Bitter gourd extract improves glucose homeostasis and lipid profile via enhancing insulin signaling in the liver and skeletal muscles of diabetic rats
Saber Mohamed Eweda, Mennatallah Ahmed Ali, Hala Mohamed Abd El-Bary, Nahed Hussein El-Sokkary,Madiha Hassan Helmy, Maher Abdel-Nabi Kamel
1Department of Biochemistry, Faculty of Science, Alexandria University, Alexandria, Egypt
2Department of Pharmacology & Therapeutics, Faculty of Pharmacy and Drug Manufacturing, Pharos University in Alexandria, Alexandria, Egypt
3Department of Biochemistry, Medical Research Institute, Alexandria University, Alexandria, Egypt
4Department of Physiology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
5Medical Laboratories Technology, College of Applied Medical Sciences, Taibah University, Madinah, KSA
ABSTRACT
KEYWORDS: Neonatal; Streptozotocin; Diabetic; Sulfonylurea;Bitter gourd; Phosphorylated insulin receptor; Protein kinase C;IRS-1; GLUT2/GLUT4; Insulin signaling
1. Introduction
Type 2 diabetes mellitus (T2DM) is a complex heterogeneous group of metabolic conditions that are mainly caused by impaired insulin action and/or insulin secretion[1]. To date, the International Diabetes Federation (IDF) have estimated that 463 million adults(20-79 years) were living with diabetes; by 2045 this number will rise to 700 million[2]. Obesity is usually associated with insulin resistance that precedes hyperglycemia and T2DM and this close relationship has been referred to as “diabesity”[3]. Moreover, a variety of derangements in metabolic and regulatory mechanisms due to insulin resistance are responsible for the disruption of lipids metabolism, leading to classical dyslipidemia found with T2DM[4].
Although insulin signaling pathway is well-studied, the exact molecular mechanisms of insulin resistance are still not fully elucidated. Impaired proximal signaling of insulin receptor (IR)and decreased insulin receptor substrate (IRS) protein levels or their phosphorylation may attenuate the activation of protein kinase B (Akt) and atypical protein kinase C (aPKC) eventually leading to insulin resistance[5]. Protein kinase C (PKC) isoforms have an important role in glucose transport and are also involved in insulin resistance[6]. The aPKC isoforms are required for insulin-stimulated glucose transport in peripheral tissues (muscle and adipocytes).Under a variety of insulin-resistant conditions, the activation and/or expression of aPKCs is defected due to both impaired activation of IRS-1-dependent phosphoinositide 3-kinase (PI3K)and the direct activation of aPKCs by the lipid product of PI3K;phosphatidylinositol-3,4,5-triphosphate[7]. Reduced phosphorylation of Akt and aPKC in skeletal muscle and liver diminishes glucose transporter 4 (GLUT4) translocation and glucose uptake, decreases glycogen synthesis, and increases hepatic gluconeogenesis,leading to excessive glucose release through glucose transporter 2(GLUT2)[8].
Glibenclamide, also known as glyburide, is the most widely used sulfonylurea drug that binds sulfonylurea (SU) receptor, an ATPsensitive Kchannel. It stimulates insulin secretion by the elevation of cytosolic Caconcentration in the β-cells. Glibenclamide has a relatively long terminal half-life than other SUs, owing to its high affinity to SU receptor and the accumulation of active metabolites[9].Bitter gourd (Momordica charantia) is a powerful nutrient-dense fruit that is used to treat many illnesses in traditional cultures. It contains a diverse complex of beneficial compounds that include bioactive chemicals, vitamins (vitamin C, vitamin A, vitamin E,vitamins B, B, B, and folate), and minerals (potassium, calcium,zinc, magnesium, phosphorus, and iron)[10]. Medicinal value of bitter gourd has been mainly attributed to its high antioxidant properties; partly due to phenols, flavonoids, isoflavones, terpenes,anthroquinones, and glucosinolates[11]. Some compounds, especially charantin, vicine, and polypeptide-p, are believed to stimulate insulin secretion and alter hepatic glucose metabolism[12]. The aim of the present study is to explore the hypoglycemic effect of bitter gourd extract and to investigate its regulatory effects on insulin signaling pathway in liver and skeletal muscle tissues of neonatal streptozotocin (STZ) induced diabetic rats.
2. Materials and methods
2.1. Induction of type 2 diabetes
The neonatal rats were reared in air-conditioned rooms [(21 ±1) ℃], and relative humidity [(60 ± 10)%] with a 12 h light-dark cycle. Five-day-old male Wistar rats (Medical Research Institute,Alexandria, Egypt) were intraperitoneally injected with freshly prepared STZ (90 mg/kg; Sigma, St. Louis, MO, USA) in citrate buffer (0.1 M, sodium citrate, pH 4.5). These rats were fed a diet that consists of a mixture of cookies and a standard rat chow, containing approximately 57.7% carbohydrate, 19.5% protein, and 22.8%fat to induce the neonatal STZ (n5-STZ) type 2 diabetic model.After 3 months; fasting blood glucose level was determined using an automatic glucose meter (Accu-Check, Roche Diagnostics,Germany) and the rats with fasting blood glucose level ≥160 mg/dL were considered diabetic and were included in the current study[13].The control group received the vehicle solution in an equivalent volume and was maintained on a standard rat chow diet.
2.2. Preparation and extraction of bitter gourd
Egyptian variety of young, green bitter gourd fruits were kindly supplied by Prof. Dr. Esam M. Abd El-Kader, Vegetable Department,Faculty of Agriculture, University of Alexandria. The fruits were cleaned by tap water, cut into small pieces, and oven-dried at 50 ℃for a day. The dried sample was then pulverized into fine powder in a grinder and kept in an airtight container at 4 ℃ prior to use.One hundred grams of the fine powder were weighed and soaked in 67% ethanol at 76 ℃ for 72 h. During the extraction, the dispersion was vortexed. The mixture was filtrated and the residues were resoaked in 67% ethanol for 72 h at 76 ℃ and then filtered again. The combined extracts were evaporated in a vacuum at 40 ℃ to obtain viscous crude extract. Subsequently, the bitter gourd extract was further purified by adding 50% methanol to the viscous extract,vortexed for 15 min, centrifuged at 1 000 rpm for 10 min and the supernatant was discarded. The washing process was repeated using 50% methanol and hexane for complete elimination of chlorophyll,sugars, and other nonpolar impurities from the extract. The purified extract was finally evaporated in a vacuum at 40 ℃ again and collected in vials and stored at 4 ℃[14]. Different concentrations were prepared from the purified extract (100, 200, 400, and 600 mg/kg).These doses cover the wide range of the therapeutic doses of bitter gourd used in the literature[15-18].
2.3. Determination of total polyphenol and flavonoid contents in bitter gourd extract
Total polyphenol content of extract was determined by Folin-Ciocalteu method[19]. The results were expressed as milligram gallic acid equivalents/100 g dry weight. The total flavonoid content was determined using aluminum chloride colorimetric method[20]. The flavonoid content was expressed as milligram rutin equivalents/100 g dry weight.
2.4. Experimental design
Seventy male Wistar albino rats were divided into control group(GroupⅠ; n=10) and n5-STZ induced T2DM group (n=60). The diabetic rats were randomly allocated into six subgroups (n=10 each) and were fed on the same high caloric diet till the end of the experimental period. Subsequently, these subgroups were divided into; groupⅡ(untreated diabetic rats) received the vehicle, while group Ⅲ was treated with glibenclamide (0.1 mg/kg/day, p.o.,Daonil, Sanofi Aventis, Guildford, UK). Groups Ⅳ, Ⅴ, Ⅵ, andⅦ were orally administered bitter gourd extract in different doses(100, 200, 400, and 600 mg/kg/day, respectively) for 30 d. The final body weights were recorded. At the end of treatment periods, the rats fasted overnight were sacrificed by cervical dislocation and blood samples were obtained to separate the serum for further analysis,then the animals were dissected out to obtain liver and skeletal muscle tissues for assessment of tissue parameters.
2.5. Ethical statement
The current study was approved by the “Institutional Animal Care and Use Committee (IACUC)-Alexandria University, Egypt” (Approval No.: AU01219073033; date of approval 30 July 2019). Experiments were performed in strict accordance with the guidelines and regulations of Egypt’s guide for the care and use of laboratory animals[21]. All efforts were made to decrease the distress of rats during the experimental period.
2.6. Oral glucose tolerance test (OGTT)
After rats fasted overnight, OGTT was performed. The baseline blood glucose level was determined, then glucose (2 g/kg) was administered through a gavage tube and blood samples were collected at 30, 60, 90, and 120 min. The area under the curve (AUC)was calculated for blood glucose values during the OGTT using the following equation[22]:
AUC = 0.25 (fasting value) + 0.5 (1/2 h value) + 0.75 (1 h value) +0.75 (2 h value)
2.7. Determination of serum biomarkers
Serum samples were obtained from the overnight fasted animals at the end of the treatment period and were used to assess fasting serum glucose (Randox colorimetric reagent kits, Antrim, UK) and insulin using an ELISA kit (Abnova, Jhongli, Taiwan). Colorimetric kits (Boehringer Mannheim, Germany) were used to determine serum triglycerides (TGs) and total cholesterol (TC). High-density lipoprotein cholesterol (HDL-C) was determined using the method described by Lopes-Virella et al[23]. Low-density lipoprotein cholesterol (LDL-C) was then calculated according to the Friedewald equation[24]:
LDL-C = TC-(HDL-C + 1/5 TGs)
The homeostasis model assessment index (HOMA) for insulin resistance was determined using the following formula[25]:HOMA = (fasting glucose × fasting insulin)/405
2.8. Determination of tissue parameters
Immediately after blood collection, rats were deeply anesthetized using ketamine 100 mg/kg and xylazine 10 mg/kg and then euthanized. Their livers and soleus skeletal muscles were excised,homogenized, divided into aliquots, and preserved at -80 ℃ until assay. Commercially available ELISA kits were used to determine GLUT2/GLUT4 (Uscn Life Science Inc., Wuhan, China) and phospho-IR-β (Tyr1150/1151) (CST PathScan, Cell Signaling,Beverly, MA). IRS-1 and PKC were determined using the corresponding ELISA kit (Wkea Med Supplies, Changchun, China).A commercially available Upstate colorimetric Signal Transduction Assay Reaction ELISA kit was used to measure p-Akt (Threonine 308) in cellular lysate (Millipore, Billerica, MA, USA). The protein concentrations were determined using the modified Lowry method[26].
2.9. Statistical analysis
Values are expressed as mean ± SD of 10 animals. The GraphPad Prism v5.0 (GraphPad Prism Inc., La Jolla, CA, USA) was used to analyze and present all the data. Multiple comparisons were performed using one-way ANOVA, followed by Tukey post-hoc test using Pearson correlation coefficient. The correlation coefficients (r)between different assayed parameters were also evaluated; P<0.05 was considered significantly different.
3. Results
3.1. Total polyphenol and flavonoid contents of bitter gourd extracts
The present study showed that the total polyphenol and flavonoid contents of ethanolic extract of bitter gourd were 592.23 mg gallic acid and 243.41 mg rutin equivalent/100 g dry weight, respectively.
3.2. Effect of bitter gourd extracts on OGTT of n5-STZ diabetic rats
As shown in Table 1, the diabetic rats showed 2.7 folds increase in fasting blood glucose level compared to normal control rats and the level reached its peak half an hour after oral glucose administration and then decreased gradually. However, blood glucose level in diabetic rats was elevated by about 181.2% above normal value after 30 min and failed to reach the pre-prandial level 2 hours later, confirming a state of impaired glucose tolerance. Bitter gourd extract at different doses abated the high glucose level in the OGTT to various extents, while the dose of 400 mg/kg was able to normalize blood glucose level equivalently to the effect achieved by glibenclamide as confirmed by the AUC (Figure 1).
3.3. Effect of bitter gourd extracts on body weight and glucose homeostasis parameters of n5-STZ diabetic rats
The results of the current study indicated that n5-STZ diabetic rats showed a significant increase in body weight, fasting blood glucose level, and HOMA index compared with control rats (Table 2). The diabetic rats also showed reduced fasting insulin levels by 33.65%compared to control rats. Treatment with glibenclamide normalized these parameters. Moreover, different doses of bitter gourd extracts showed varying effects on these parameters. The group treated with bitter gourd extracts at the doses of 100, 200, and 600 mg/kg failed to significantly reduce the body weight compared with the diabetic group. In contrast, the bitter gourd extract at a dose of 400 mg/kg remarkably decreased body weight, which showed a similar effect to glibenclamide. However, treatment with different doses of bitter gourd extracts showed a significant reduction in fasting blood glucose level. The same pattern was observed in the ability of bitter gourd to increase insulin levels. In addition, the most predominant effect in reducing HOMA index was observed in 200 and 400 mg/kg of bitter gourd extract.
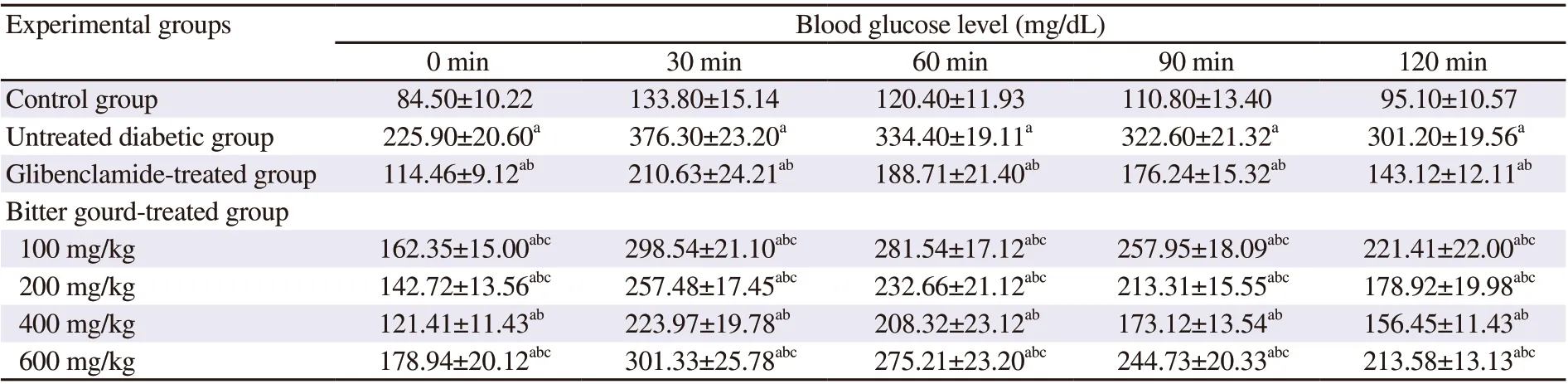
Table 1. Effect of bitter gourd extract on oral glucose tolerance.
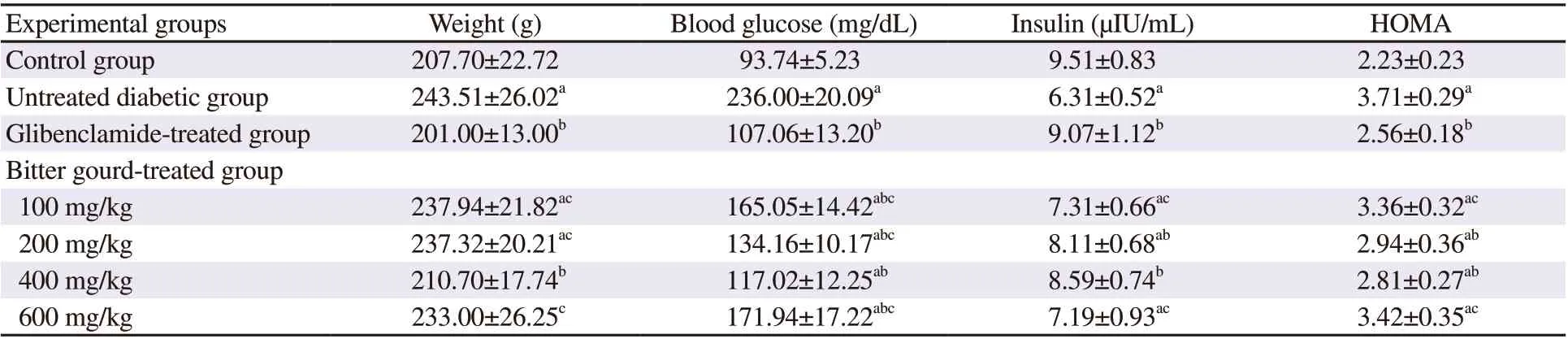
Table 2. Effect of bitter gourd extract on body weight and glucose homeostasis parameters in n5-STZ diabetic rats.
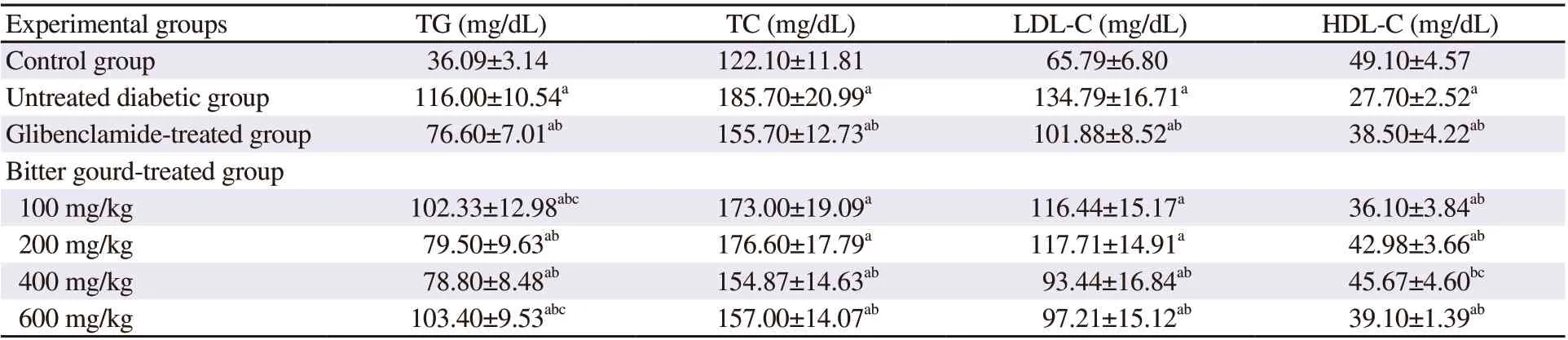
Table 3. Effect of bitter gourd extract on lipid profile in n5-STZ diabetic rats.
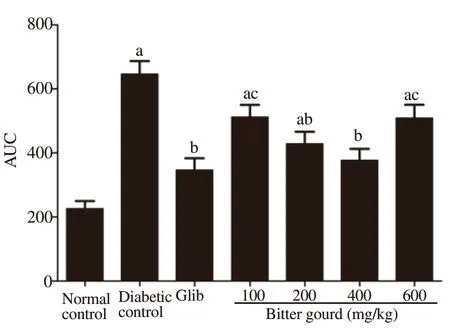
Figure 1. Effect of bitter gourd extract on the area under the curve (AUC)of the oral glucose tolerance test (OGTT) in n5-STZ diabetic rats. Values are expressed as mean ± SD (n=10). Values with different superscript letters indicate significant differences compared with the normal control(a), untreated diabetic rats (b) or glibenclamide treated rats (c) at P<0.05 by ANOVA followed by Tukey post-hoc test. Glib: Glibenclamide.
3.4. Effect of bitter gourd extracts on serum lipid profile of n5-STZ diabetic rats
The diabetic model showed altered serum lipid profile (Table 3),demonstrated by significant increases in serum TG by about 3.2 folds, TC by about 1.5 fold, and LDL-C by about 2 folds compared to control rats. On the other hand, HDL-C was decreased in the n5-STZ diabetic rats by 43.58% compared to the normal control value. Treatment with glibenclamide improved lipid profile as demonstrated by the significant decline in the levels of TG by 34%,TC by 16.16%, and LDL-C by 24.42 %, in addition to the increase in HDL-C by 38.99% when compared to diabetic untreated rats.Bitter gourd extracts at the dose of 400 mg/kg showed the best improvement in lipid profile.
3.5. Effect of bitter gourd extracts on hepatic insulin signaling pathway in n5-STZ diabetic rats
The diabetic rats showed marked disruption in the liver insulin signaling pathway, confirmed by a significant increase in GLUT2 by 70.3% compared to control rats [(138.15±12.70) pg/mg protein vs. (81.12±7.45) pg/mg protein] as well as a significant decrease in p-IR-β [(4.39±0.59) pg/mg protein vs. (6.77±0.74) pg/mg protein],IRS-1 [(1.48±0.15) pg/mg protein vs. (1.82±0.21) pg/mg protein],p-Akt [(49.77±3.78) U/mg protein vs. (60.63±7.33) U/mg protein],and PKC [(29.10±2.70) pg/mg protein vs. (52.30±6.14) pg/mg protein] compared to the control value (Figure 2). Treatment with glibenclamide and bitter gourd extract (400 mg/kg) increased hepatic IRS-1, p-IR-β, and PKC, and decreased GLUT2. On the other hand,the other doses of bitter gourd failed to improve the levels of hepatic p-IR-β, IRS-1 and GLUT2 compared to the untreated diabetic group.
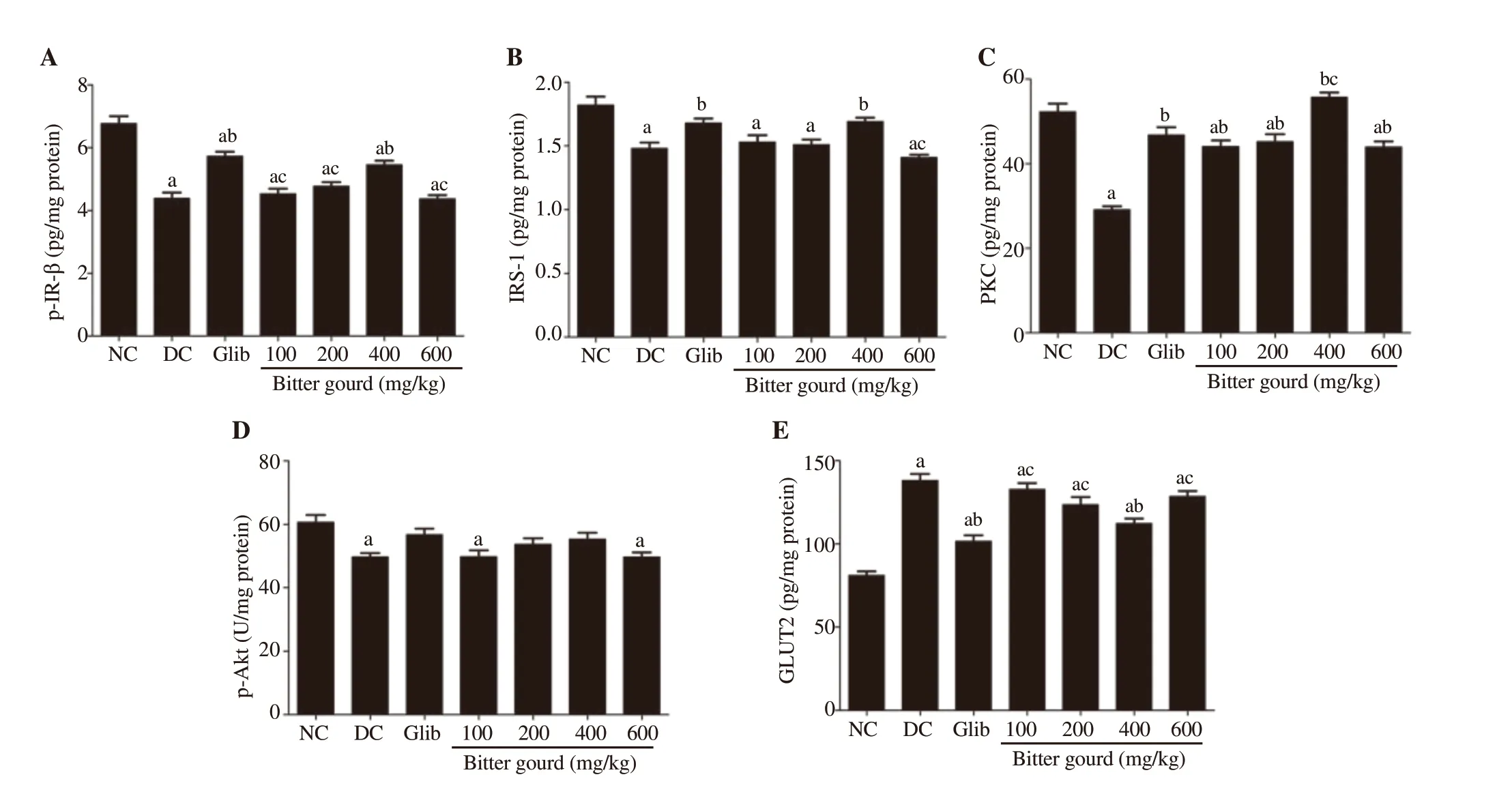
Figure 2. Effect of bitter gourd extract on hepatic insulin signaling pathway [phosphorylated insulin receptor-β (p-IR-β) (A), insulin receptor substrate-1 (IRS-1)(B), protein kinase C (PKC) (C), p-Akt (D) and glucose transporter 2 (GLUT2) (E)] in n5-STZ diabetic rats. GLUT2, p-IR-β, IRS-1, PKC and p-Akt were determined in cellular lysate of liver using their corresponding ELISA kit.Values are expressed as mean ± SD (n=10). Values with different superscript letters indicate significant differences compared with the normal control (a), untreated diabetic rats (b) or glibenclamide treated rats (c) at P<0.05 by ANOVA followed by Tukey post-hoc test. NC: normal control; DC: diabetic control.
3.6. Effect of bitter gourd extracts on insulin signaling pathway in skeletal muscle of n5-STZ diabetic rats
The results showed a prominent disruption in insulin signaling pathway in skeletal muscle of the untreated diabetic group,illustrated by a significant decrease in p-IR-β, IRS-1, p-Akt,PKC, and GLUT4 [(3.95±0.33) pg/mg protein, (1.25±0.09) pg/mg protein, (88.52±11.21) U/mg protein, (35.55±4.09) pg/mg protein, and (46.21±3.24) pg/mg protein; respectively] compared to the control values [(6.06±0.72) pg/mg protein, (1.69±0.18) pg/mg protein, (105.22±13.32) U/mg protein, (55.97±6.61) pg/mg protein, and (104.30±9.69) pg/mg protein; respectively] (Figure 3). Treatment with glibenclamide non-significantly elevated p-Akt and significantly elevated PKC, and GLUT4 in skeletal muscle and normalized skeletal muscle IRS-1 level, but failed to significantly increase p-IR-β. Different doses of bitter gourd extracts improved these parameters.
3.7. Correlations
Statistical analysis of bitter gourd treated groups showed that the HOMA was negatively correlated with hepatic p-IR-β (r = -0.369, P= 0.019, Figure 4A), IRS-1 (r = -0.353, P = 0.025, Figure 4B), and PKC (r = -0.366, P = 0.020, Figure 4C). Moreover, HOMA showed a negative correlation with skeletal muscle p-IR-β (r = -0.393, P =0.120, Figure 4A), IRS-1 (r = -0.373, P = 0.180, Figure 4B), PKC (r= -0.349, P = 0.270, Figure 4C) and GLUT4 (r = -0.328, P = 0.039,Figure 4D) (Figure 4).
4. Discussion
T2DM is a pandemic disorder that requires novel approaches for prevention of T2DM and amelioration of its progression as well as subsequent complications and consequences[27]. Apart from the currently available therapeutic options, traditional and complementary medicines may offer a revolutionary breakthrough in the treatment of T2DM and may propound the prospect of future drugs to counteract insulin resistance, consistent with the global rising interest in drug discovery from natural products[12].
Bitter melon is a tropical plant that has a remarkable versatility in treating a wide range of illnesses[10]. Abundant pre-clinical studies have documented the anti-diabetic and hypoglycemic effects of bitter gourd through various postulated mechanisms, however, the precise mode of action remains unclear[28]. The current study hypothesized that the effects of bitter gourd on the stimulation of insulin secretion and improvement of hepatic and skeletal muscle insulin signaling pathways reinforce its beneficial effects in treating T2DM.
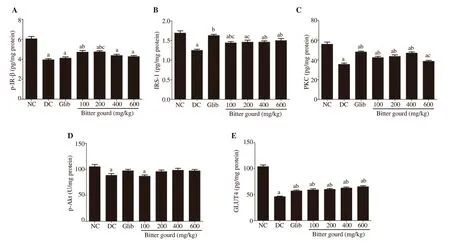
Figure 3. Effect of bitter gourd extract on insulin signaling pathway in skeletal muscle [p-IR-β (A), IRS-1 (B), PKC (C), p-Akt (D) and glucose transporter 4(GLUT4) (E)] of n5-STZ type 2 diabetic rats. Values are expressed as mean ± SD (n=10). GLUT4, p-IR-β, IRS-1, PKC and p-Akt were determined in cellular lysate of skeletal muscle using their corresponding ELISA kit. Values with different superscript letters indicate significant difference compared with the normal control (a), untreated diabetic rats (b) or glibenclamide treated rats (c) at P< 0.05 by ANOVA followed by Tukey post-hoc test..
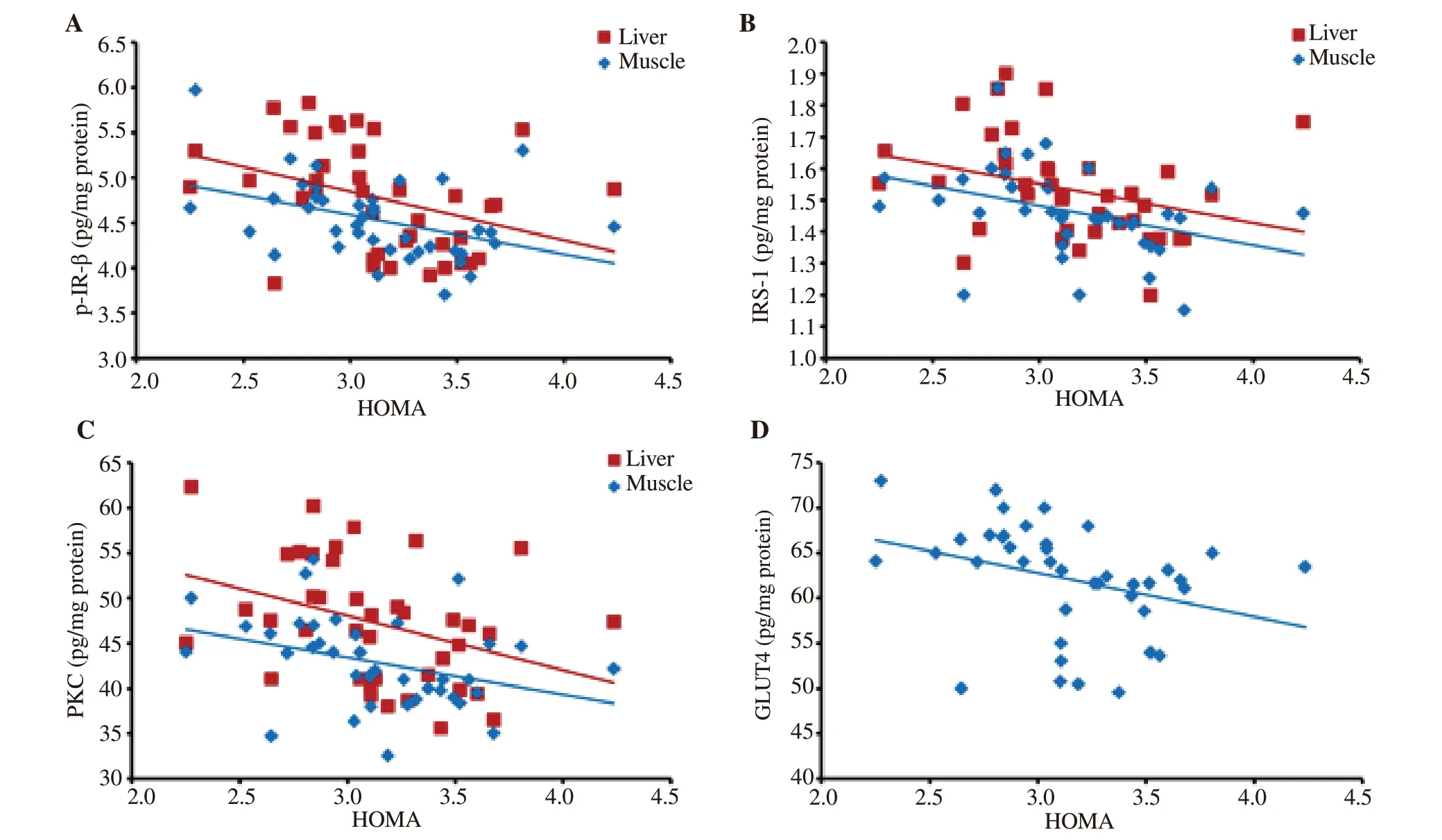
Figure 4. Correlation between HOMA insulin resistance index and the components of the insulin signaling pathway; p-IR-β (A), IRS-1 (B), and PKC (C) in liver and muscle and GLUT4 (D) in the muscle tissue of bitter gourd treated rats. HOMA: homeostasis model assessment index.
Phenolic compounds including polyphenols and flavonoids were reported to have important physiological activities such as antidiabetic,antioxidant, anticarcinogenic, and antimutagenic activities[29]. The result of the present study showed that the ethanolic extract of bitter gourd contains polyphenols equivalent to 592.23 mg gallic acid and flavonoids equivalent to 243.41 mg rutin/100 g dry weight. A previous study reported that the total phenolic and flavonoid contents of bitter gourd extract were 639.37 mg gallic acid and 203.31 mg catechin equivalents/100 g dry weight, respectively. The study also showed that the major phenolic acids in bitter gourd extract were gallic acid, chlorogenic acid, catechin, caffeic acid, p-coumaric acid, and ferulic acid[30]. Steroidal glycoside, saponin, charantin, polypeptide k, and terpenoids are the main phytochemical compounds found in ethanolic bitter gourd extract[14].
The results indicated that the n5-STZ diabetic rat model showed a typical manifestation of T2DM, including increased body weight,hyperglycemia, and elevated HOMA-IR, compared to control rats. This impaired glucose homeostasis is also associated with disturbed lipid metabolism as indicated by elevated TG, TC, and LDL-C, and declined HDL-C. These abnormalities of glucose and lipid homeostasis were associated with detected impairments in insulin signaling including p-IR-β, IRS-1, p-Akt, PKC, GLUT2 in the liver, and GLUT4 in skeletal muscles. These findings are consistent with those of other studies that used a similar rat model of T2DM[13,31]. Different doses of ethanolic extracts of bitter gourd showed reduction in fasting blood glucose,HOMA-IR, and serum lipids as well as an increase in fasting insulin level and HDL-C, with the best results obtained with 400 mg/kg dose compared with other doses. The therapeutic effects of 400 mg/kg of bitter gourd extract were equivalent to those of glibenclamide.
The insulinotropic effect of bitter gourd extract might be related to its ability to stimulate the proliferation and the spontaneous recovery of insulin-secreting pancreatic β-cells, increasing their number and function[32], as well as preventing their death via its antioxidant properties[33]. It has been also suggested that the zinc content of bitter gourd, which is an important cofactor in various enzymes involved in glucose metabolism[34], could play a role in glucose-lowering effect in addition to increasing insulin secretion. Moreover, bitter gourd contains different amino acids, such as leucine that enters islets by a sodiumindependent transport system, stimulating a biphasic increase in insulin release[35] and gamma-aminobutyric acid that acts on GABAA receptor in the cells, causing membrane hyperpolarization and hence suppressing glucagon secretion[36].
The lipid-lowering properties of bitter gourd can be explained by lowering plasma apoB-100/48. Moreover, plant insulin, saponins, and plant sterol in bitter gourd are known to reduce blood TG and intestinal cholesterol absorption via inhibition of pancreatic lipase activity. Also, it has been suggested that bitter gourd affects the breakdown of LDL-C and may enhance fat oxidation[37,38].
Bitter gourd treatment showed significant enhancement in insulin signaling in the liver and muscle. This induction at the protein level indicates that some constituents of bitter gourd may act as positive regulators at the transcriptional, post-transcriptional, and/or translational level of gene expression. However, the specific constituents responsible for such effects require further investigation. The current findings suggest that the high levels of insulin-stimulated by bitter gourd,autophosphorylates insulin receptors at tyrosine residues (p-IR),stimulating downstream IRS-1 pathways to further activate PI3 kinase(PI3K), PKB/Akt, and PKC. This cascade promotes skeletal muscle GLUT4 translocation to enhance glucose uptake and utilization and subsequently inhibits glycogen synthase kinase-3β activity in the liver,leading to enhanced glycogen synthesis and inhibited gluconeogenesis.These corrections in insulin signaling pathway were negatively correlated with HOMA-IR, confirming our hypothesis of the underlying mechanism of the antidiabetic action of bitter gourd.
In line with our data, previous studies showed that bitter gourd increased GLUT4 mRNA and protein expression levels in skeletal muscle of fructose-fed rats[39]. It also significantly increased basal Akt phosphorylation as well as insulin-stimulated phosphorylation of IRS-1,Akt, and PI3K[40]. Bitter gourd may act as a peroxisome proliferator activator receptor-gamma agonist that regulates the genes involved in carbohydrate metabolism[41]. Also, the triterpenoids of bitter gourd were documented to activate AMP-activated protein kinase (AMPK) with subsequent GLUT4 translocation and glucose uptake[42].
Notably, the results of our study demonstrated that glibenclamide and bitter gourd at the dose of 400 mg/kg exerted nearly equivalent effects on insulin secretion, however, bitter gourd’s effect on peripheral response of insulin signaling components, IRS-1 and GLUT4, surpassed that of glibenclamide. This effect might be ascribed to the presence of polypeptide-p or plant insulin, which is an insulin-like hypoglycemic protein[43].
The observed discrepancy between the effect of the highest dose of bitter gourd (600 mg/kg) and lower doses (100-400 mg/kg) requires further investigation. This discrepancy may be related to the environmental and/or agricultural contaminants to which the plant was exposed during breeding and processing. Such suggestions need further verification, and the presence of inhibitors or contaminants requires concrete evidence.
In summary, bitter gourd extract is a powerful glucose and lipidlowering agent that is equivalent to classical sulfonylurea (glibenclamide).It can be recommended at controlled doses to treat T2DM. Its effect is mediated through acting as an insulin secretagogue and sensitizer,mimicking insulin action and inducing peripheral tissues IRS1/Akt/PKC/GLUT4 insulin signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr. Esam M Abd El-Kader, Faculty of Agriculture, Department of Vegetable, Alexandria University,Alexandria, Egypt, for providing the bitter gourd fruits.
Authors’ contributions
SME, MAK, and HMA contributed to the experimental design and performing the experiments. MHH, MAK, and HMA conceived and analyzed the data. SME, MHH, and MAK wrote the manuscript.NHE and MAA contributed reagent, materials, analysis tools and revised the manuscript. Asian Pacific Journal of Tropical Biomedicine2021年8期
Asian Pacific Journal of Tropical Biomedicine2021年8期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: A scoping review
- Valencene-rich fraction from Vetiveria zizanioides exerts immunostimulatory effects in vitro and in mice
- Cytotoxic effects of Thai noni juice product ethanolic extracts against cholangiocarcinoma cell lines
- Antioxidant and antigenotoxic properties of Alpinia galanga, Curcuma amada, and Curcuma caesia
