Recent advances of sorafenib nanoformulations for cancer therapy:Smart nanosystem and combination therapy
Fngmin Chen ,Yifn Fng ,Xing Chen ,Rui Deng ,Yongjie Zhng ,Jingwei Sho ,b,∗
a Fujian Provincial Key Laboratory of Cancer Metastasis Chemoprevention and Chemotherapy,College of Chemistry,Fuzhou University,Fuzhou,Fujian 350116,China
b Institute of Oceanography,Minjiang University,Fuzhou 350108,China
Keywords:Sorafenib Multi-kinase inhibitor Smart nanodelivery systems Tumor microenvironment Combination therapy
ABSTRACT Sorafenib,a molecular targeted multi-kinase inhibitor,has received considerable interests in recent years due to its significant profiles of efficacy in cancer therapy.However,poor pharmacokinetic properties such as limited water solubility,rapid elimination and metabolism lead to low bioavailability,restricting its further clinical application.Over the past decade,with substantial progress achieved in the development of nanotechnology,various types of smart sorafenib nanoformulations have been developed to improve the targetability as well as the bioavailability of sorafenib.In this review,we summarize various aspects from the preparation and characterization to the evaluation of antitumor efficacy of numerous stimuli-responsive sorafenib nanodelivery systems,particularly with emphasis on their mechanism of drug release and tumor microenvironment response.In addition,this review makes great effort to summarize the nanosystem-based combination therapy of sorafenib with other antitumor agents,which can provide detailed information for further synergistic cancer therapy.In the final section of this review,we also provide a detailed discussion of future challenges and prospects of designing and developing ideal sorafenib nanoformulations for clinical cancer therapy.
1.Introduction
Cancer is still a leading cause of mortality all over the world [1] .Statistics revealed that there were 18.1 million new cases and 9.6 million deaths of cancer in 2018.Conventional chemotherapy as the main method for cancer therapy has achieved no significant advances over the past decades.One of the well-accepted disadvantages of chemotherapy is the lack of specificity,further leading to considerable damage to normal tissues [2] .Moreover,the primary or acquired resistance to chemotherapeutic drugs such as cisplatin,doxorubicin and 5-fluorouracil is often developed,which resulting in a significant reduction of the progression-free survival and overall survival rates of cancer patients [3] .In recent years,molecular targeted therapy has been successfully developed and demonstrated prospective profiles of efficacy in the front-line clinical treatment of human cancers [4,5] .Sorafenib,as one of the molecular targeted drugs,displays considerable activity in improving progression-free survival and overall survival rates of cancer patients in the clinic [6] .Accumulated mechanistic elucidation investigations revealed that the antitumor efficacy of sorafenib is attributed to its ability to suppress cell proliferation,induce cell apoptosis and prevent angiogenesis [7,8] .It was found that sorafenib triggers cell cycle arrest,autophagy and apoptosis by regulating various protein kinases and signal transduction pathways such as the signal transducer and activator of transcription 3 (STAT3),vascular endothelial growth factor receptor 2 (VEGFR-2) and platelet-derived growth factor receptor (PDGFR) [9,10] .For instance,sorafenib has been demonstrated to negatively regulate STAT3 activation by protein tyrosine phosphatases through de-phosphorylation of phosphorylatedp-STAT3 (p-STAT3),which leading to the down-regulation of expression levels of apoptosis-related proteins and genes such as cyclin D1,caspase-3,Mcl-1 and Bcl-2 [11,12] .In addition,it was also reported that sorafenib can block the development of solid tumors mostly through the inhibition of RAF/MEK/ERK signaling cascade [13,14] .
Despite sorafenib displays significant profiles of efficacy for cancer therapy as compared to chemotherapeutic drugs,it should be noted that sorafenib still has some disadvantages in clinical therapy:(i) The clinical application of sorafenib is greatly limited by its low bioavailability(~8.43%),resulting from its poor water solubility and rapid elimination and metabolism [15] ;(ii) A dose reduction or temporary discontinuation of sorafenib can be frequently observed in many cancer patients due to the serious side effects including skin toxicity,diarrhea,hypertension and hand-foot syndrome [16,17] .The chemical structure of sorafenib is presented in Fig.1 .From which we can find that soafenib is a hydrophobic drug,and the high hydrophobicity of sorafenib contributes to its effective encapsulation into various materials.
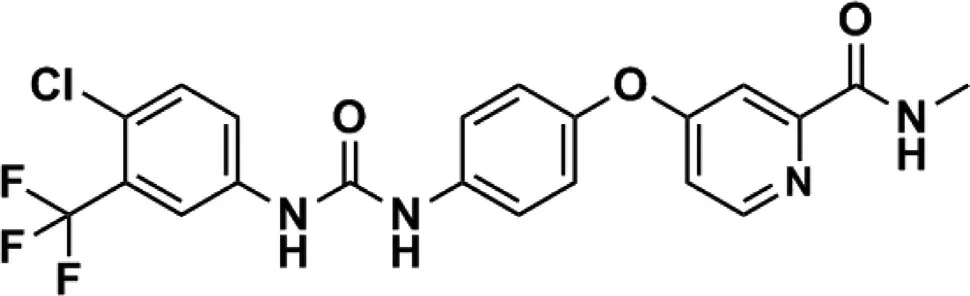
Fig.1–Structure of sorafenib.
In order to overcome the above disadvantages to obtain desired delivery system of sorafenib with great advantages in the clinic,various types of stimuli-responsive sorafenib nanodelivery systems have been prepared and studied in great detail [18,19] .These smart sorafenib nanodelivery systems possess a number of advantages over traditional formulations such as the improved targetability and bioavailability,controlled drug release manner and enhanced tumor accumulation capacity [20] .It is noted that the critical advantage of sorafenib-based nanodelivery systems is that they are inactive before delivering into tumor tissues so that they can protect sorafenib from early leaking through the systemic circulation,thus reducing the occurrence of adverse events.Moreover,in addition to smart sorafenib nanoformulations,a series of combination nanosystems of sorafenib with other antitumor agents also have been prepared and found to display significantly enhanced therapeutic effect based on cooperative mechanism of antitumor actions [21] .In this review,great effort has been made to summarize the preparation methods,pharmaceutical properties and preclinical performance of various types of smart sorafenib nanodelivery systems including pH-,redox-,enzyme-,light-,temperature-and ultrasound-responsive nanosystems,particularly with emphasis on their mechanism of drug release and tumor microenvironment response.In addition,an emphasis is also placed on the nanosystem-based combination therapy of sorafenib with other antitumor agents such as chemotherapy agents,phototherapy agents and genetherapy agents (Fig.2).These combination nanosystems with multi-mechanism of antitumor actions were able to display satisfactory therapeutic outcomes,thereby could provide useful information for future synergistic cancer therapy.The overall information presented in this review is aim at providing an insight into the future challenges and prospects in the design and preparation of ideal sorafenib nanoformulations for clincal cancer therapy.
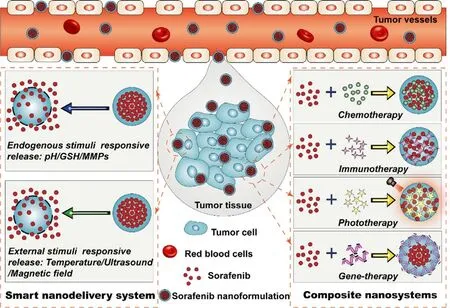
Fig.2–Schematic of smart responsive delivery systems and composite nanosystems for tumor-targeted sorafenib delivery.
2.Representative materials in sorafenib nanodelivery systems
Biomaterials techniques have achieved significant advances in recent years and which have been widely expoited to develop natural or synthetic materials for constructing the efficient drug delivery systems [22—25] .In order to improve the pharmacokinetic shortcomings,such as weak water solubility,rapid elimination and metabolism as well as low bioavailability of sorafenib.Hence,various types of representative materials have been used to develop sorafenib delivery strategies.Among different representative materials,liposomes had the greatest potential to be clinically translated due to their unique advantages including good biocompatibility and biodegradability,low toxicity and immunogenicity [26] .Meanwhile,the high hydrophobicity and great lipid affinity of sorafenib could ensure the effective encapsulation of sorafenib into liposomes,which protected the drug from leaking into normal tissues and thereby reduced the adverse effects [27] .Solid lipid nanoparticles are another representative materials to be utilized for improve the safety and efficacy of sorafenib.In addition to show the similar advantages with liposomes,solid lipid nanoparticles are also featured with their great tumor targeting and controlled drug release properties through being modified with various functional ligands [28,29] .Poly(lactic-co-glycolic acid) (PLGA)is one of the representative polymeric materials and has been approved by FDA for clinically using in drug delivery strategies [30] .As a biocompatible and biodegradable polymer,PLGA has been widely used in drug delivery systems based on nanoparticles for sorafenib delivery [31] .The obtained PLGA nanoparticles had high drug loading capacity of sorafenib owing to its hydrophobic properties,and could achieve controlled release sorafenib into tumor tissues through surface-modified with tumor-targeting ligands or stimuliresponsive linkers [31] .Silica-based materials have attracted considerable attention for their application in drug delivery and nanomedicines preparation.The outstanding advantage of silica-based materials is their large surface area and pore volume [32],contributing to the high drug loading efficiency of sorafenib.Moreover,silica-based materials are easy to be surface-modified with various stimuli-responsive linkers and thereby serve as targeted delivery systems for co-delivery of sorafenib with chemotherapeutic agents [33] .As presented above,we summarized and discussed the representative materials that constructing the delivery systems for sorafenib.The selection of materials to be used for sorafenib delivery is essential to the clinical translation of sorafenib formulations.With aim at promoting the clinical success of sorafenib nanoformulations,we should carefully consider the surface characteristics,biosafety,targetability,preparation procedure and manufacturing cost of various materials that used for sorafenib delivery.
3.Development of smart sorafenib-based nanodelivery system
Over the past decades,various types of traditional sorafenib nanodelivery systems such as liposomes [34,35],solid lipid nanoparticles [36,37],polymeric nanoparticles [38,39] and micelles [40,41],gold nanoparticles [42] and inorganic nanoparticles [43] have achieved significant enhancement in therapeutic effect as compared to free sorafenib.However,the overall evidence suggested that the traditional sorafenib nanodelivery systems still need to overcome some shortcomings or challenges including inadequate targetability,uncontrolled drug release and non-guided blood circulation.Hence,there is an urgent demand to design and fabricate a series of improved nanodelivery systems to further enhance the sorafenib delivery into tumor sites.Accumulating evidence demonstrated that an ideal nanodelivery system should be sensitive to tumor microenvironment and subsequently release its payload at the most into tumor tissues,thereby leading to a more satisfactory therapeutic outcome in cancer therapy.Taking this into account,developing smart nanodelivery systems with stimuli-responsive ability are highly needed for enhanced cancer therapy.In recent years,various endogenous stimuli including pH,glutathione (GSH),matrix metalloproteinases (MMPs) or external stimuli including light,temperature and magnetic field have been addressed to prepare smart responsive delivery systems for tumor-targeted sorafenib delivery.
3.1.pH-responsive sorafenib-based nanodelivery systems
pH-responsive drug delivery systems are sensitive to the abnormal change of pH values in diseased tissues and it have been widely explored for targeted drug release[44,45] .Studies revealed that the pH values of tumor microenvironment is frequently lower than normal tissues[46],indicating that developing pH-responsive sorafenib delivery systems could become effective strategies to display improved tumor targetability and controlled release manner in cancer therapy.For instance,Liu et al.reported on the encapsulation of sorafenib into m-polyethelene glycol -PLGApoly(L-phenylalanine) (PEG-PLGA-PPA) micelles via the ring opening polymerization.It was showed that the hydrophobic drug can assemble in the hydrophobic block copolymers forms the core of micelles [47] .The in vitro release efficiency of sorafenib from micelles could be improved by regulating the hydrophilic/hydrophobic ratios of the triblock copolymers.The pH-responsive sorafenib micelles displayed significantly higher antitumor effect than free sorafenib while the empty micelles demonstrated no cytotoxicity [48] .Their results were in congruence to those of Li et al.as a novel pH-responsive polymeric micelle was synthesized [49] .It was found that the amide bond and CDM bond of this micelle were able to rapidly break on the weak-acid conditions,which led to the depolymerization of micelle,thereby displaying a pHresponsive sorafenib release manner and an enhanced tumor selectivity.The half-life of this sorafenib-loaded micelle was extended to 36.7 h and of free sorafenib was only 8.8 h.The obvious long circulation effect of this micelle was arise from its significant sustained release effect under physical conditions.Meanwhile,the good physical stability of the micelle also promoted the long circulation of sorafenib in vivo .Inspired by such advantages discussed above,Thapa et al.made great efforts on the preparation and evaluation of a pH-responsive layer-by-layer liquid crystalline nanoparticle of sorafenib (Fig.3).This nanosystem was able to efficiently release sorafenib at acidic tumor microenvironment due to the selective depolymerization of polymer layers and then facilitated subsequent intracellular uptake of released sorafenib,thereby leading to an enhanced antitumor efficacy against HepG2 cells [19] .Further,Yao et al.developed a pH-triggered liposome,in which sorafenib and siRNA were complexed with the carboxymethyl chitosan (CMCS) to possess controlled release property [50] .CMCS was stable during circulation but could be triggered by the acidic tumor microenvironment to realize targeted drug release into tumor sites [51] .Sorafenib is hydrophobic in nature,therefore,it could be encapsulated into the lipid bilayer.In vivo results using H22 liver tumor-bearing mice revealed that the CMCS-modified liposome achieved superior effect in reducing tumor volume when 4.5 mg/kg of this formulation was administrated intravenously.In summary,due to the acidic pH of the tumor microenvironment,various pH-responsive nanodelivery systems were constructed to delivery sorafenib for effective cancer therapy.As discussed above,most studies have concentrated on developing nanodelivery systems for pH-responsive sorafenib delivery.Of note,neutralizing the acidic pH values and then normalizing the tumor microenvironment is proposed as another useful approach to achieve enhanced cancer therapy.Therefore,novel sorafenibbased nanodelivery systems designed for the normalization of acidic tumor microenvironment also have great potential to be developed as smart strategies for improving sorafenib therapeutic outcomes.
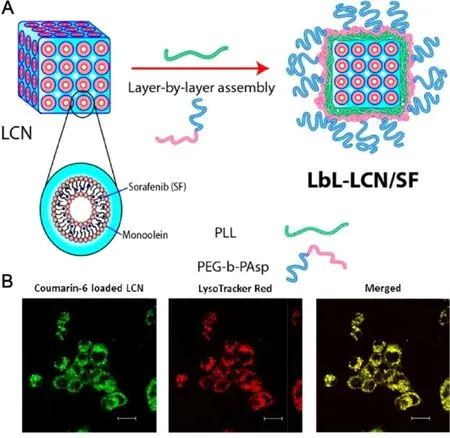
Fig.3–Layer-by-layer polymer-assembled sorafenib-loaded liquid crystalline nanoparticles (LbL-SF-LCN) for targeted delivery to enhance chemotherapy.(A) Schematic illustration of fabrication of LbL-SF-LCN.(B) Intracellular uptake of LbL-LCN determined by confocal laser scanning microscopy.Adapted with permission from [19] .Copyright 2015,American Chemical Society.
3.2.Redox-responsive sorafenib-based nanodelivery systems
It is recognized that the cellular redox environment is mainly regulated by the concentration of GSH.As one of the thiol-containing tripeptides,GSH is abundant in cancer cells while has a low concentration in normal cells [52,53] .Some linking bonds between the amphiphilic polymers were found to be responsive to reductive GSH,indicating that the GSH could act as a stimulus to effectively trigger drug release from nanosystems.In addition,it was reported that sorafenib could block cysteine (Cys)/glutamate (Glu)reverse exchanging system and increase the level of reactive oxygen species (ROS) in cancer cells,therefore,GSH will be reduced [54] .Moreover,many researchers reported that the deletion of intracellular GSH would ultimately lead to the ferroptosis of cells [55] .Based on the above research,it was showed that exhaustion of the intracellular GSH in dual directions could exert a superior ferroptosis-inducing effect.Therefore,a variety of GSH-responsive nanodelivery systems have been successfully synthesized for controlling the release of sorafenib into tumor tissues [56,57] .In a work by Liu et al.,the amphiphilic polymers poly (acryic acid) (PAA) linked with D-α-tocopherol succinate (VES) by disulfide linkage and which can self-assemble into micelles suitable for sorafenib delivery.The micelles were found to effectively improve the aqueous solubility of sorafenib and control its release.In addition,owing to the GSH-sensitivity of disulfide linkage,a controlled and GSH-dependent drug release manner could be observed from the micelles group,which contributed to its enhanced cytotoxicity.In vivo pharmacokinetics studies demonstrated that the sustained release profile of sorafenib from the GSH-responsive micelles could improve the half-life and AUC values of sorafenib,and which were about 1.3 and 2.8-fold higher than free sorafenib,respectively [58] .In another study,a series of manganese doped mesoporous silica nanoparticles (MMSNs)have been synthesized and utilized as delivery vehicles of sorafenib.In addition,the sorafenib was loaded into MMSNs by physical adsorption.The structure of MMSNs contains some manganese-oxidation bonds and which could rapidly break under high GSH concentration conditions,subsequently leading to the degradation of MMSNs.Therefore,a GSHresponsive drug release manner could be achieved in MMSNs and its in vitro inhibitory effect was significantly enhanced via the consumption or synthesis inhibition of GSH [59] .Inspired by the above results,Sang et al.developed a sorafenib-loaded nanophotosensitizer complex for the ferroptosis of multidrug resistant cancer cells.This nanocomplex with disulfide bonds could be triggered in the presence of GSH stimuli,and simultaneously produce ROS,iron and raise the concentration of lipid peroxidation (LPO),thus resisting the multi-drug resistance,invasion and metastasis of breast cancers during the epithelial-to-mesenchymal transition (EMT) (Fig.4) [60] .These studies presented smart nanodelivery systems that effectively enhance the effect of cancer therapy through GSH-responsive sorafeinb delivery.These GSH-responsive nanodelivery systems exhibited enhanced antitumor activity and fewer side effects as compared to free sorafenib,showing great potential for inhibiting cancer progression.However,the specific GSH-responsive drug release mechanism has not been illustrated in most studies,and this challenge must be addressed before the GSH-responsive delivery systems can be accepted for clinical use.In addition,the excess GSH in the tumor microenvironment would scavenge the cell-killing ROS during the therapeutic process,leading to the failure of ROSmediated cancer therapy.Thus,combining the GSH depletion agents with sorafenib therapy can be developed as effective strategies to enhance cancer therapeutic outcomes.

Fig.4–GSH-triggered magnetic complex of sorafenib and nanophotosensitizer (CSO-SS-Cy7-Hex/SPION/Srfn) for the comprehensive ferroptosis therapy.(A) Schematic illustration for the preparation of CSO-SS-Cy7-Hex/SPION/Srfn.(B) Sorafenib release profiles at different light or GSH conditions.(C) Iron release profiles at different light or GSH conditions.(D) Tumor sizes with time after different treatments.Adapted with permission from [60] .Copyright 2019,Ivyspring International Publisher.
3.3.Enzyme-responsive sorafenib-based nanodelivery systems
Accumulating evidence has demonstrated MMPs,especially MMP-2 and MMP-9 were over-expressed in various types of tumor micorenvirenment [61] .MMPs were found to be highly correlated with tumor metastasis,invasiveness and angiogenesis [62,63] .Therefore,a variety of smart nanodelivery systems which could be respond to MMPs have been designed to enhance the drug delivery into tumor tissues.In a recent work by Shi et al.,a novel MMP-2 responsive nanodelivery system which self-assembled from amphiphilic mPEG-peptide deblock copolymer (PPDC),then sorafenib and camptothecin be co-loaded into the core area through hydrophobic interaction (Fig.5).Upon exposure to tumor microenvironment,the peptide segments could be digested by MMP-2 and then simultaneously release two drugs into tumor tissues.It was found that sorafenib delivered by the MMP-2 responsive systems could retain at a higher concentration in the bloodstream as compared to free sorafenib.The longer retention time of sorafenib nanosystems promoted the improved drug accumulation in tumor tissues.Moreover,this nanosystem displayed an enhanced therapeutic effect for tumor suppression through the synergistic actions of simultaneous anti-angiogenesis and chemotherapy [64] .The representative example highlighted the potential of enzyme-responsive nanodelivery systems for tumor-specific sorafenib-delivery.Nevertheless,the information of enzyme levels in tumor tissue is still need to be further explored,maybe some of which act as a stimulus to trigger the precise release of drug from nanosystems.
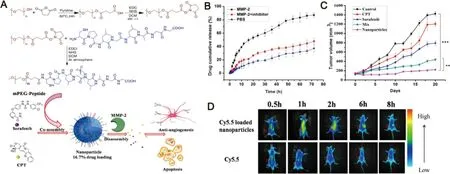
Fig.5–MMP-2-responsive nanoparticles for synergistic antitumor effect of anti-angiogenesis and chemotherapy.(A) Schematic illustration of the co-assembly and disassembly of nanoparticles subject to MMP-2 stimuli.(B) Accumulative release of drug from nanoparticles in different media.(C) Tumor sizes with time after different treatments.(D) In vivo biodistribution of tumor-bearing mice after administration of Cy5.5 loaded nanopraticles and free Cy5.5.Adapted with permission from [64] .Copyright 2016,American Chemical Society.
3.4.Temperature-responsive sorafenib-based nanodelivery systems
In addition to endogenous stimuli such as pH values,GSH and MMPs,the external stimuli such as temperature has also been considered as a promising stimulus for thermal responsive drug delivery based on thermosensitive polymers[65] .Polypropylene oxide (PPO),polycaprolactone (PCL),PEG and PLGA as the ideal thermosensitive polymers with tunable critical solution temperature in water,have been widely utilized as thermosensitive delivery materials for localized and sustained drug release [66,67] .For instance,Zheng et al.developed a PLGA-PEG-PLGA-based thermosensitive nanosystem in order to control the release profiles of sorafenib and selenium nanoparticles.The results showed that sorafenib was released continuously with the temperature-dependent degradation of the copolymers for a prolonged time.Under the X-ray irradiation,the thermosensitive nanosystem remarkably decreased the expression of Ki67 and CD34 to inhibit cell proliferation and tumor angiogenesis,and also activated caspase-3 signaling pathway to promote the cells apoptosis.[39] .Gold nanorods that display good optical absorption in the NIR region are the most promising carriers for widespread applications including photothermal therapy (PTT) and photoacoustic imaging (PAI) [68,69] .Under the NIR light irradiation,gold nanorods induce PPT and generate thermal effect to promte the relase of drug.Based on this concept,Chen’s group designed a temperature-responsive nanocapsule for controlled sorafenib and antioncogene p53 delivery,which composed of the gold nanorods cores and polycationic mesoporous silica shells.This nanocapsule could be activated to induce PTT and trigger the drug release under the NIR light irradiation.Taking advantages of the inherent photothermal properties of gold nanorods,synergistic effect arising from the combination of chemotherapy and photothermal therapy was achieved.The temperatureresponsive nanocapsule could selectively release sorafenib in tumor tissues and continuously inhibit the tumor growth,leading to a signficant treatment outcome (Fig.6) [70] .In addition,the similar drug release response was also observed in Liu’s and Ebrahim’s work,in which sorafenib-loaded gold nanorods were found to effectively inhibit the growth of cancer cells under irradiation [71,72],further suggesting the great prospects for enhancing targeted drug release by using gold nanorod-based temperature-responsive delivery systems.Inspired by the delivery efficiency of human serum albumin protein (HSAP) nanoparticle towards cancer cells[73,74],Callaghan et al.designed a HSAP-modified gold nanorod of sorafenib to induce a temperature-triggered chemo-photothermal therapy.This nanoplatform was found to display triggered release of sorafenib and synergistic inhibitory effect against cancer cells [75] .Considering that the thermosensitive nanodelivery systems can be triggered by the hyperthermia effect induced by phototherapy,a thermosensitive nanosystem-based combination of sorafenib with phototherapy agents may of great potential for enhanced cancer therapy.The challenge in the design of temperatureresponsive nanosystems is the selection of safe and sensitive materials to be respond to slight temperature changes around physiological temperature.Among the most investigated temperature-responsive materials,liposomal carriers are approved by FDA and have wide clinical applications,therefore,it could be used as a preferred nanocarrier.
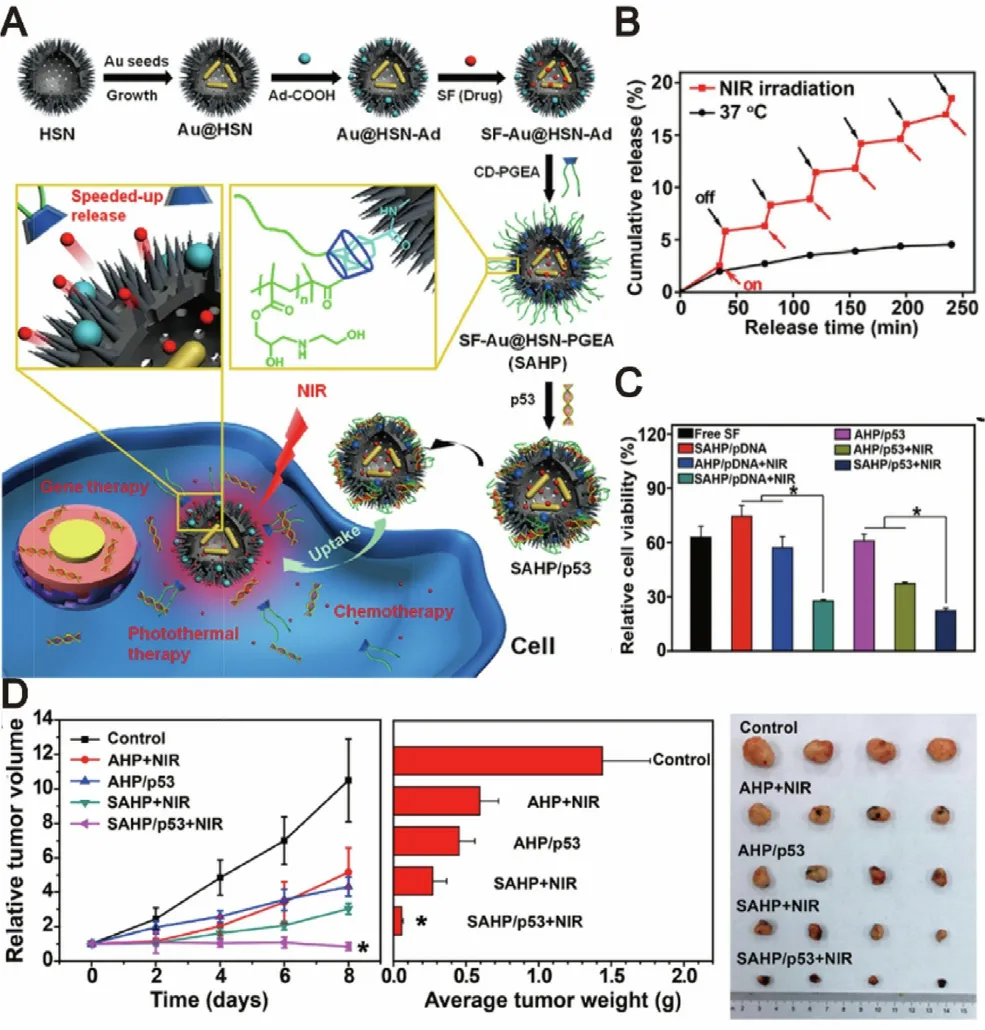
Fig.6–NIR-responsive nanocapsules for photothermally enhanced drug/gene co-delivery.(A) Schematic illustration for the preparation and mechanism of actions of SF-AU@HSN-PGEA (SAHP).(B) Sorafenib release profiles under NIR irradiation.(C) MTT assay of HepG2 viabilities after different treatments.(D) Time-dependent growth curves,average tumor weights and visual illustration of tumor sizes after different treatments.Adapted with permission from [70] .Copyright 2018,American Chemical Society.
3.5.Ultrasound-responsive sorafenib-based nanodelivery systems
Among the external stimuli,ultrasound is particularly attractive due to its superior ability to induce bubble cavitation and subsequently facilitate drug release,enhance cellular uptake and affect tumor microvasculature [76] .Ultrasound displays great potential to simultaneously improve local therapeutic effect as well as tumor contrast imaging [77] .Yang’s group pioneered the development of ultrasoundtriggered phase transition nanodroplets for co-delivering sorafenib and doxorubicin.Upon exposure to ultrasound irradiation,the nanodroplets occurred a liquid-to-gas phase transition,which could be served as a cavitation nucleus to promote drug release and enhance cellular uptake.In vitro studies using liver cancer cells revealed that the nanodroplets could effectively inhibited cell proliferation,migration and induced apoptosis.Further,the strong cavitation of drug-loading nanodroplets could significantly disrupt tumor microvessels,which was beneficial for tumor tissuepenetrating drug delivery and consequently led to reduced tumor angiogenesis and inhibited tumor growth in tumor bearing mice [78] .Under the ultrasound irradiation,the average tumor volume of drug-loaded nanodroplets was 417 ±132 mmand the inhibition rate was increased up to nearly 68.7%.Ultrasound waves utilized the thermal and mechanical effects generated by radiation forces resulted in triggering drug release.Additionally,it was noteworthy that combination of thermosensitive carriers and high-intensity focused ultrasound triggered the release of drug with only a mild temperature change,indicating that the construction of temperature and ultrasound multi-responsive nanodelivery systems may have potential for improving the therapeutic outcomes of sorafenib.
3.6.Dual/multi-responsive sorafenib-based nanodelivery systems
Recently,multi-responsive nanodelivery systems based various endogenous/external stimuli have been explored to display synergistic enhancement of drug delivery in cancer therapy.As an example,Park et al.reported a dualresponsive nanosystem which composed of iron oxide nanocube and pH-sensitive peptides for targeted delvery of sorafenib.This nanosystem with magnetic properties was responded to acid tumor microenvironment,which led to a MRI-guided and pH-triggered drug release manner,and subsequently displayed an enhanced tumor growth inhibition in HCC models [79] .In addition to the traditional apoptotic therapeutics mean,ferroptosis is also widely applied in cancer therapy [80,81] .It is an iron-dependent cell death caused by accumulation of LPO and is highlighted with clinical significance for tumor treatments [82,83] .In order to improve the therapeutic outcomes of current ferroptosis therapy,IR780-Hex,magnetic iron oxide nanoparticles and sorafenib were co-loaded into a novel multi-stimuli responsive nanosystem.This system disassembled by the GSH attack and triggered by the NIR irradiation to release payloads for the production of LPO and synergistic effects to improve therapeutic efficacy in the breast tumor-bearing mice [84] .Moreover,Liu’s group designed a ferrous-supplyregeneration nanosystem compose of Fe,derived tannic acid (TA) and sorafenib nanocrystal.Studies revealed that pH declines could destroy the nanosystem followed by the corona erosion,leading to the rapid release of sorafenib into tumor cells to initiate ferroptosis.In addition,this network could be modified with a photodynamic agent of methylene blue (MB) to display significant photodynamic effect and tumor imaging ability.This imaging-guided and acid-responsive system could induce complete tumor elimination through the simultaneous actions of photodynamic therapy (PDT) and ferroptosis therapy(Fig.7) [85] .Despite the advantageous versatility of these multi-responsive sorafenib-based nanodelivery systems,it is noted that these nanosystems often appear as so complex that they are still developed at the proof-of-concept level.Therefore,more studies and investigations should be carried out to prove that these multi-responsive nanosystems were viable strategies.
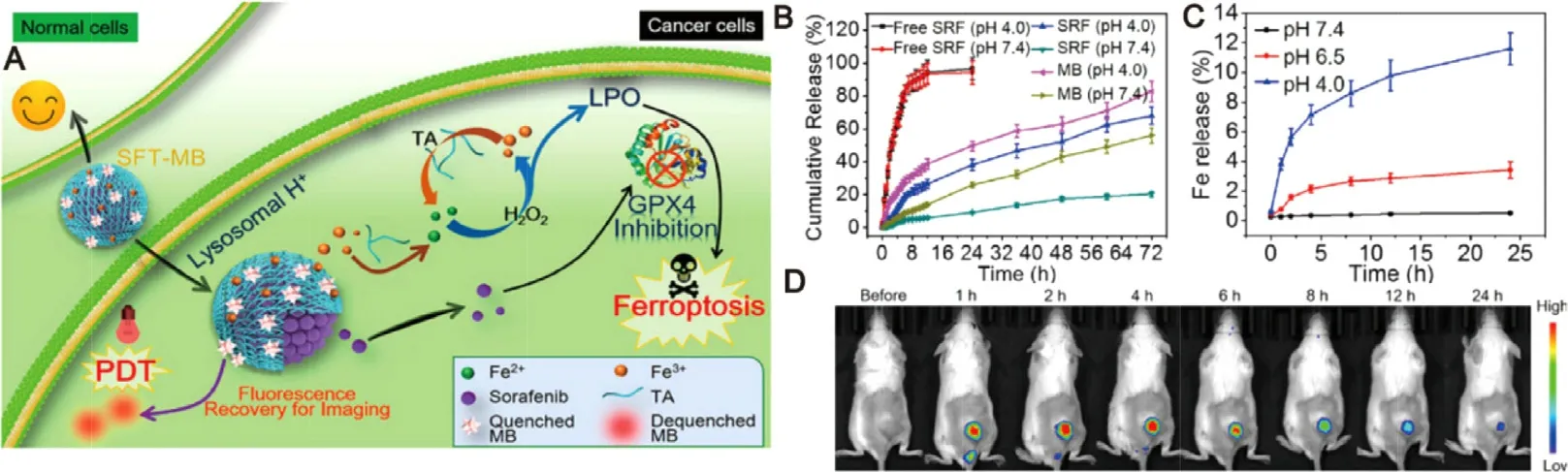
Fig.7–Ferrous-supply-regeneration nanosystems for acid-responsive and imaging-guided tumor therapy.(A) Schematic illustration of the sorafenib-mediated combination of ferroptosis and PDT.(B) Sorafenib and MB release profiles at different pH conditions.(C) Iron release profiles at different pH conditions.(D) In vivo biodistribution of tumor-bearing mice after administration of SFT-MB.Adapted with permission from [85] .Copyright 2018,American Chemical Society.
3.7.Imaging-guided smart sorafenib nanodelivery systems
In recent years,great attention has been paid in the development of nanoparticle-based theranostic strategy for simultaneous cancer therapy and imaging [85] .For instance,a liposomal delivery system composed of sorafenib and gadolinium was prepared to serve as not only a therapeutic agent for HCC therapy,but also a contrast agent for magnetic resonance imaging (MRI).The in vivo efficacy demonstrated that the liposome displayed the highest antitumor effect in H22 tumor-bearing mice [86] .In addition,superparamagnetic iron oxide nanoparticle (SPION),as a MRI contrast agent,was co-load with sorafenib into solid lipid nanoparticles to enhance the sorafenib delivery with the guidance of remote MRI imaging,which subsequently led to the comprehensive antitumor effect against HepG2 cells [87] .Moreover,surface-modification of nanoparticles with specific ligands to recognize cancer cell receptors could further improve its tumor active targetability [88] .Zhang et al.developed a folate-modified micelles based on PEGPCL polymer,in which sorafenib and SPIONs were coloaded.The targeted micelles possessed significant effect in inhibiting proliferation and inducing apoptosis of cancer cells in vitro [89] .Melanin,as a naturally occurring pigment,not only can offer its intrinsic property for PAI,but also is able to actively chelate to metal ions for MRI and positron emission tomography (PET) [90] .Inspired by this,Zhang’s group pioneered the development of PEGmodified melanin delivery system of sorafenib (SF-MNPs)to realize imaging-guided chemotherapy.The melanin-based system displayed a gradual drug release manner and subsequently caused a significant tumor volume reduction of mice (Fig.8) [91] .As compared to the traditional imaging-guided nanodelivery systems that need complicated modification and introduce contrast agents of potential toxicity,SF-MNPs as natural platforms are more preferable to simplify the preparation procedure for safe imagingguided sorafenib therapy.Moreover,considering melanin is an endogenous biopolymer,the melanin-based system may have great potential to efficiently deliver other antitumor agents for furture multimodality-imaging guided cancer therapy.
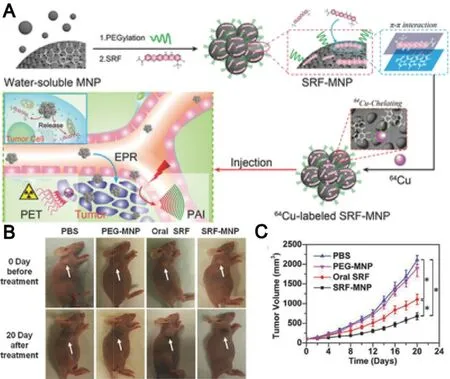
Fig.8–Ultra-small PEG-modified melanin nanoparticles(SF-MNPs) as imaging-guided delivery systems for enhanced chemotherapy.(A) Schematic illustration of the process of imaging-guided tumor therapy by SF-MNPs.(B)Visual illustration of tumor development of tumor-bearing mice after different treatments.(C) Tumor growth curves of tumor-bearing mice after Adapted with permission from.Reproduce with permission [91],Copyright 2015,WILEY-VCH.
The various stimuli-responsive sorafenib nanodelivery systems were summarized in Table 1 .The current stimuliresponsive sorafenib-based systems have limited chances of being applied in the clinic due to absence of insufficient biocompatibility or degradability.The ability of these systems to be sensitive to discrete change of pH,GSH,enzyme or temperature potential is not straightforward to achieve,and the penetration depth of exogenous stimulus into targeted tissues also should be addressed.For most of these systems,the difficulties in the scaling-up of their synthesis and complexity of their architectural design are likely to hinder their clinical translation.It is hard to indicate which stimuliresponsive nanosystems have the most potential to be used in the clinic.Thus,the simpler and easier system is developed,the better its potential to be used in the clinic.Numerous progress in nanotechnology has promoted the development of smart sorafenib-based nanodelivery system.In the future days,perhaps we should pay more attention on the clinically acceptable nanodelivery systems that are more sensitive to discrete variations in specific stimuli.
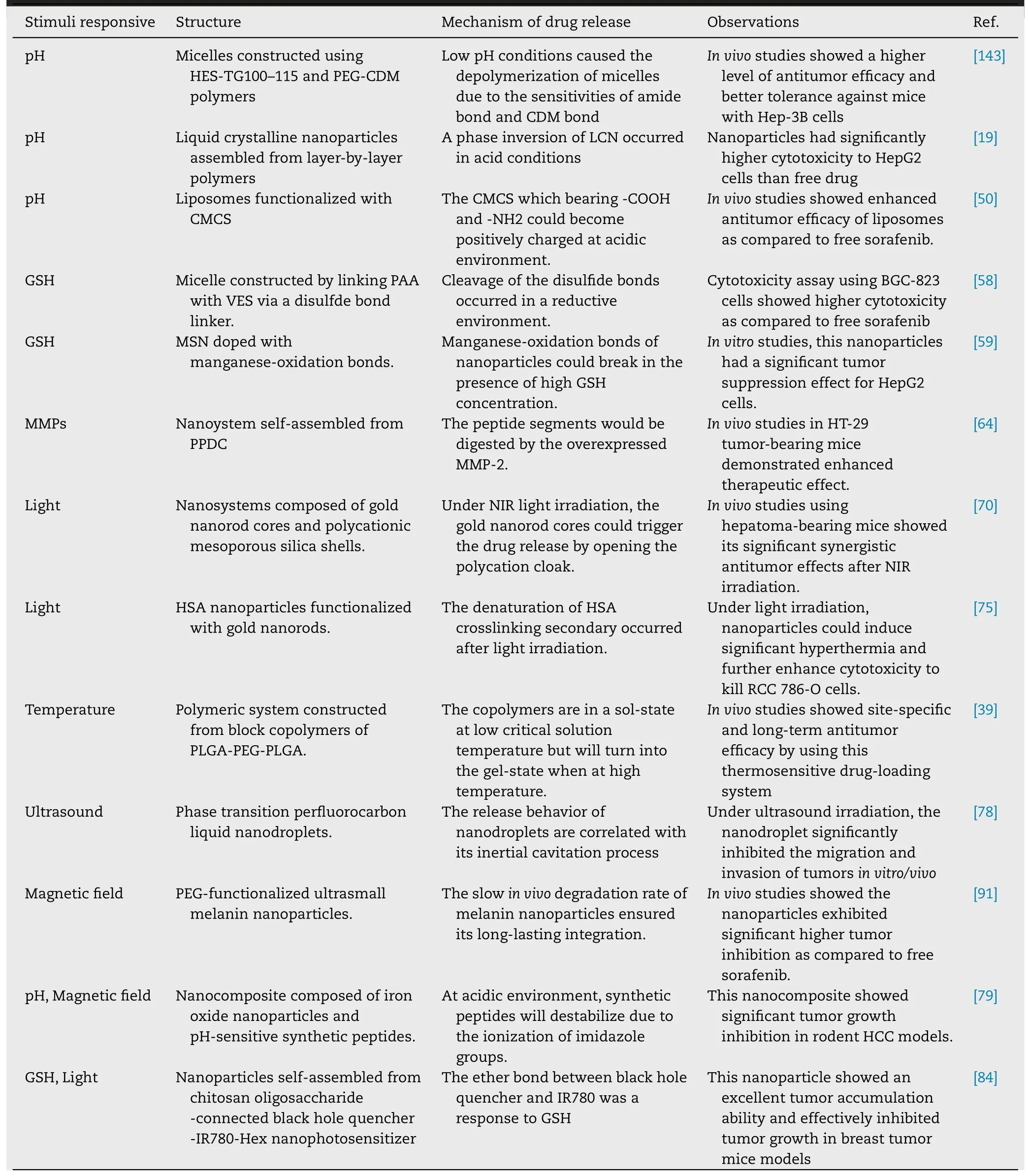
Table 1–A summary of various stimuli responsive sorafenib nanodelivery systems.
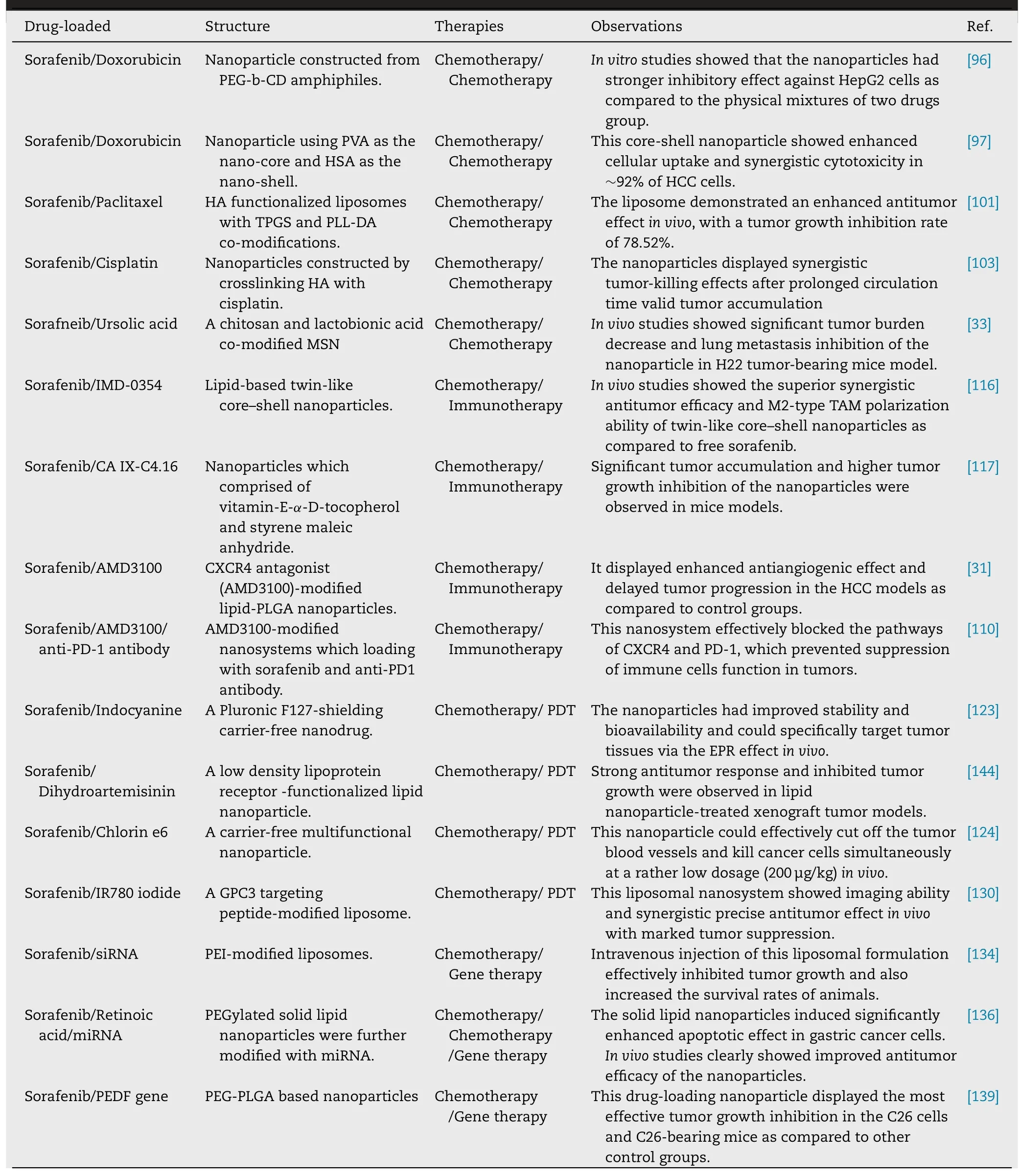
Table 2–A summary of various composite nanosystems of sorafenib with other agents.
4.Development of combination therapy of sorafenib
Over the past few years,combination delivery of different therapeutic agents using nanosystems have emerged as prospective strategies for ehanced cancer therapy [92] .These combination delivery systems are considered to be of great potential to overcome the issues of drug resistance,as synergistic actions of multiple agents may improve the tumor cell killing activity and also decrease the toxicity toward normal cells [93] .Accumulated evidence have demonstrated that sorafenib can inhibit the expression of VEGFR and PDGFR as well as block the cell signaling pathway mediated by RAF/MEK/ERK to inhibit the proliferation of tumor cells[9,10] .However,there are some compensation signaling pathways existing in tumor progression so that tumor cells can escape from the signaling pathway blockade mediated by sorafenib,thus leading to a unsatisfactory therapeutic outcomes in some cancer patients.Moreover,high-dosage use of sorafenib in clinic has caused obvious drug resistance issues in some patients,thus combining sorafenib with its antitumor analogues or adjuvant agents can also display synergistic therapeutic effect based on cooperative mechanism of actions.Inspired by this,a series of composite nanosystems of sorafenib with other antitumor agents such as chemotherapy agents,immune-modulating agents,phototherapy agents and gene-therapy agents have been developed.
4.1.Composite nanosystems of sorafenib with chemotherapy agents
Co-adminstartion of sorafenib with other chemotherapeutic agents has been widely studied for improved therapeutic effect in cancer therapy.Doxorubicin as a commonly used antitumor drug can exhibit significant antitumor efficacy through intercalating DNA as well as inhibiting topoisomerase II preventing DNA replication [94] .Due to the complementary mechanisms of sorafenib with doxorubicin,there is interest in combination treatments with the two drugs to display synergistic antitumor effect.In a phase II clinical trial,the combination therapy of sorafenib and doxorubicin led to a significant improvement on the median overall survival of advanced HCC patients [95] .Xiong’s group developed a reduction-responsive nanodelivery system for loading sorafenib and disulfide-modified doxorubicin prodrug.In this system,sorafenib was delivered with PEG-b-cyclodextrin(PEG-b-CD) amphiphiles in the core of polymeric nanoparticle by hydrophobic interaction and π-π stacking interaction with doxorubicin.This nanosystem could be triggered by dithiothreitol and subsequently displayed enhanced cytotoxicity against HepG2 cells than physical mixtures of sorafenib and doxorubicin [96] .Still,more detailed in vivo results need to be achieved for evaluating the prospect of this co-delivery strategy.A core-shell lipid-based nanosystem has been prepared by Malarvizhi et al.for improving the tumor co-accumulation of sorafenib and doxorubicin.The core-shell nanoparticle effectively imparted cytotoxic stress and displayed synergistic cytotoxic effect against 92% of tested HCC cells [97] .The similar synergistic effect also could be achieved in Lei et al.’s work,in which sorafenib and paclitaxel were co-loaded into a multifunctional liposome with modification by hyaluronic acid (HA).Paclitaxel can promote tubulin polymerization and microtubule assembly so that stabilize microtubules and inhibit mitosis of tumor cells,thus triggering cell apoptosis,and effectively preventingthe proliferation of cancer cells [98] .HA was found to directly bind to CD44 receptor which over-expressed on cancer cells,thereby leading to the attractive applications of HAmodified liposomes for active targeting of sorafenib [99,100] .The modified liposome demonstrated a endo-lysosomal escape in the tumor microenvironment,leading to high drug tumor co-accumulation and improved cancer therapeutic outcome [101] .The liposomal nanosystems have also been applied for the efficient co-delivery of sorafenib and ceramide for enhanced cancer therapy [102] .Further,Zhang et al.developed a nanoscale co-delivery system of sorafenib and cisplatin.This system displayed great pH-sensitive
property due to the chelation interactions between HA and cisplatin,which guaranteed the major drug release and valid drug co-accumulation into tumor sites [103] .Increasingly attention has been paid on the nanoencapsulation of sorafenib with natural antitumor agents for combination cancer therapy.As an example,Cao et al.reported the design and development of nanoparticle which self-assembled from sorafenib,curcumin and PEGyated vitamin-E succinate(PEG-VES) through the hydrophobic interactions.Curcumin not only modulates a range of signaling pathways such as the NF-κB,TGF-β and JAK/STAT pathways in tumor cells,but also confers properties of electron receptors to destabilize ROS,thus displaying anti-apopototic and antioxidant effects[104] .The nanoparticle could improve cytotoxicity and cell apoptosis in vitro,enhance drug tumor co-accumulation,and obviously suppress tumor progression with enhanced inhibitory effect [105] .Asialoglycoprotein receptor (ASGPR)is one of the over-expressed C-type lectin receptors (CTLR)in liver cancer cells [106] .Tunki et al.prepared a galactosemodifed solid lipid nanoparticle of sorafenib,which not only showed enhanced cellular uptake and cytotoxic activity against ASGPR over-expressed HepG2 cells,but also displayed improved bioavailability in tumor-bearing mice as evidence from its higher mean residence time and AUC [107] .In addition,a MSN decorated with chitosan and lactobionic acid was utilized to co-load sorafneib and ursolic acid for targeting ASGPR over-expressed cancer cells,which subsequently displayed superior activities in inducing apoptosis,inhibiting cell migration,proliferation,angiogenesis against cancer cells (Fig.9) [33] .Based on these observations,we conclude that developing composite nanosystems of sorafenib with chemotherapy agents can display synergistic antitumor effect due to their complementary mechanisms of action.The combination therapy strategies are beneficial to reduce the dosage of one single drug and overcome the drug resistance issues.It is noted that stimuli-activatable drug delivery systems have great potential to enhance drug accumulation into tumor tissues and thereby improve therapeutic outcomes.Thus,tumor microenvironmenttargeted co-delivery of sorafenib with chemotherapy agents using stimuli-activatable nanoparticles may further enhance antitumor efficacy.
4.2.Composite nanosystems of sorafenib with immune-modulating agents
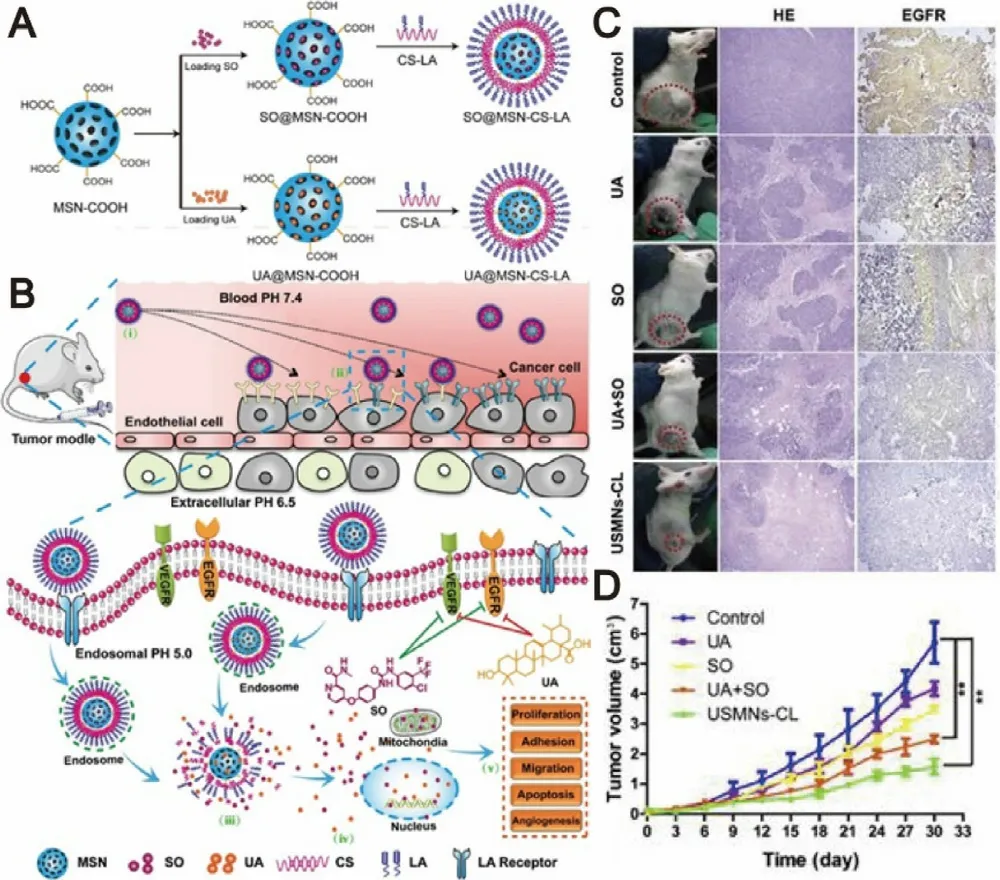
Fig.9–Chitosan and lactobionic acid dual-functionalized mesoporous silica nanoparticles (MSN) for simultaneous drug delivery.(A) Schematic diagram of the preparation of dual-functionalized MSN.(B) Schematic illustration of the synergistic effect induced by the dual-functionalized MSN.(C) Visual illustration of tumor sizes after different treatments.(D) Time-dependent growth curves after different treatments.Adapted with permission from [33],Copyright 2017,Elsevier.
In recent years,immune-modulating agents have attracted much attention in cancer therapy due to their outstanding ability to inhibit tumor progression through modulating the immune system [108] .It is noted that the PD-L1 expression in tumor-infiltrating immune cells was increased after sorafenib treatment,indicating that sorafenib monotherapy may promote the development of tumor immunosuppression microenvironment and thereby inhibit the ability of immune systems to identify and attack tumor cells [109] .Based on these observations,a variety of combination therapy strategies of sorafenib with immune-modulating agents have been developed with aim at improving cancer therapeutic outcomes through simultaneously blocking tumor progression-relevant signaling pathways and boosting antitumor immune responses.C-X-C receptor type 4 (CXCR4)-targeted nanoparticles have attracted great interests in recent years as they preserving the properties of regulating tumor microenvironment,overcoming drug resistance and PD-L1-mediated immunosuppression [110,111] .Gao’s group exploited CXCR4-targeted nanoparticles to co-deliver sorafenib and CXCR4 antagonist AMD3100 for combination therapy.The targeted nanoparticles could effectively reduce infiltration of Tumor-associated macrophages (TAM 2),enhance anti-angiogenic effect,inhibit tumor progression and improve overall survival in the HCC models [31] .CXCR4-targeted nanoparticles also exhibited satisfactory co-delivery outcomes of sorafenib and selumetinib for preventing the activation of RAF/ERK,down-regulating PD-L1 expression and facilitating infltration of CD8T cells,subsequently inhibiting the development and progression of fibrosis-associated HCC[112,113] .In another study,anti-PD-1 antibody and sorafenib were co-loaded into CXCR4-targeted nanoparticles to further prevent the suppression of immune cells function in tumors through blockade of both CXCR4 and PD-1 pathway.This multi-drug delivery strategy displayed enhanced immune cell tumor penetration and activation,and ultimately delayed tumor development and progression (Fig.10) [110] .TAMare essential components of tumor microenvironment and which can promote tumor initiation,growth and metastasis by inhibiting immune cells [114,115] .In order to reverse phenotype of TAMs and display synergistic effects of chemotherapy and immunotherapy,Wang et al.developed a twin-core—shell nanoparticle for co-delivering sorafenib and TAMre-polarization agent IMD-0354.In Hepa1—6 tumor bearing mice,this nanoparticle could exhibit superior synergistic antitumor effect and TAMpolarization ability[116] .In addition,a tumor microenvironment-responsive micelle system has been designed for the combination therapy of sorafenib and PI3K γ inhibitor TG100—115.It was found to effectively activate adaptive immunity and further exhibit enhanced antitumor effect in tumor-bearing mice [49] .Taking tumor hypoxia-directed nanoparticles as vehicles,Alsaab et al.fabricated a targeted co-delivery system of sorafenib with a class of apoptosis inducer CA IX-C4.16,which provided multimodal antitumor effects including the resurrection of apoptosis,repolarization of TAM 2 and reversal of multi-drug resistance.Moreover,owing to the proper physical-chemical properties of CA IX-oligomer,this nanosystem could be furher explored for diagnostic use and image-guided cancer therapy [117] .The overall evidence demonstrated that the combination strategies of sorafenib with cancer immunotherapies could show enhanced antitumor efficacy.However,in order to achieve clinical use of these combination strategies,the more biocompatible materials should be developed to safely and precisely deliver agents into desired sites.Moreover,the specific synergistic mechanisms of action between sorafenib with most immune-modulating agents are still unclear,and more efforts should be further paid on these settings.
Fig.10–Schematic illustration of the sorafenib-induced SDF1 α/CXCR4 axis-mediated immunosuppression in tumor microenvironment.Reproduce with permission from [110] .Copyright 2015,Wiley-VCH.
4.3.Composite nanosystems of sorafenib with phototherapy agents
Recent studies suggested that sorafenib could enhance ROS generation,which implies that redox mechanism may also contribute to its antitumor efficacy.PDT is a newer class of cancer therapy that can produce a great amount of ROS upon light irradiation,and subsequently kill cancer cells and inhibit tumor development [118—120] .Moreover,some of phototherapy agents have the ability to induce significant photothermal effect upon light irradiation and subsequently lead to temperature change of delivery systems.Therefore,designing temperature-responsive materilas to co-delivery sorafenib with phototherapy agents may achieve tumor-speicific drug release.Based on this concept,various nanodelivery systems have been widely used as codelivery strategies for the combination therapy of sorafenib and phototherapy agents in order to exhibit synergistic therapeutic effect [93,121,122] .For example,a series of selfimaging polymeric nanoparticles have self-assembled from Pluronic F127,indocyanine green (ICG) and sorafenib through the one-step nanoprecipitation method.Upon exposure to NIR laser irradiation,ICG could generate a great amount of ROS and hyperthermia to produce photothermal effect and simultaneously promote sorafenib for rapid release into tumor tissues,thereby resulted in synergistic and improved cytotoxic effects (Fig.11) [123] .With aim at synergistic cancer therapy,Wei et al.synthesized a multifunctional nanoparticle of sorafenib and chlorin e6 (Ce6) through a reprecipitation technique.The nanoparticle with great passive targetability could efficiently cut off the tumor blood vessels and simultaneously kill tumor cells at a lower dosage in vivo (200 mg/kg),which demonstrating the significant synergistic effects of PDT and anti-angiogenic therapy[124] .PTT typically involves the conversion of absorbed light energy into localized heat energy and further induces hyperthermia to kill cancer cells [125] .Nanoparticle-based chemo-photothermal therapy has attracted great attention and interest in recent years for enhanced cancer therapy[126,127] .The glypican-3 (GPC3) is highly expressed in most of liver cancers,with intense research and application in targeted delivery of drugs to cancers cells [128,129] .Mu’s group pioneered the development of a GPC3-targeted liposomal theranostic nanoplatform to co-deliver sorafenib and IR780 iodide for combinatorial chemo-photothermal HCC therapy.Upon irradiation with NIR light,IR780 could absorb the light energy and produce a great amount of localized cytotoxic heat,which enhanced the sensitivity of cancer cells to sorafenib,and subsequently achieved a superior in vivo antitumor effect with marked tumor suppression (Fig.12)[130] .
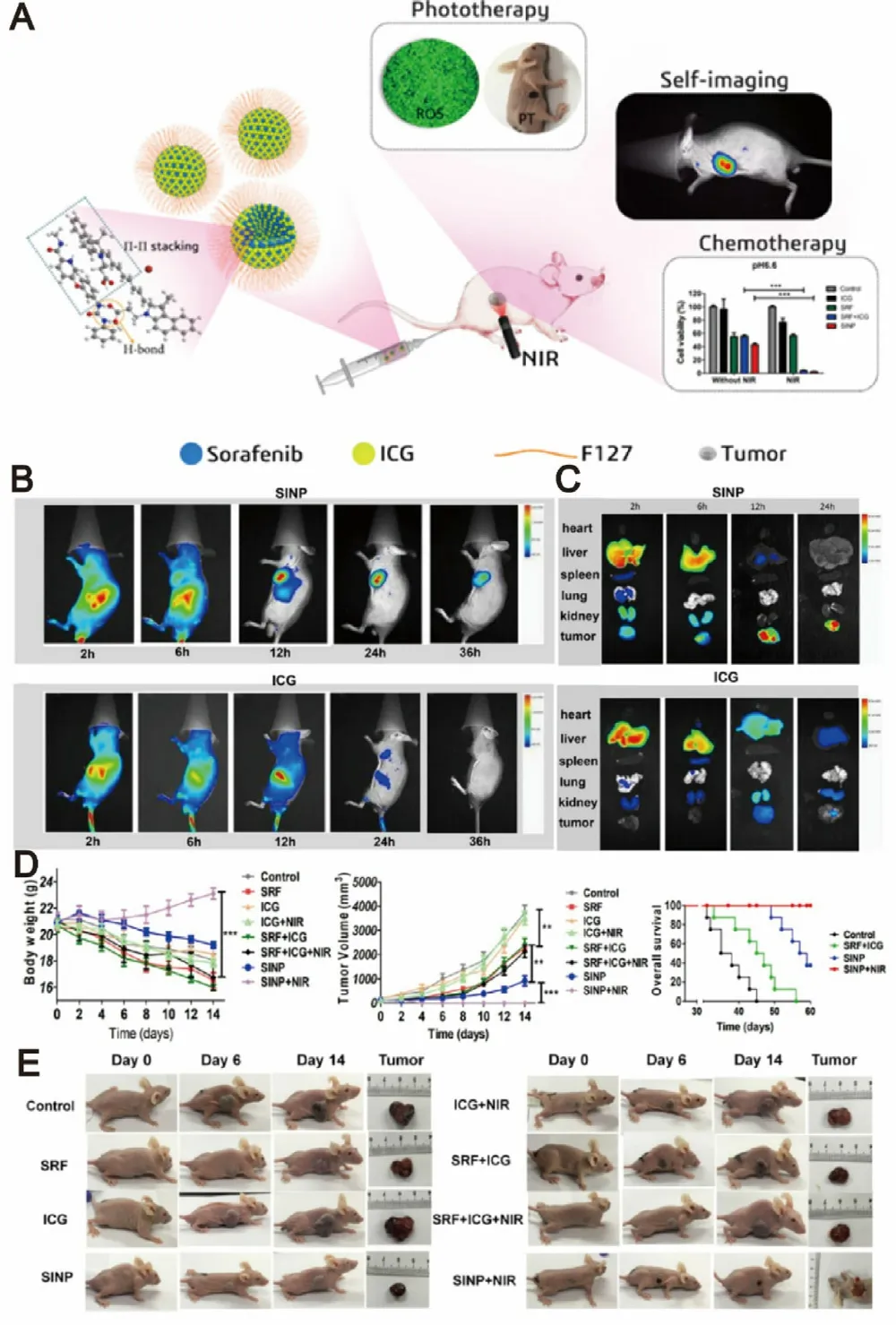
Fig.11–Self-imaging and self-delivered nanodrugs for sorafenib and ICG co-delivery.(A) Schematic illustration of the sorafenib-mediated combination of chemotherapy and self-imaging-guided PDT.(B) In vivo biodistribution of tumor-bearing mice after administration of SINP and free ICG.(C) Ex vivo fluorescent images of tumors after 2,6,12,and 24 h of administration.(D) Time-dependent growth curves,average tumor weights and overall survival curve of mice after different treatments.(E) Visual illustration of tumor sizes after different treatments.Adapted with permission from [123] .Copyright 2019,American Chemical Society.
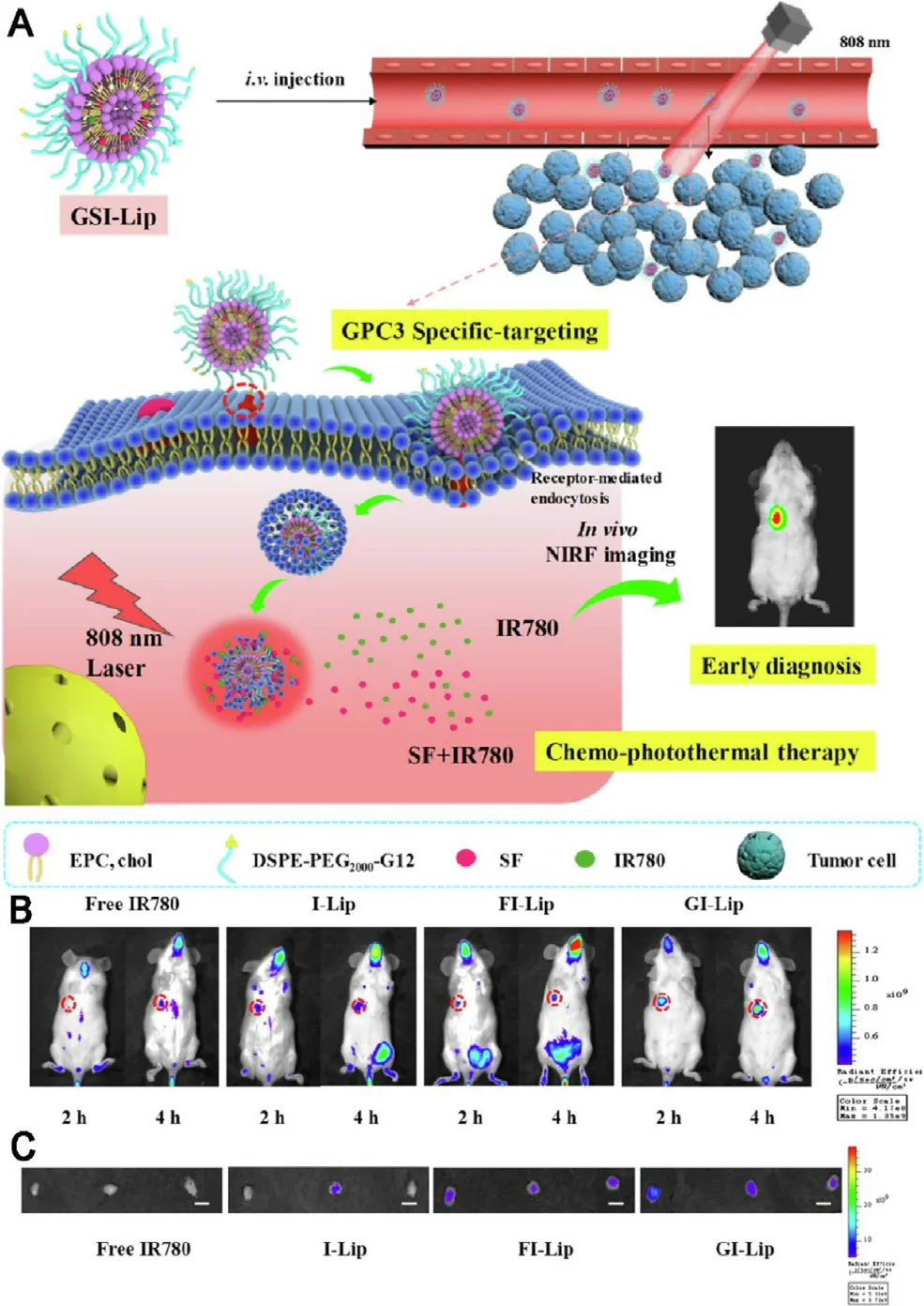
Fig.12–GPC3-targeted liposomal theranostic nanoplatforms for photothermally enhanced drug delivery.(A) Schematic illustration of the GPC3-targeted nanoplatforms for early diagnosis and combination chemo-photothermal therapy (B) In vivo biodistribution of tumor-bearing mice after different treatments.(C) Ex vivo fluorescent images of tumors after 2 and 4 h of different treatments.Adapted with permission from [130] .Copyright 2019,American Chemical Society.
4.4.Composite nanosystems of sorafenib with gene-therapy agents
As a new model of tumor therapy,gene therapy can restore or enhance the function of immune cells to improve the sensitivity of cancer to immune effects.With high tumor selectivity and biological activity,the gene-therapy agents including small interfering RNA (siRNA),microRNA (miRNA)and CRISPR/Cas9 have gained attractive interest in cancer therapy [131,132] .Co-delivery of sorafenib and siRNA have demonstrated synergistic effect in down-regulating tumor progression-relevant genes expression to provide satisfactory therapeutic effect [70,133] .Polyethylenimine (PEI) has been considered to be the promising gene carriers due to its unique proton sponge effect for excellent gene transfection efficiency[134] .Sun’s group reported that the co-delivery of sorafenib and siRNA through a PEI-modified liposome could effectively improve the gene transfection efficiency.This liposome was stable in blood circulation and displayed low systemic toxicity due to the dense structure of PEI/siRNA complex.The overexpression of cyclin D1 and GPC3 in caner cells could be efficiently down-regulated after this liposome administration,which greatly enhanced the sensitivity of tumors to sorafenibinduced therapy [134] .In addition to siRNA,miRNA also plays an important role in cell growth,proliferation and apoptosis[135] .Li et al.selected PEGylated solid lipid nanoparticles to co-deliver sorafenib,all-trans retinoic acid and miRNA into gastric cancer cells.The nanoparticles remarkably inhibited the tumor growth as evidence from the treatedmice showing an obvious reduction of tumor volume [136] .Pigment epithelium-derived factor (PEDF) gene,as an antiangiogenic agent,was proved to show significant therapeutic effect against many tumors [137,138] .Inspired by this,Chen et al.designed a chemo-gene combination therapy of sorafenib and PEDF gene by using PEG-PLGA nanoparticles as delivery vehicles.The PEG-PLGA polymer with tumor targetability could enhance the drug accumulation of sorafenib and PEDF gene towards tumor tissues and subsequently exhibited significantly improved antitumor efficacy in tumor-bearing mice models [139] .Despite enticing prospects of genetherapy agents for combination cancer therapy,one of the key concern is their biosafety and more efforts should be paid to understand their biodistribution,excretion pathways,metabolism,degradation and stability in animal models before they are used for combination delivery strateges with sorafenib.
The various composite nanosystems of sorafenib with other agents were summarized in Table 2 .The clinical application of current sorafenib-based composite nanosystems is limited due to the issues correlated with toxicity.For most of these systems,their toxicity is multifactorial,depend on composition,physicochemical properties,route of administration and dose.Numerous progress in nanomaterial technology has promoted the development of sorafenib-based combination therapies.In the future days,perhaps we should pay more attention on the illustration of different synergistic mechanisms of sorafenib with other antitumor agents that are necessary to achieve.
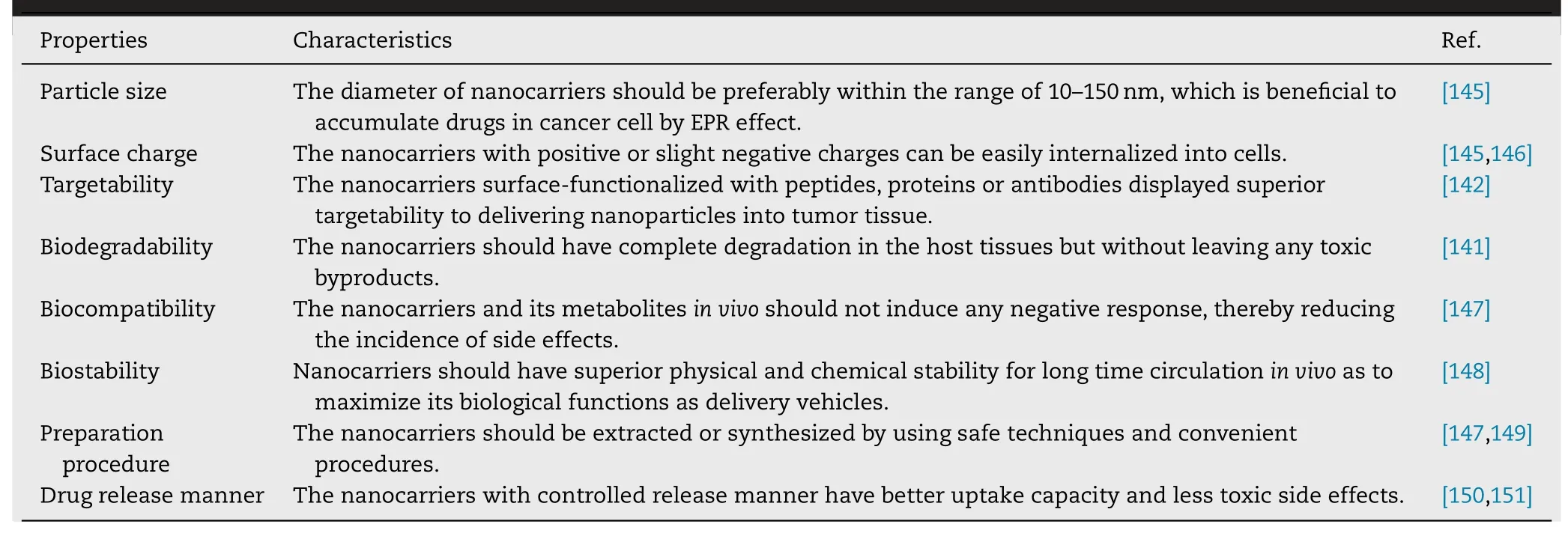
Table 3–A summary of common key properties of superior nanodelivery systems.
5.Conclusion and perspectives
Sorafenib has been recommended as the front-line therapeutic drug by the FDA based on its survival advantages identified in the clinical therapies of liver cancer and kidney cancer.However,the poor pharmacokinetic properties significantly limited the further clinical application in cancer therapy.In order to overcome these limitations,there were various multifunctional nanosized sorafenib delivery systems have been designed and synthesized with the help of nanotechnology.This article summarizes some valuable studies about improving the pharmacokinetic behavior of sorafenib by using natural or synthesized delivery systems and also discusses their preparation technique,physicochemical properties,delivery efficiency and potential advantages or limitations.In addition,this review provides a detailed discussion of the smart sorafenib delivery systems,which aims to improve its specific tumor targetability and tumor microenvirenment response.Moreover,in this review,much attention has been paid to overview the nanosystem-based combination therapy of sorafenib with other agents to exhibit synergistic antitumor effect.The overall evidence from the available literatures demonstrated that the application of sorafenib nanoformulations is a facile modality to overcome the disadvantageous events of free sorafenib regimens.However,nanodelivery system of sorafenib is now in the early stage and its applications to deliver sorafenib still suffer from the following challenges:(i)The processing procedures of some nanoformulations involve tedious technique and sophisticated facilities,which may lead to a physical instability and low drug loading efficiency[140] ;(ii) The materials used as delivery vehicles may generate some toxic byproducts under its circulation and metabolism in vivo,thereby cause some undesirable systemic side effects[141] ;(iii) The nonselective biodistribution of some sorafenib nanoformulations can be observed from in vivo studies,which means that the tissue specificity of some nanoformulations remains to be enhanced [142] .
With regard to these challenges,we summarize the common key properties of superior nanosystems in Table 3,and the following several aspects should be considered in future studies to expedite the development of sorafenib nanoformulations.(i) The main purpose of designing sorafenib-based nanodelivery systems is to promote presily drug release into tumor tissues.It is noted that effectively encapsulating sorafenib into nanodelivery systems is still a major problem that shoule be concerned in order to protect the loaded drug from early leaking through the systemic circulation,thus enhacing drug accumulation at the tumor sites while reducing the side effects.(ii) It is highly desired to translate the laboratory achievements into industrial products.Hence,further studies should focus more attention on exploring safe techniques and convenient procedures of nanocarriers or discovering ideal natural delivery materials,which will significantly help implementation of sorafenib nanoformulations into the drug market.(iii) More efforts should be paid to systematically investigate the biodistribution,pharmacokinetic response and in vivo efficacy of various sorafenib nanoformulations,rather than only be limited to evaluate its inhibition effect against in vitro cancer cells.In this case,more comprehensive preclinical and clinical trials will be conducted to assess the potential value of sorafenib nanoformulations as effective therapeutic modalities for cancer therapy.(iv) Additionally,some literatures have reported that sorafenib can exert synergistic effect with other agents including doxorubicin,ceramide and ursolic acid.Therefore,in future,in-depth studies on combination delivery of sorafenib with other antitumor agents or natural products are needed to be carried out systematically to acquire a better understanding of their joint mechanisms of action,and further reasonably guide their effective combination therapy.To conclude,the overall relevant studies concluded in this review suggest that sorafenib nanoformulations hold great potential and prospects to become viable therapeutics in the clinic by unremitting effort.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (81972832),Project supported by College Students’ innovation and entrepreneurship training program of Fujian Province (S202010386051).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2020.07.003 .
 Asian Journal of Pharmacentical Sciences2021年3期
Asian Journal of Pharmacentical Sciences2021年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Advances of mRNA vaccines for COVID-19:A new prophylactic revolution begins
- Exploring the potential of functional polymer-lipid hybrid nanoparticles for enhanced oral delivery of paclitaxel
- Innovative color jet 3D printing of levetiracetam personalized paediatric preparations
- pH-sensitive micelles self-assembled from star-shaped TPGS copolymers with ortho ester linkages for enhanced MDR reversal and chemotherapy
- In vitro - in vivo - in silico approach in the development of inhaled drug products:Nanocrystal-based formulations with budesonide as a model drug
- Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies
