Drug-drug cocrystals:Opportunities and challenges
Xiaojuan Wang,Shuzhang Du,Rui Zhang,Xuedong Jia,Ting Yang,Xiaojian Zhang
Department of Phamacy,The First Affiliated Hospital of Zhengzhou University,Zhengzhou 450052,China
Keywords:Drug-drug cocrystal Drug combination Cocrystal Physicochemical property
ABSTRACT Recently,drug-drug cocrystal attracts more and more attention.It offers a low risk,low-cost but high reward route to new and better medicines and could improve the physiochemical and biopharmaceutical properties of a medicine by addition of a suitable therapeutically effective component without any chemical modification.Having so many advantages,to date,the reported drug-drug cocrystals are rare.Here we review the drug-drug cocrystals that reported in last decade and shed light on the opportunities and challenges for the development of drug-drug cocrystals.
1.Introduction
At the cellular and organ levels,complex diseases such as cancer,diabetes,infectious diseases,and cardiovascular diseases are associated with multiple alterations in molecular pathways and complex interactions [1] .When considering drug treatment strategies in these complex diseases,single-target mono-therapy approaches have fundamental limitations as the disease phenotypes are rarely driven by single molecular entities.In addition,while there may be benefits from using a single compound in the treatment of a disease,treatments consisting of mixtures of active pharmaceutical ingredients (APIs) often have superior effects[2] .Consequently,the combination of multiple APIs into unit doses has become a popular drug development strategy.The use of multiple-targeting fixed dose drug combinations(FDCs) can help reduce pill load without the additional risk of adverse events or drug resistance [3] .As a result,FDCs could simplify dosage regimens (e.g.,reduce the number and timing of dosage units the patient must take) and improve patient compliance [4],particularly among the patient populations with special needs such as geriatric and pediatric patients[5] .In addition,these FDCs are being used to achieve highly specific drug-delivery profiles and targeted release to specific sites within the body,and extend product life cycles.However,the development of FDC remains a major challenge in drug development because of its various disadvantages,such as stability,solubility differences and incompatibility between the parent drugs [6] .Among the common FDC formulation designs,the bilayer tablet has many advantages over the monolithic tablet,such as avoiding the problem of chemical stability between two incompatible drugs,providing both a rapid release layer and a slow release layer to produce the desired overall drug release kinetics for optimal therapeutic outcome [3—4,7] .However,the successful development of bilayer tablet products must also overcome new challenges in formulation design,manufacturing process control,and product performance requirements unseen in traditional monolithic tablets.Therefore,it is necessary to develop new technologies to overcome these problems.Drug-drug cocrystal (DDC) is one of the most suitable methods.
According to the FDA,co-crystals are defined as‘dissociable multicomponent solid crystalline supramolecular complexes composed of two or more components within the same crystal lattice where in the components are in neutral state and interact via nonionic interactions.’ [8] In the past decade,pharmaceutical co-crystals have been designed as a new solid form by virtue their capability to modulate physicochemical properties of APIs.Since several key publications [7,9—10] in 2003—2004 demonstrated that cocrystallization could be applied to improve the physicochemical properties of drug substances,the researches of cocrystals related to pharmaceutical science have been grown rapidly.Nair et al.developed a cocrystal of posaconazole-4-aminobenzoic acid with superior solubility and dissolution behavior [11] .Das et al.prepared a cocrystal of moxifloxacin with trans-cinnamic acid improved residence time in the lungs [12] .Chen et al.reported a cocrystal of temozolomide and baicalein with modulated plasma drug concentration [13] .Mei et al.synthesized a cocrystal of nifedipine and isonicotinamide with superior photostability[14] .In addition,several drugs are marketed as a cocrystal,for instance,ipragliflozin-L-proline [15],eglidene-L-pyroglutamic acid [16],escitalopram-oxalate [17] and chloral-betaine[18] cocrystals.
While both components of a cocrystal are APIs,the cocrystal could be called DDC [19] .DDC represents a viable approach to overcome the problems associated with traditional combination drugs.It can also modulate physicochemical properties of parent drugs and can be used as potential solid forms for the delivery of dual drugs therapy.Compared to other drug combination technologies,DDC may improve the physicochemical properties of relevant APIs,such as improved dissolution property of at least one component,increased bioavailability,enhanced stability of unstable components and assistance in lifecycle management of existing products.Carmen Almansa et al.reported a cocrystal of tramadol hydrochloride and celecoxib (CTC) in 2017 [20] .The dissolution studies showed that the intrinsic dissolution rate (IDR) of celecoxib was increased and that of tramadol hydrochloride is released slower.Such IDR result was subsequently correlated with a positively modified clinical pharmacokinetic profile in comparison with commercially available single-entity reference products and the open combination [21] .In humans,Tramadol was absorbed more slowly from CTC,resulting in a reduction in peak plasma concentration (C),while celecoxib was absorbed faster from CTC.In addition,the phase II clinical studies showed that CTC also presented a superior analgesic effect compared to tramadol and celecoxib for the management of moderate to severe pain following extraction of two or more impacted third molars requiring bone removal [22] .This DDC also got new intellectual property protection and may prolong the lifecycle of both celecoxib and tramadol.Zaworotko et al.developed a DDC of meloxicam with aspirin [23] .This DDC presented a 44-fold increase in pH 7.4 phosphate buffer solubility and a fourfold improved bioavailability.Lu et al.reported a DDC synthesized by nicotinamide with tranilast[24] and reported a 1.9 times higher solubility and improved photostability compared to those of pure tranilast.Compared to general cocrystals,DDCs could offer potential advantages of synergistic and/or additive effects and sometimes they may extend their useful range to treat new diseases.Zegarac et al.developed a DDC of sildenafil and aspirin with improved IDR compared with a marketed sildenafil citrate salt [25] .The DDC also displays a potential application in the treatment of erectile dysfunction in patients with cardiovascular complications.
Although more and more pharmaceutical cocrystals had been developed,there is limited published work available on DDCs.Here we review the DDCs that reported in last decade and shed light on the opportunities and challenges for the development of DDCs.
2.Reported DDCs in the last decade
Recently,several DDCs formed with the APIs in a variety of therapeutic areas have been reported.Among them,the nonsteroidal antiinflammatory drugs (NSAIDs) formed the most multi-drug cocrystals and approximate half of the reported DDCs have at least one NSAID as their cocrystal co-former.The antitubercular and diuretic APIs formed the second and third most DDCs,more than one third of the reported DDCs are formed with at least one of these two types of APIs.
2.1.DDCs of NSAIDs
NSAIDs are the most common and effective drugs used to relieve pain of the patients suffering from arthritis,low back pain and soft tissue pain.These drugs work by blocking the class of the enzymes called cyclooxygenases(COX) and subsequently the production of prostaglandins.Table 1 listed the DDCs of NSAIDs reported in the last decade.Among the 30 reported DDCs,7 DDCs were formed with two NSAIDs and the other 23 DDCs were formed with one NSAID and one other API.The pharmaceutical properties of 12 DDCs had not been evaluated.The other 18 DDCs exhibited various pharmaceutical enhancements.14 DDCs presented improved dissolution properties,3 DDCs showed enhanced stability,3 DDCs exhibited improved drug release and 1 DDC displayed an improved in vivo stability.Nangia and his team reported a 1:1 DDC of andrographolide and salicylic acid [26] .This DDC presented 12 times higher solubility,3 times faster IDR and relatively 2 times higher estimated drug release.In addition,the andrographolide—salicylic acid cocrystal completely inhibited the chemical transformation of andrographolide to its inactive sulfate metabolite.Zhang et al.developed a DDC of piroxicam and clonixin [27] .Both of the two elements are NSAIDs.8 solvates of this DDC were obtained and the pharmaceutically acceptable ethyl acetate solvate demonstrated superior physical stability against hydration.Surov et al.developed 2 DDCs of diflunisal and diclofenac with theophylline [28] .The IDR of diclofenactheophylline was around 1.2 times higher than for each drug alone.Enhanced hygroscopic stability of theophylline was observed at 100% relative humidity (RH) (Scheme 1 —4).
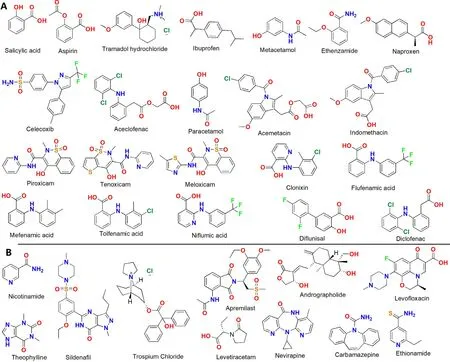
Scheme 1–The molecular structures of the APIs that formed the NSAID DDCs (a) NSAIDs,(b) the other APIs.
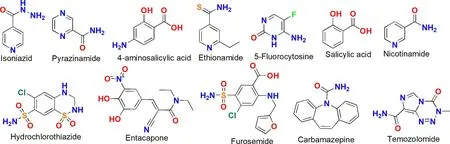
Scheme 2–The molecular structures of the APIs that formed the DDCs of antitubercular drugs.
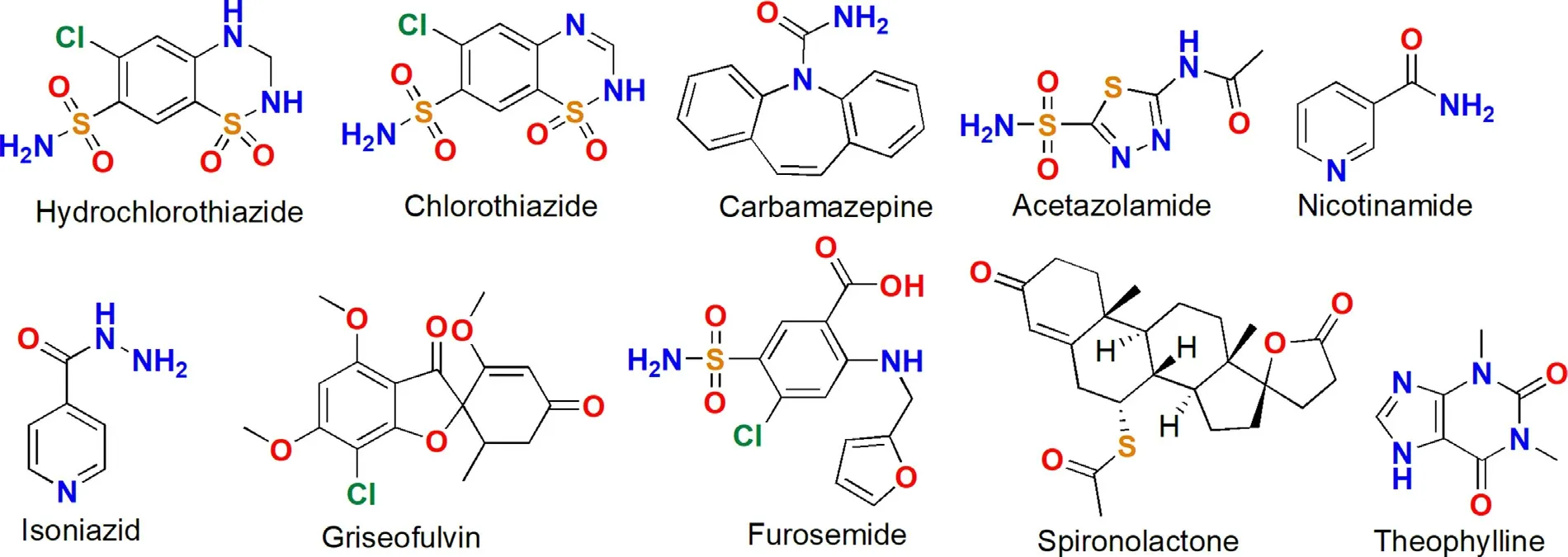
Scheme 3–The molecular structures of the APIs that formed the DDCs of diuretic drugs.
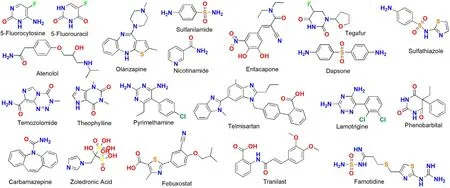
Scheme 4–The molecular structures of the APIs that formed the other DDCs.
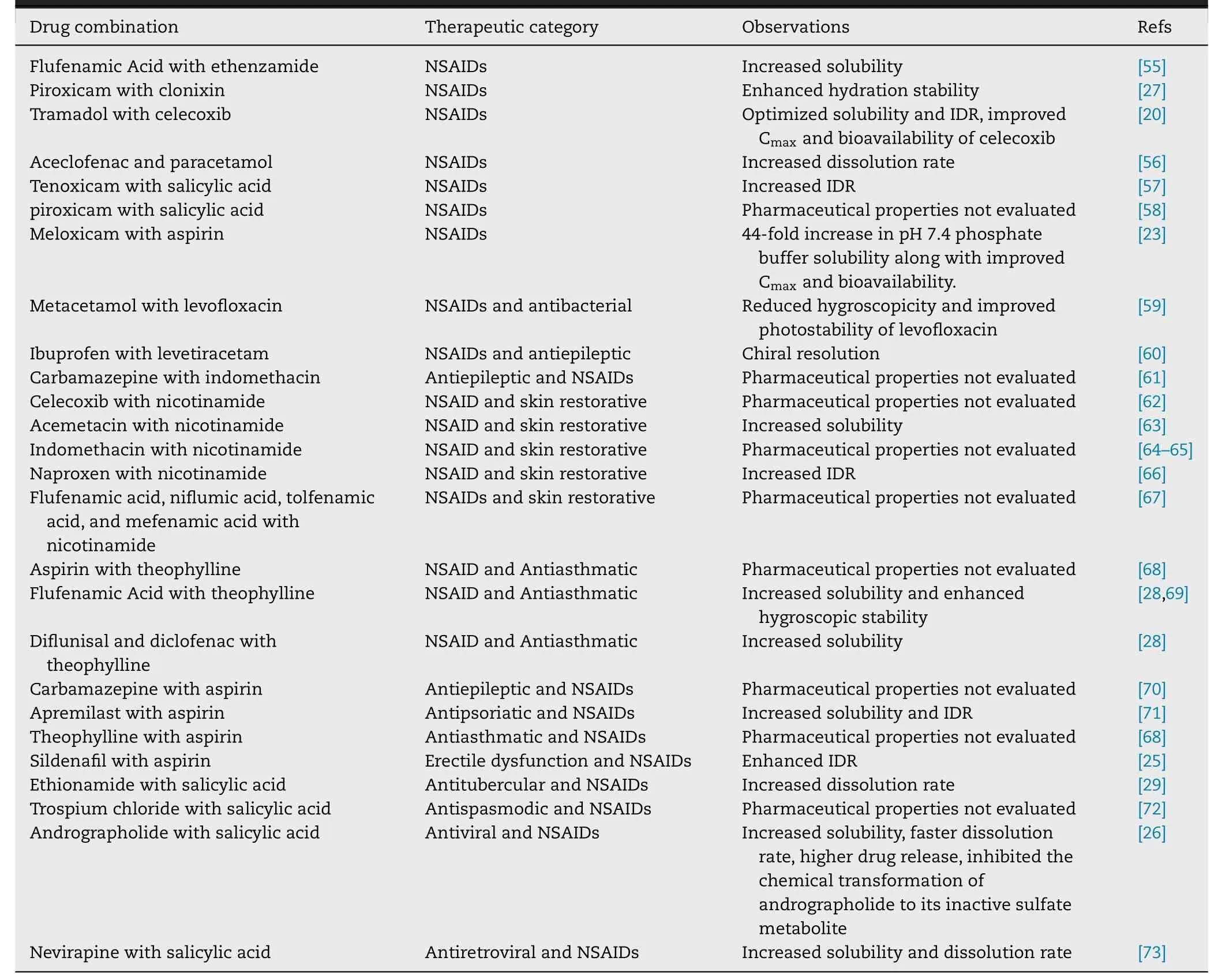
Table 1–DDCs of NSAIDs reported in the last decade.
2.2.DDCs of antitubercular drugs
Tuberculosis is a chronic infectious disease caused by mycobacterium tuberculosis and antitubercular drugs are used to treat tuberculosis.Table 2 listed the DDCs of antitubercular drugs reported in the last decade.Among the 13 reported DDCs,the pharmaceutical properties of 3 DDCs had not been evaluated.The other 10 DDCs Exhibited various pharmaceutical enhancements.8 DDCs presented improved dissolution properties,4 DDCs showed optimized diffusion permeability and 1 DDC exhibited enhanced hydration stability.Chierotti et al.developed a DDC of ethionamide with salicylic acid and reported an increased dissolution rate compared to the pure ethionamide [29] .Ellena et al.reported a 1:1 DDC of 5-fluorocytosine with isoniazid [30] .The DDC has superior phase stability properties against moisture when compared to the starting pharmaceutical ingredients.Yan et al.developed a drug-bridge-drug ternary cocrystal of isoniazid with fumaric acid and pyrazinamide [31] .This cocrystal exhibits optimized formulation capacity and in vitro/vivo synergistic effects,which provides a new insight into antituberculosis combination drugs.
2.3.DDCs of diuretic drugs
Diuretics are used as the sole therapeutic agents to treat hypertension.Diuretics can also be used in combination with other antihypertensive drugs to treat more severe forms of hypertension.Table 3 listed the DDCs of diuretic drugs reported in the last decade.Among the 9 reported DDCs,the pharmaceutical properties of 3 DDCs had not been evaluated.The other 6 DDCs all presented improved dissolution properties.In addition,4 DDCs also showed optimized diffusion permeability.Desiraju et al.developed a DDC formed by hydrochlorothiazide and nicotinamide[32],and the DDC have higher solubility (1.3 fold) and improved permeability (1.8 fold) compared to those of pure hydrochlorothiazide.
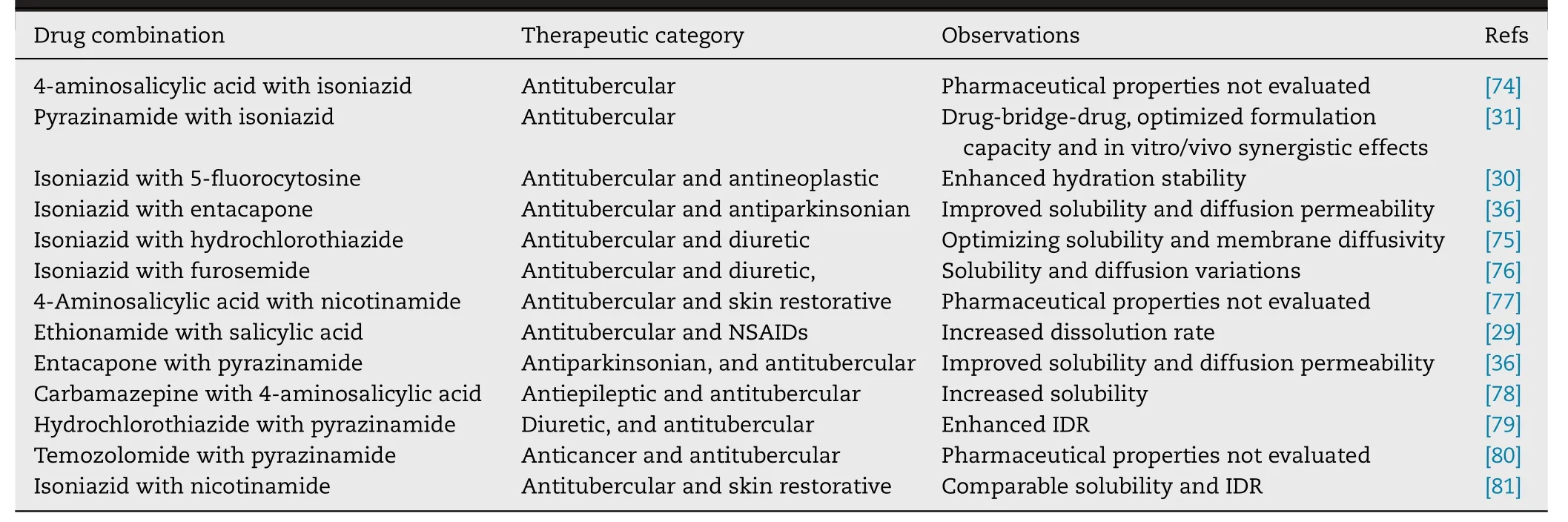
Table 2–DDCs of antitubercular reported in the last decade.
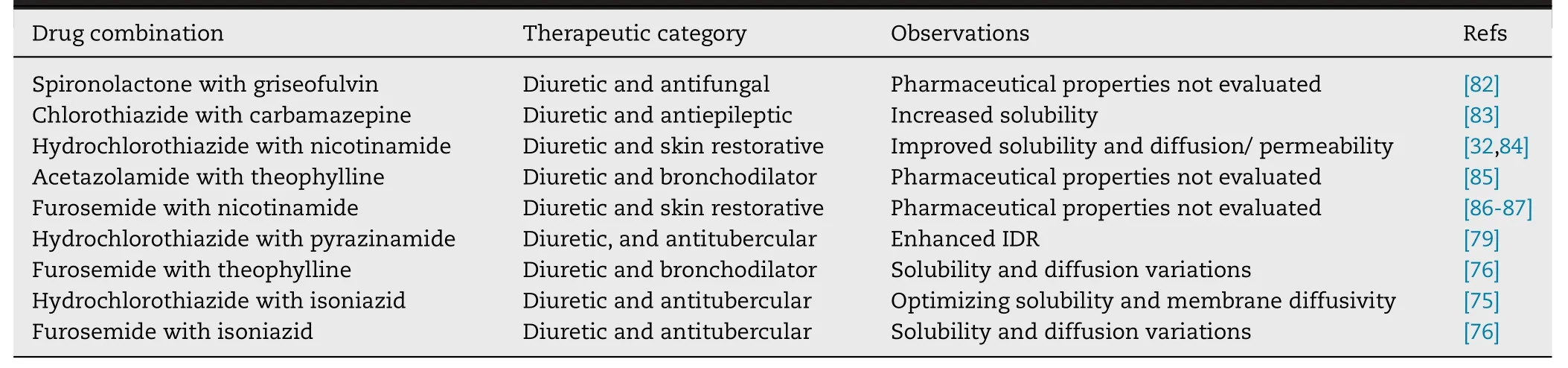
Table 3–DDCs of diuretic reported in the last decade.
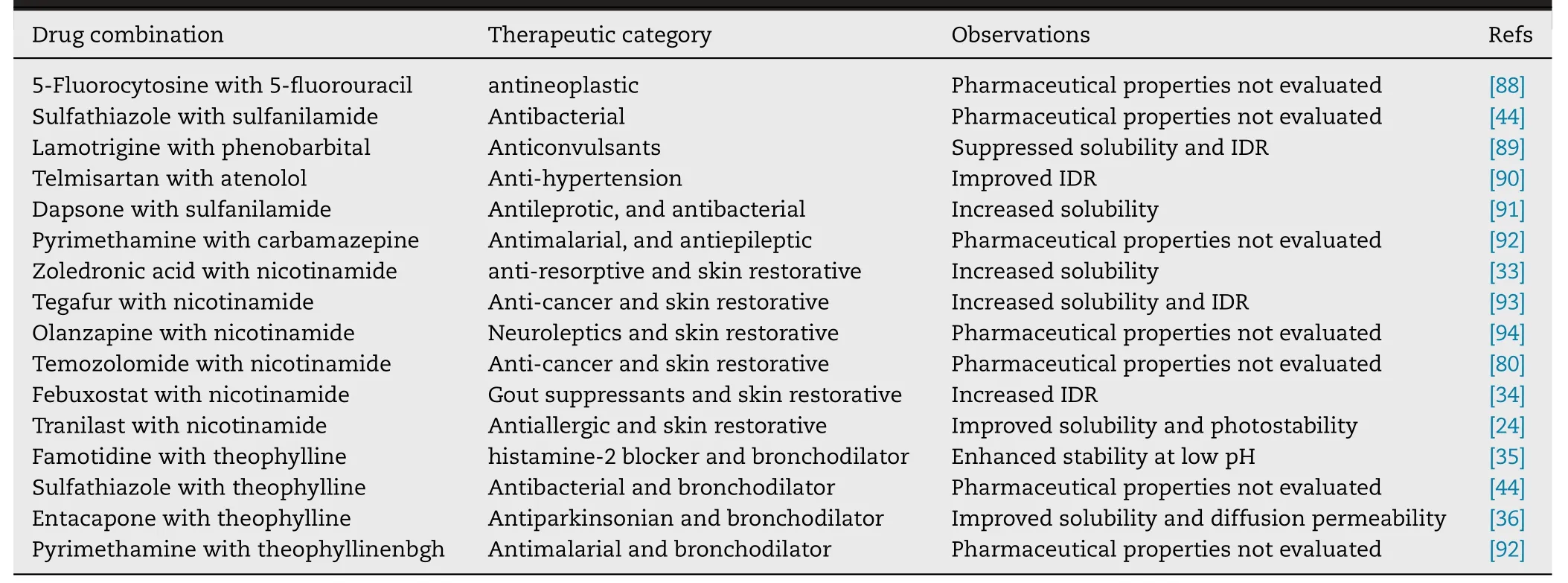
Table 4–The other DDCs reported in the last decade.

Table 5–Summary of commercially available DDCs.
2.4.DDCs of the other APIs
Except for these DDCs discussed above,other 16 DDCs reported in the last decade are listed in Table 4 .The pharmaceutical properties of 7 DDCs had not been evaluated.Among the other 9 DDCs,8 DDCs presented improved dissolution properties and 2 DDCs showed enhanced stability.Sathyanarayana et al.reported a DDC formed with zoledronic acid and nicotinamide and this DDC presented a 20 fold increased solubility than zoledronic acid [33] .Nangia et al.reported a DDC comprising febuxostat and nicotinamide that had an enhanced IDR [34] .Dissolution rates were found to be 36.6 times faster for Febuxostat-nicotinamide DDC when compared to that of pure febuxostat.Sarma et al.developed a DDC of famotidine with theophyllinenbgh and such DDC improved the phase stability of famotidine at low pH [35] .In addition,some DDCs also showed improved diffusion/permeability.Nangia et al.reported a DDC synthesized by theophylline with antiparkinsonian agent entacapone [36] .This DDC presented 1.41 times higher solubility and 1.71 times faster IDR compared to pure entacapone.Diffusion rates in a Franz cell showed that the stable and high solubility DDC also has good permeability.
2.5.Commercially available DDCs
Entresto is the most recently marketed DDC drug that developed by Novartis in 2015.It is synthesized by the angiotensin receptor inhibitor valsartan with a neprilysin inhibitor prodrug (sacubitril) [37] .The combination of sacubitril plus valsartan inhibits neprilysin (neutral endopeptidase) via the active metabolite of the prodrug sacubitril,and blocks the angiotensin II type-1 (AT1) receptor via valsartan [38] .This DDC is a classic example of how cocrystals can influence pharmacokinetics.It improves the bioavailability of valsartan,and achieves the same therapeutic effect with a lower dose.
Dimenhydrinate is another marketed DDC that widely used for the prevention of motion sickness,including nausea and vomiting [39] .It is combined by diphenhydramine and 8-chlorotheophylline.Diphenhydramine is an antihistamine with anticholinergic and sedative effects and it mainly performs the antiemetic effect in this drug.However,it has a side effect of drowsiness and the stimulant 8-chlorotheophylline overcomes this drawback.Sodium valproate is the other DDC and it is crystallized by sodium valproate with its free acid valproic acid [40] .Muscle relaxant dichloralphenazone is a DDC combined with NSAID antipyrin and hypnotic chloral hydrate that used for the treatment of tension and vascular headaches [41] .
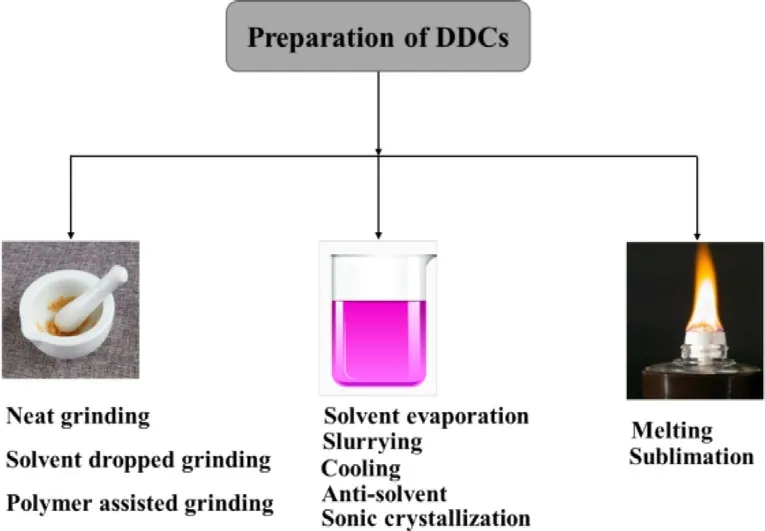
Fig.1–Preparation methods of DDCs.
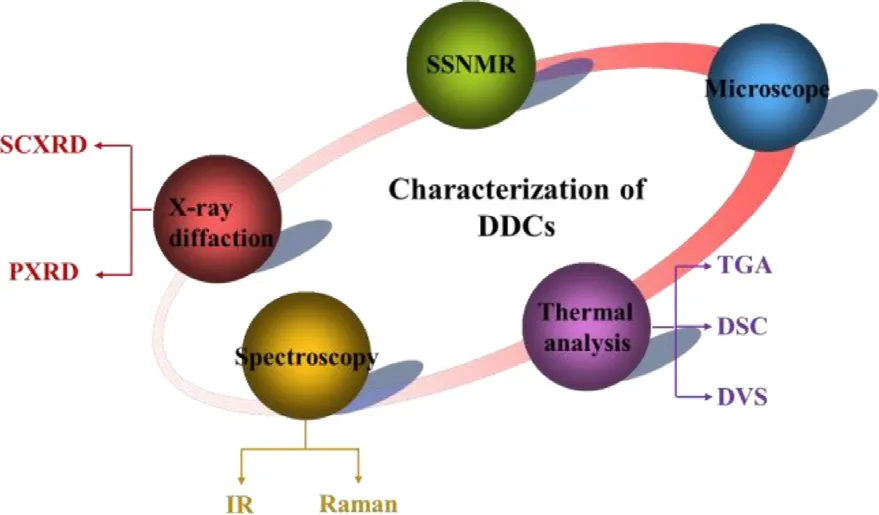
Fig.2–Characterization technologies for DDCs.
3.Synthesis,characterization,and evaluation of DDC
Generally,the preparation methods of DDCs are similar to the conventional methods used in the preparation of other common co-crystals (Fig.1).Solvent evaporation,cooling,slurrying,anti-solvent,grinding and liquid-assisted grinding are the common techniques that have been used for the preparation of DDC.Rohani and Lu synthesized the DDC of sulfamethazine-theophylline using slow evaporation,neat grinding and solvent-drop grinding methods,respectively[42] .Nehm et al.obtained a DDC of carbamazepinenicotinamide by slurrying [43] .Lee et al.prepared two DDCs of sulfathiazole −theophylline,and sulfathiazole −sulfanilamide by cooling experiments [44] .
The characterization methods of DDCs are also the same to that of the common cocrystals (Fig.2),including microscope,single crystal x-ray diffraction (SCXRD),powder x-ray diffraction (PXRD),solid state nuclear magnetic(SSNMR),infrared spectroscopy (IR),raman spectroscopy,thermogravimetric analysis (TGA),differential scanning calorimetry (DSC) and dynamic vapor sorption (DVS) etc.[45] .The evaluated properties of the reported DDCs in the last decade discussed above are presented in Fig.3 .Among the 63 reported DDCs,26 of them did not evaluate their pharmaceutical properties,32 DDCs determined the solubility or/and dissolution rate,7 DDCs investigated their permeability and 6 DDCs studied their physical or chemical stability.In addition,1 DDC optimized formulation capacity and another 3 DDCs improved the bioavailability.
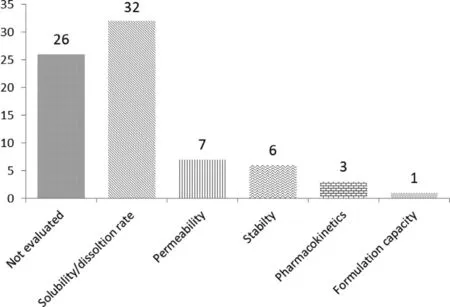
Fig.3–Evaluated properties of the reported DDCs in the last decade.
4.Opportunities and challenges
Recent estimates show that the cost of developing a new drug is about US$2.8 billion [46] .Furthermore,the number of approved drugs per billion of spending has halved each year since the 1950s.Here,DDCs offer a low risk,low-cost,but high reward route to new and better medicines.It could improve the physiochemical and biopharmaceutical properties of a medicine by addition of a suitable drug co-former without any chemical modification.DDCs could overcome the disadvantages of FDCs,and treat additional indications by drug-drug interactions with modulated physicochemical properties of each API.In terms of regulation,the FDA firstly published guidance on the regulatory classification of co-crystals in 2013 and released the final version of the Guidance in February 2018 [47] .In Europe,the European Medicines Agency (EMA) released a reflective document in 2015 that classified cocrystals in a similar way to the salts of APIs [48] .It is expected that the FDA and EMA guidelines would support the growth of a DDC market.In addition,a DDC could obtain a new patent which could mean longeror even new exclusivity and would enhance the existing commercial value of a product in the pharmaceutical industry.
However,there are many challenges for the development of DDC.One of the major challenges is the designing and synthesizing of DDCs.From the DDCs discussed above,it could be found that most of the DDCs reported were primarily focused on identifying hydrogen-bonding patterns,understanding the role of supramolecular interactions.The obtained DDCs always designed from molecular structure and prepared by high throughput cocrystal screening.It could not be guaranteed that the synthesized DDC is a pharmaceutically acceptable combination that could provide potential benefits.For example,Tomita et al.synthesized a 2:1 DDC of theophylline—phenobarbitalin 1977 [49] .Theophylline is an antiasthmatic,and phenobarbital is a nervous system depressant.Such combination does not have any supporting evidence for synergism or practical applications in therapy.It might not provide any significant therapeutic benefits.In addition,theophylline is metabolized by hepatic enzymes,whereas phenobarbital is a cytochrome P450 (CYP) inducer.Hence,in this DDC,the plasma concentration of theophylline may be decreased below therapeutic concentrations.If we design the DDCs from the synergism or practical applications in therapy,such as the marketed FDCs,the designed DDCs may difficult to be prepared.Dosage is another challenge.Cocrystals always have fixed stoichiometric ratio,such as 2:1,1:1 and 1:2 and the oral dose is also recommended in a suitable range.Hence,the dose-ratio of an obtained DDC may not accord with the clinical applications.For example,Desiraju et al.developed a DDC of lamivudine and zidovudine [50] .Both of the two APIs are antiviral agent and this may be a beneficial combination for drug development.However,the mass ratio of lamivudine and zidovudine (1:1.2) is differed from that of their oral dose (1:2).This difference may cause difficulty in the clinical application of this DDC.
5.Future prospects
From the reported DDCs discussed above,it was observed that although thousands of pharmaceutical cocrystals had been reported,drug—drug cocrystals are scarce.The reason may be that the selection of appropriate drug combination for development is challenging,with multiple factors to consider,including,differential solubility,molecular interaction,packing patterns,dosage compatibility,therapeutic applications and drug—drug interactions.In addition,the marketed APIs are numbered,and the suitable drug-drug combinations are limited.To overcome these disadvantages,we can make efforts from three aspects.Firstly,co-crystallization technique selection is the most important as the nature,properties and morphology of co-crystals formed were influenced by this process.Choosing suitable cocrystal methods and develop more effective screening procedures may increase the success rate of the DDCs preparation.Secondly,two different compounds always could form more than one cocrystals with different stoichiometric ratio.Exploring DDCs with different stoichiometric ratios could be used to overcome the dosage problem.Thirdly,extend the DDCs to drug—nutraceuticals cocrystals could be advantageous and relatively easier to develop.Numerous clinical trials confirmed the potential benefits of various nutraceuticals in multiple disorders [51—53] .However,most nutraceuticals are weakly ionizing compounds that display poor solubility and bioavailability [54] .The clinical applications of nutraceuticals are limited by their low bioavailability.Soluble drugs could improve the absorption of these nutraceuticals and the nutraceuticals could modulate some physicochemical properties and provide drug-drug synergism for marketed APIs.This could lead to the emergence of an entirely new range of safe and effective therapeutic combinations of APIs and nutraceuticals with synergistic benefits and reduced adverse effects.
6.Conclusion
DDC has attracted the attentions of many scientists for several years by its low risk,low-cost and other advantages.However,to date,there is limited published work available on DDCs and the marketed DDCs are rare.The development of DDCs has many challenges.There is a need for pharmaceutical scientists to develop robust models for predicting DDC formation,to give useful insights into the relevance of supramolecular interactions and their molecular patterns,and to provide mechanistic understanding of the association and dissociation patterns of DDCs.We could invent new suitable cocrystallization technology,develop more effective screening program and extend DDCs to drug-nutraceuticals cocrystals to overcome the challenges of DDC development.As a result,it may bring about a new opportunity for drugdrug combination and new applications of the conventional drug.
Conflict of interest
The authors declare that there is no conflict of interest.The authors alone are responsible for the content and writing of this article.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2020.06.004 .
 Asian Journal of Pharmacentical Sciences2021年3期
Asian Journal of Pharmacentical Sciences2021年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- The application of label-free imaging technologies in transdermal research for deeper mechanism revealing
- Alginate and alginate composites for biomedical applications
- Recent advances of sorafenib nanoformulations for cancer therapy:Smart nanosystem and combination therapy
- Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies
- In vitro - in vivo - in silico approach in the development of inhaled drug products:Nanocrystal-based formulations with budesonide as a model drug
- pH-sensitive micelles self-assembled from star-shaped TPGS copolymers with ortho ester linkages for enhanced MDR reversal and chemotherapy
