
The concentration of plasma decorin in 275 ESCC patients was found to be significantly lower than that in normal controls[83]. The expression of decorin in malignant ESCC tissue samples is also lower compared with normal tissue[84,85]. The study by Jiet al[84] showed that decorin expression in ESCC is negatively correlated with histological grade, lymph node metastasis, tumor stage, and clinical stage. Low expression of decorin is associated with poor survival rate and is an independent prognostic marker in patients with ESCC[84]. The tumor suppressive property of decorin, as revealed from studies in other types of cancer such as skin squamous cell carcinoma[86] and breast cancer[87,88], is predominantly due to its ability to trap transforming growth factor β (TGF-β) in the ECM. The binding of decorin to TGF-β prevents the latter from binding to its receptors. Interestingly, this decorin-TGF-β interaction is dependent on decorin-collagen binding[89]. In addition, decorin also acts as a receptor tyrosine kinase inhibitor[90]. It can inhibit the activity of epidermal growth factor receptor[91], insulin-like growth factor receptor 1, and platelet-derived growth factor receptor α/β[92], thereby suppressing their downstream signaling cascades, and finally inhibiting cancer cell proliferation, migration, and invasion[93]. Systemic administration of recombinant decorin protein can inhibit tumor growth and reduce metastasis of squamous cell carcinoma[86]. Based on these characteristics of decorin, it is regarded as a promising anti-tumor molecule and a potential neoadjuvant therapy for human cancers[94,95].
Biglycan
Biglycan, a SLRP protein coded by theBGNgene, is structurally related to decorin but holds two GAG chains rather than one at the N-terminus of the core protein. The molecular weight of biglycan core protein is about 42 kDa. Although biglycan shares similar structure with decorin, the functions of biglycan in human cancers differ from that of decorin. In ESCC, the gene expression ofBGNis upregulated in tumor samples compared with non-tumor tissues[96,97], although there is no significant association with patient survival[98]. High expression of biglycan in tumor tissue is positively correlated with tumor invasion, lymph node metastasis, and advanced clinical stage[96,97] and is an independent prognostic marker of ESCC[97]. In addition, higher serum biglycan was found in patients with EAC[99], suggesting that biglycan has diagnostic significance in esophageal cancer. Functionally, biglycan has anti-apoptotic effects on mesangial cells and pro-angiogenesis effects on tumor endothelial cells[100],which contribute to cancer cell survival and metastasis. These studies suggest that targeting biglycan may be a novel approach in anti-angiogenic and anti-tumor therapy for ESCC patients[97,100].
Lumican
Lumican is a class II SLRP that has up to three KS-GAG chains. There are contradictory reports on the roles of lumican in human cancers. In pancreatic cancer, patients with high expression of stromal lumican have favorable survival after surgery[101]. However, the gene expression ofLUMwas found to be 7-fold higher in ESCC than in normal epithelia[102,103]. Strong positive lumican immunostaining was found in the stromal and epithelial compartments of ESCC specimens but was almost negative in normal epithelium[103]. The concentration of lumican in plasma was identified as a potential biomarker of ESCCviaa proteomic screen[104]. Nevertheless, a previous study by our group comparing a highly invasive ESCC subline with its parental cells showed thatLUMdecreased 8-fold in the highly invasive subline and was accompanied by activation of AKT pathway[105]. Liet al[101] reported that overexpression ofLUMsuppressed AKT activation in pancreatic cancer cells. These findings infer that lumican might be negatively correlated with AKT activation.
Testican-1
Testican-1, also known as proteoglycan 1, belongs to the SPOCK family and is encoded by theSPOCK1gene. It has been reported to be able to induce EMT in gastric cancer[106]. In colorectal cancer, knockdown ofSPOCK1could significantly reduce cell proliferation and invasion through the inhibition of phosphatidylinositol-3-kinase/AKT pathway[107]. In ESCC, upregulation ofSPOCK1also induces EMT and promotes cancer cell migration and invasion[108].
CONCLUSION
This article reviews the classification and structure of proteoglycans (Table 1 and Figure 1) and the functions of proteoglycans related to ESCC (Figure 3). As one of the components of the ECM, proteoglycans have been studied in several kinds of human cancers due to their roles in matrix organization and regulation of tumor cell-matrix interactions. Proteoglycans have diagnostic and prognostic significance in ESCC and can modulate ESCC cell migration/invasion and EMT. The secreted serglycin can also activate cell signaling in an autocrine manner (Figure 2). In addition, the significance of GAGs attached to the core protein of proteoglycans (e.g., serglycin and syndecan-1)and the expression level of GAGs regulated by multiple enzymes are increasingly gaining attention. Proteoglycans can enhance or inhibit the activity of soluble factors through interacting with them, and such interactions depend largely on the GAG chains. To date, most studies on proteoglycans in ESCC have focused primarily on their diagnostic and/or prognostic significance. In order to utilize or target these dynamic molecules in designing new strategies for treatment of this cancer, more indepth research is needed to decipher the complex roles of proteoglycans in ESCC,especially their interactions with other ECM components, receptors, and soluble factors.

Figure 3 Classification and schematic representation of proteoglycans studied in esophageal squamous cell carcinoma. Proteoglycans are classified as extracellular, pericellular, cell-surface, and intracellular according to their cellular and subcellular localization. The ones that have pro-invasive function in esophageal squamous cell carcinoma (ESCC) are highlighted in red bubbles, and the ones that act as the tumor suppressors are highlighted in green bubbles.Lumican is in the transparent bubble because its function in ESCC is still controversial. EMT: Epithelial-mesenchymal transition.
 World Journal of Clinical Oncology2021年7期
World Journal of Clinical Oncology2021年7期
- World Journal of Clinical Oncology的其它文章
- BRCA mutations and gastrointestinal cancers: When to expect the unexpected?
- Esophagogastric junction adenocarcinoma: Preoperative chemoradiation or perioperative chemotherapy?
- Mechanisms of acquired resistance of BRCA1/2-driven tumors to platinum compounds and PARP inhibitors
- Therapeutic potential of thymoquinone in combination therapy against cancer and cancer stem cells
