Exploring the potential of functional polymer-lipid hybrid nanoparticles for enhanced oral delivery of paclitaxel
Lu Qin Hiyng Wu Enyu Xu b∗Xin Zhng Jin Gun Ruizhi Zho Shirui Mo
a School of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
b Department of Forensic Toxicological Analysis,School of Forensic Medicine,China Medical University,Shenyang 110122,China
c Guangdong Provincial Key Laboratory of Clinical Research on Traditional Chinese Medicine Syndrome,The Second Affiliated Hospital of Guangzhou University of Chinese Medicine,Guangzhou 510000,China
Keywords:Polymer-lipid hybrid nanoparticles(PLHNs)Chitosan P-gp inhibitors CYP450 inhibitors Mucoadhesion Oral bioavailability
ABSTRACT Most biopharmaceutics classification system (BCS) class IV drugs,with poor solubility and inferior permeability,are also substrates of P-glycoprotein (P-gp) and cytochrome P450 (CYP450),leading to their low oral bioavailability.The objective of this study is to explore the potential of using functional polymer-lipid hybrid nanoparticles (PLHNs) to enhance the oral absorption of BCS IV drugs.In this paper,taking paclitaxel (PTX) as a drug model,PTX-loaded PLHNs were prepared by a self-assembly method.Chitosan was selected to modify the PLHN to enhance its mucoadhesion and stability.Three P-gp inhibitors (D-α-tocopherol polyethylene glycol 1000 succinate,pluronic P123 and Solutol®HS15) were incorporated into selected PLHNs,and a CYP450 inhibitor (the extract of VBRB,BC0) was utilized to jointly promote the drug absorption.Properties of all the PLHNs were characterized systemically,including particle size,zeta potential,encapsulation efficiency,morphology,stability,in vitro drug release,mucoadhesion,in situ intestinal permeability and in vivo systemic exposure.It was found mucoadhesion of the CS-modified PLHNs was the strongest among all the formulations tested,with absolute bioavailability 21.95%.P-gp and CYP450 inhibitors incorporation further improved the oral bioavailability of PTX to 42.60%,8-fold increase compared with that of PTX itself (4.75%).Taken together,our study might shed light on constructing multifunctional PLHNs based on drug delivery barriers for better oral absorption,especially for BCS IV drugs.
1.Introduction
Oral delivery is the preferred route for drug administration.However,the rate and extent of drug absorption from the gastrointestinal tract are quite intricate and affected by many physicochemical and physiological factors [1] .Particularly,the delivery of BCS IV drugs is confronting tremendous challenges,including low aqueous solubility,poor intestinal permeability and erratic absorption,leading to low oral bioavailability.Moreover,most of BCS IV drugs are the substrate of P-glycoprotein (P-gp),even worse,they are also frequently metabolized by cytochrome P450 (CYP450)enzymes,which further decrease the therapeutic potential of these drugs [2] .To solve the above-mentioned problems,many drug delivery vehicles have been designed,including polymeric micelles,polymer-lipid hybrid nanoparticles(PLHNs),liposomes and dendrimers [3,4] .
Among them,PLHNs present more promising potential for enhancing oral absorption of BCS IV drugs.They are core-shell nanoparticles composed of lipid core and polymeric shell,combining both the advantages of liposomes and polymeric nanoparticles,and exhibiting superior characteristics in solubilizing capacity and physical stability [5] .Meanwhile,the lipid based nanocarriers show good biocompatibility and can promote drug absorption via selective lymphatic uptake[6] .Moreover,substantial researches have demonstrated that certain lipids,such as Peceol and Gelucire 44/14,are capable of inhibiting efflux pump activity to further improve drug transport efficiency in the gut [7] .Thus,adding P-gp inhibitors in the PLHN system is considered as a viable tool to further facilitate the absorption of BCS IV drugs.In addition,mucoadhesive modification of PLHN with chitosan (CS) can further enhance oral drug absorption [8] .
CYP450 enzymes family,which are responsible for the oxidative metabolism of numerous endogenous compounds,offer a defensive barrier against xenobiotics including therapeutic drugs.Various studies have shown that they are the major barriers for the absorption of BCS IV drugs[9] .Fortunately,it has been proven that the extract of vinegar-baked Radix Bupleuri,a widely used traditional Chinese medicine,can effectively modulate and inhibit the metabolizing activity of CYP450 enzymes [10],with enhanced oral absorption of 10-Hydroxycamptothecin demonstrated[11] .
Taking the advantages of PLHN,we assumed CS-modified PLHN system in combination with P-gp inhibitors and CYP450 inhibitors might be an effective strategy to enhance the oral absorption of BCS IV drugs.To demonstrate this hypothesis,taking PTX,a typical BCS IV drug,as a drug model,a novel PLHN system was developed from a combination of glyceryl monooleate (GMO) and soluplus with higher solubility,permeability and specific functional components.Thereafter,CS,as a biocompatible cationic natural polymer,was applied to decorate the PLHNs in order to enhance mucoadhesion and stability of the system.And three P-gp inhibitors (namely D-α-tocopherol polyethylene glycol 1000 succinate (TPGS),pluronic P123 (P123) and SolutolHS15 (HS15)),along with a CYP450 inhibitor(namely the extract of VBRB,BC0) were utilized to further promote the drug absorption.Properties of all the prepared PLHNs were characterized systemically,including particle size,zeta potential,encapsulation efficiency,solid state,morphology,stability,in vitro drug release,mucoadhesion,in situ intestinal permeability and in vivo systemic exposure.Furthermore,contribution of different strategies in this system to the enhanced in vivo absorption of PTX was analyzed.
2.Materials and methods
2.1.Materials
Paclitaxel was purchased from Dalian Meilun Biotechnology Co.,Ltd.(Dalian,China).Chitosan (molecular weight 400 kDa,deacetylation degree ≥85% and moisture content ≤10%) was obtained from Jinan Haidebei Marine Bioengineering Co.,Ltd.(China) and degraded as reported previously to get chitosans with molecular weight of~50 and~100 kDa [12] .Glyceryl monooleate (GMO) was acquired from Dandong Kehai Food Technology Co.,Ltd.(China).TPGS,P123 and HS15 were provided by BASF,China.Adhesin (Type 2 mucin form porcine stomach) and soluplus were purchased from Sigma-Aldrich (USA).Vinegar baked Radix Bupleuri (VBRB) was from Kangmei Medical Company (Guangzhou,China) and VBRB extract (BC0) was prepared as described previously [10] .All other reagents,unless otherwise specified,were of analytical grade.
2.2.Preparation of PTX-loaded PLHNs
PTX-loaded PLHNs were prepared by a self-assembly method as described previously [13] .Briefly,30 mg soluplus,10 mg 50 kDa CS and 15 mg TPGS,P123,HS15 were fully dissolved in 4.72 ml of acetic acid solution.Then 0.44 mg GMO was added to the resulting aqueous phase under stirring.Thereafter,100 μl of ethanol solution containing 5.4 mg PTX was dropwise added to the mixture and stirred for 4 h.The mixture was subsequently passed through 0.8 μm microporous membrane to obtain PTX-loaded PLHNs.The preparation method of unmodified PLHNs was the same as described above without the addition of CS and P-gp inhibitors.
2.3.Characterization of PTX-loaded PLHNs
2.3.1.Particle size and zeta potential
The particle size (z-average) and zeta potential of the PLHNs were characterized by Zetasizer Nano ZS 90 instrument.The samples were diluted with distilled water to appropriate concentration and then analyzed at 25 °C with scattering angle of 90.
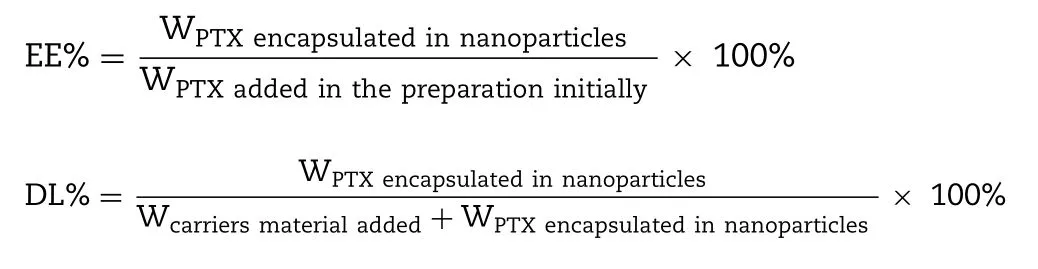
2.3.2.Encapsulation efficiency and drug loading
Encapsulation efficiency (EE%) and drug loading (DL%)of PTX in the PLHNs were measured according to the following procedures:the newly prepared PLHNs were passed through a 0.8 μm aqueous microporous membrane to remove unencapsulated PTX,which were further diluted 50-fold with acetonitrile.The mixture was centrifuged at 10 000 rpm for 10 min.A sample from the supernatant was subsequently subjected to HPLC analysis.EE% and DL% were defined as follows:
2.3.3. Transmission electron microscopy (TEM)
Morphology of the PLHNs was observed by TEM.The preparation was dropped onto a 300-mesh copper mesh and stained with 1% phosphotungstic acid for 2 min,which was subsequently dried at room temperature.Photographs were taken with an accelerating voltage of 200 kV.
2.3.4.Differential scanning calorimetry (DSC)
Thermodynamic analysis of PTX,physical mixture and the prepared PLHNs was performed by DSC.Briefly,the samples were freeze dried at −40 °C for 24 h.Thereafter,the samples(~3.0 mg) were weighed and placed in hermetically sealed aluminum pans.The samples were then scanned in the range of 25—260 °C at a heating rate of 10 °C/min under nitrogen atmosphere.The melting temperature was determined from the endothermic peak of the DSC curve recorded.
2.4.In vitro release study
For determination of drug release,PLHNs containing 100 μg PTX were added to the dialysis bag with molecular weight cutoff 8000—14 000 Da,which was placed in 80 ml HEPES buffer(pH 6.86) containing 0.1% Tween 80 (V/V),and then incubated at a rotation speed of 100 rpm/min in an air-bath oscillator(37.0 ±0.5 °C).At predetermined time points (0.5,1,2,3,4,6,12,24 and 48 h),2 ml release medium was withdrawn for drug content measurement.Meanwhile,fresh medium of equal volume was replenished for further release study.Release profiles differentiation of the tested formulations were evaluated by calculating similarity factor (f) using the following equation:
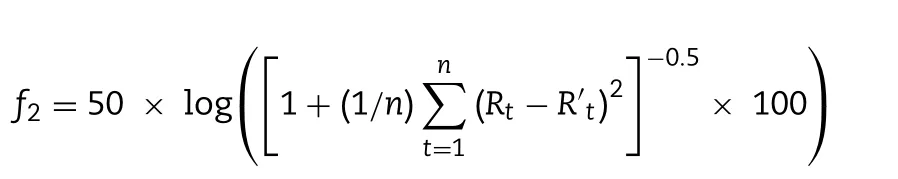
where Rand R’are the cumulative release amount of two independent formulations at time t respectively,and n is the number of time points.
2.5.Mucoadhesion measurement
Mucoadhesive properties of the PLHNs were evaluated using mucin particle method as reported previously [14] .Briefly,0.2 ml of PLHNs suspension was added into 1 ml of mucin particle suspension (ca .200 ±20 nm in diameter) and the mixture was incubated at 37 °C for 2 h.The change in mucin particle size was monitored to evaluate mucoadhesion of the PLHNs.
2.6.In situ intestinal permeation study
The transport efficiency of diverse PLHNs across different rat intestinal segments was evaluated using in situ singlepass intestinal perfusion method as reported previously[15] .All the procedures in this study were in compliance with ethical standards and guidelines issued by the ethics committee of Shenyang Pharmaceutical University for the care and use of laboratory animals.Briefly,180—220 g male SD rats were fasted for 12 h before the experiment with free access to water.Then the rats were anesthetized with an intraperitoneal injection of chloral hydrate solution (4%,w/v)at a dose of 7.5 ml/kg),placed on a heated surface maintained at 37 °C,and a 3 cm midline abdominal incision was made.An approximately 10 cm duodenal and ileal segment were isolated and cannulated on two ends,and rinsed with blank perfusion buffer.Thereafter,the Krebs-Ringer’s solution containing PTX and phenol red was perfused through the intestinal segment without sampling for 15 min at a flow rate of 0.2 ml/min,to ensure steady state conditions,followed by additional 90 min of perfusion with samples taken every 15 min.The length and radius of the perfused intestinal segment were measured at the end of the experiment.Meanwhile,the collected samples were immediately assayed by HPLC to calculate the concentration of PTX and phenol red.The apparent permeability (P) through the intestinal wall was determined according to the following equation:
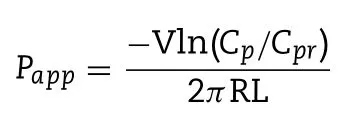
where Cand Care the ratio of the outlet and inlet concentration of PTX and phenol red respectively.V is the flow rate of perfusion buffer.R and L are the radius and length of the intestinal segment respectively.
Furthermore,to investigate the influence of CYP450 inhibitor on intestinal permeability,BC0 solution (60 mg/ml at a dose of 300 mg/kg) was utilized to equilibrate the intestinal segment before the tested formulations were perfused,and the subsequent steps were the same as described above.
2.7.In vivo pharmacokinetic study
The systemic exposure of PTX was investigated after administration of the tested formulations to rats.Briefly,35 male SD rats were randomly divided into seven groups (n=5).Thereafter,the rats were intraperitoneally anesthetized using chloral hydrate solution,and then administered with diverse formulations orally or intravenously.At specific time points(0.25,0.5,1,2,3,4,6,12 and 24 h),blood samples were collected and then centrifuged to isolate the plasma,which was stored at −20 °C until further analysis.
The plasma concentration of PTX was determined by LC-MS analysis.Briefly,100 μl plasma sample was mixed with 20 μl docetaxel (DTX) ethanol solution (150 ng/ml of internal standard) by vortexing.Thereafter,2.5 ml methyl tert—butyl ether was added to the mixture for PTX and DTX extraction.The mixture was centrifuged at 4000 rpm for 5 min and the supernatant was transferred into another tube and evaporated under nitrogen flow.The residue was redissolved in 100 μl methanol,followed by centrifugation at 10 000 rpm for 10 min.Then the obtained supernatant was analyzed by Agilent 1260—6420 tandem triple-quadrupole LCMS equipment for drug content.The pharmacokinetic parameters were calculated using a DAS.2.0 software according to the obtained drug concentration in plasma.The absolute bioavailability was calculated by the following equation:
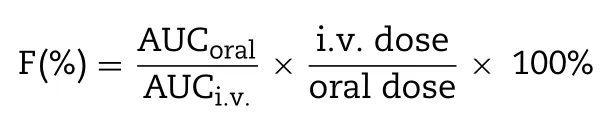
Furthermore,to investigate the influence of CYP450 inhibitor on the in vivo pharmacokinetics of PTX,the rats were administered with tested formulations after oral administration of BC0 aqueous solution for 30 min,and the subsequent steps were the same as described above.
2.8.Statistical analysis
All the experimental results were depicted as the mean ±standard deviation (SD) from at least three measurements(unless otherwise specified).Statistical significance was evaluated using one-way ANOVA at a probability level of 0.05.
3.Results and discussion
3.1.Preparation and characterization of different PLHNs
In this study,the PTX-loaded PLHNs were composed of glyceryl monooleate (GMO) as the core and soluplus as the shell,which were selected based on their compatibility with PTX via molecular dynamic simulation method using Flory-Huggins interaction parameter (χ) as the criteria (data not shown).Our preliminary experiments demonstrated that the ratio of GMO,the amount of PTX and the volume of solvent had a significant influence on properties of the preparation.Afterwards,the optimum conditions for preparing the PLHNs were screened by means of a threefactor,five-level experimental design along with response surface modeling using encapsulation efficiency,drug loading and polydispersity index as criteria (Fig.S1).Then,0.44 mg GMO,5.4 mg PTX and 4.72 ml solvent were chosen as the optimized condition to prepare the PLHNs as described in the method part and their physicochemical properties are shown in Table 1 .The PLHNs had particle size less than 100 nm,with near neutral surface charge and high drug loading efficiency.PLHNs increased PTX solubility to 1033.4 μg/ml,which is 170 times higher than that of PTX in water (~6 μg/ml).
3.1.1.Influence of mucoadhesive modification on properties of PLHNs
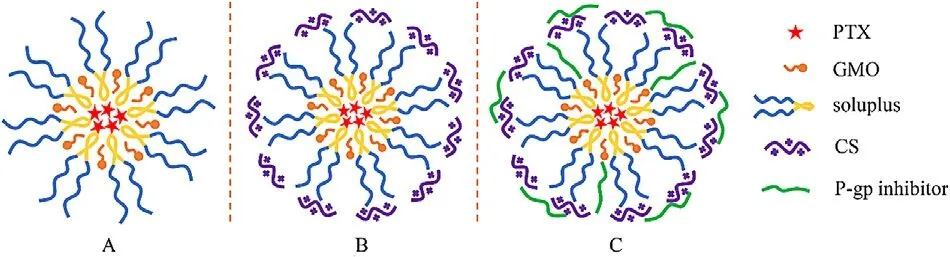
Fig.1–The schematic structure of PTX-loaded polymer-lipid hybrid nanoparticles.(A) unmodified PTX-loaded PLHN;(B) CS-modified PTX-loaded PLHN;(C)P-gp inhibitor modified PTX-loaded PLHN.
Since bioadhesive system can prolong the retention time of insoluble drugs in gastrointestinal tract,it has been widely applied for enhancing oral drug absorption.Previous study has shown that CS-based system can enhance mucoadhesion via the electrostatic interaction between positively charged chitosan and negatively charged mucosal surface [16] .Moreover,CS is well known to be able to transiently open tight junctions between epithelial cells to further enhance drug absorption [17] .To improve mucoadhesion and stability of the PLHNs,CS was selected for PLHN surface modification.
Here,two different molecular weight CS (50 kDa and 100 kDa) were selected.As presented in Table 1,compared with the unmodified PLHNs,a remarkable increase in particle size was observed for the CS-modified PLHNs,whereas the particle size of 100 kDa CS modified PLHNs was larger than that of 50 kDa CS modified ones.This can probably be explained by the fact that the larger molecular weight CS might extend its longer chains into surrounding medium after embedding in PLHN,resulting in an increase in hydrated particle size.It was noted that the CS-modified PLHNs exhibited a strong positive charge with the magnitude CS molecular weight dependent,implying successful coating of CS on the PLHN surface,as schemically depicted in Fig.1 B.Nevertheless,no statistical difference in particle size distribution,drug encapsulation efficiency was found.
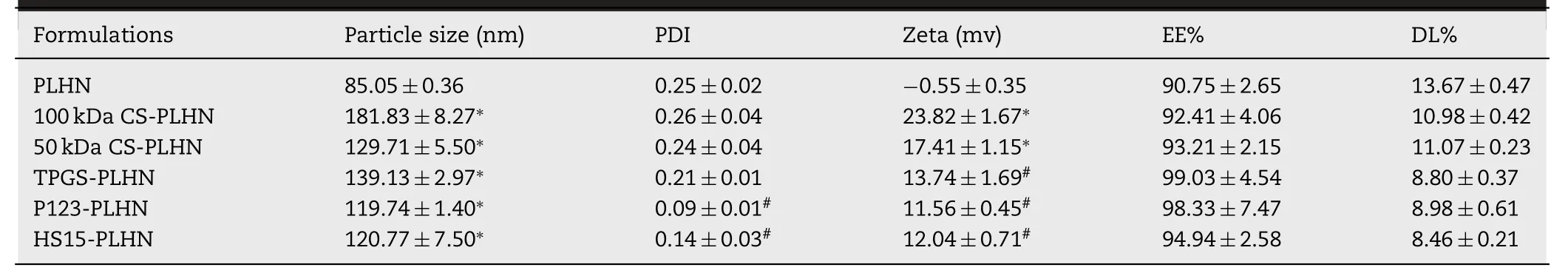
Table 1– Physicochemical properties of different PTX-loaded polymer-lipid hybrid nanoparticles (PLHNs).(∗P < 0.05 compared with PLHN;# P < 0.05 compared with 50 kDa CS modified PLHN).
Influence of CS modification on the existing state of PTX in PLHN was further analyzed by DSC.As shown in Fig.2 A,DSC thermograms revealed one endothermic peak at 221 °C and one exothermic peak at 240 °C for PTX,the former was its melting point while the latter could be assigned to its decomposition temperature.Such characteristic peaks were also observed in the physical mixture of blank PLHNs with PTX.In contrast,no drug endothermic peaks were found at around 221 °C in the thermograms of the PLHNs investigated,indicating that PTX existed in amorphous state after loading.Likewise,50 kDa CS modified PLHN system showed no drug endothermic peak but two new peaks at around 100 °C,which could be attributed to the loss of moisture content in the polysaccharide [18] .
Influence of CS modification on the short time stability of PLHN was investigated via monitoring the particle size and drug encapsulation efficiency change.As shown in Fig.2 B and 2 C,for the unmodified PLHN,although the particle size remained constant,the encapsulation efficiency decreased dramatically after a week storage (P < 0.05).In contrast,both the particle size and encapsulation efficiency of CS-modified PLHN had no apparent change during the investigated time period,indicating improved stability of PLHN via CS surface coating.
Nanoparticles with different size can have dissimilar in vitro and in vivo behavior and present particle sizedependent absorption.It has been demonstrated that drugs could be more efficiently absorbed in the intestine when being loaded into smaller sized nanoparticles [19] .Consequently,taking the particle size into consideration,50 kDa CS modified PLHN was selected for further investigation.
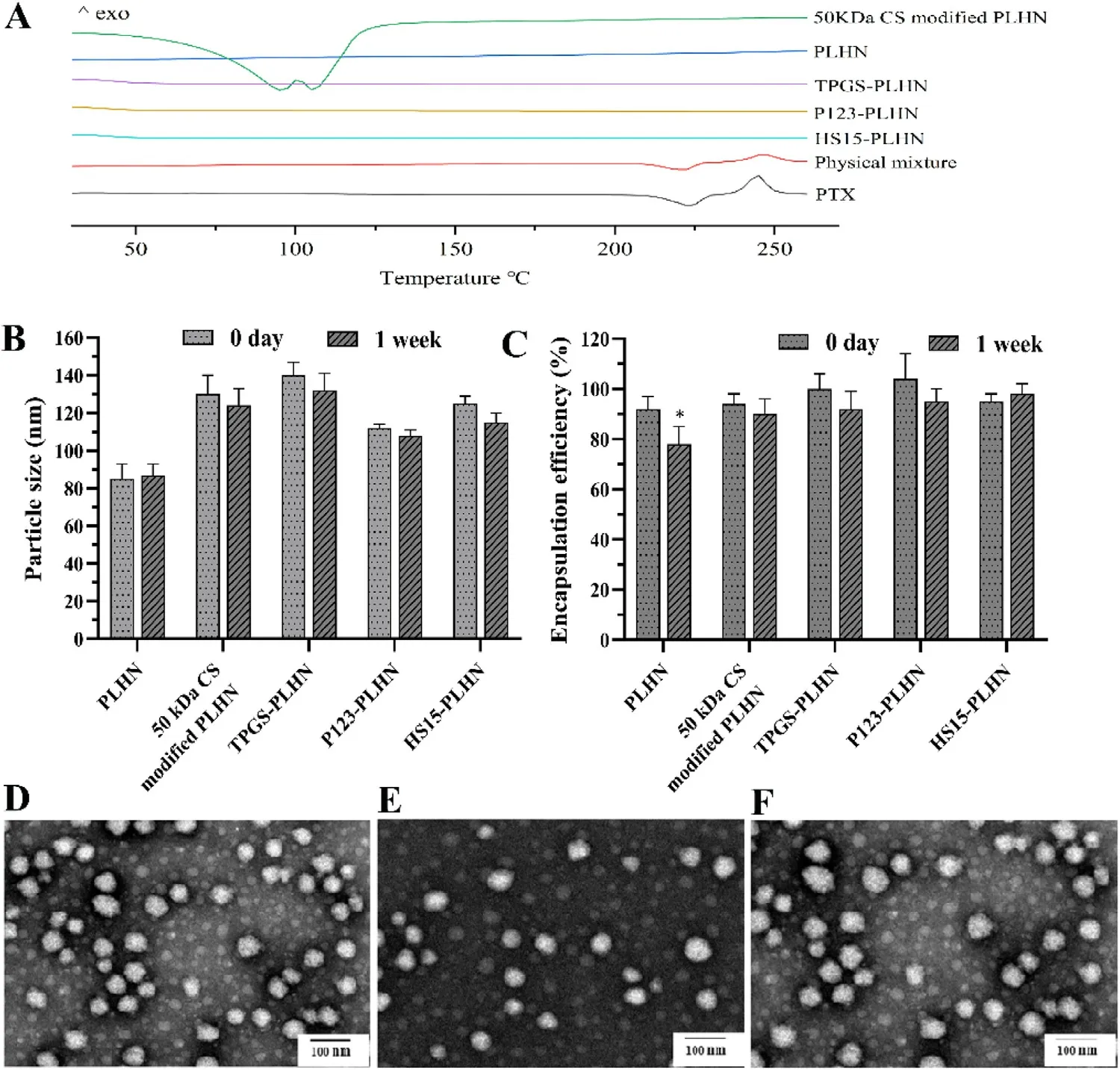
Fig.2–PLHNs characterization.(A) DSC thermograms of PTX,physical mixture of blank PLHN and PTX,and modified PLHNs.(B-C) Changes in particle size and encapsulation efficiency,respectively,upon storage of various PLHN formulations for a week.(D-F) Morphology of TPGS-PLHNs,P123-PLHNs and HS15-PLHNs,respectively.Values were exhibited as the mean ±SD (n=3).
3.1.2.Effect of P-gp inhibitors incorporation on the properties of PLHNs
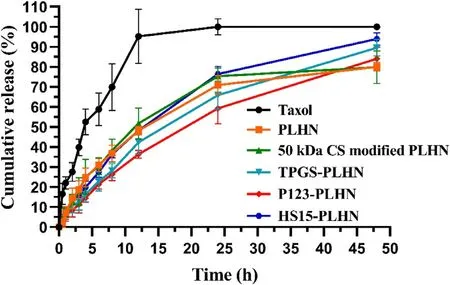
Fig.3–Cumulative release of PTX from variousformulations in HEPES solution (pH 6.86) containing 0.1%Tween 80.Values were presented as mean ±SD (n=3).
P-gp efflux pumps,located in the apical membrane of intestinal absorptive cells,can decrease drug absorption by transporting drugs from the enterocyte back to the intestinal lumen,especially those with poor solubility and permeability[20] .Therefore,it is essential to add P-gp inhibitors in PLHNs.In this study,TPGS,P123 and HS15 were selected as the representative of P-gp inhibitors.TPGS,an FDA-approved pharmaceutical excipient,may inhibit the activity of P-gp ATPase to weaken the P-gp efflux function [21] .In contrast,P123 and HS15 may directly inhibit P-gp activity by interacting with P-gp to form a complex and temporarily inactivate P-gp[22,23] .
Here,by taking 50 kDa CS modified PLHNs as an example,the effect of further incorporation of 30% (w/w) TPGS,P123 and HS15 on properties of the PLHNs was investigated.As shown in Table 1,compared with 50 kDa CS modified PLHNs,either decreased particle size (P123 and HS15 groups) or sharped particle size distribution and increased encapsulation efficiency (TPGS,P123 and HS15 groups)were observed following their addition,whereas the zeta potential reduced significantly.Moreover,TPGS-PLHN,P123-PLHN,HS15-PLHN enhanced PTX solubility to 1148.9,1159.3 and 1086.2 μg/ml,increased by 75.5,85.9 and 12.8 μg/ml compared to that of 50 kDa CS modified PLHNs,respectively.
Morphology of the PLHNs was observed by TEM.Representative images were presented in Fig.2 D-2 F,all the PLHNs were spherical or sub spherical with homogeneous particle size distribution.Similar to that in the CS-modified PLHNs,PTX mainly existed in amorphous state in the P-gp inhibitor-incorporated PLHNs (Fig.2 A),Likewise,they also presented good stability within a week (Fig.2 B and 2 C).
3.2.Influence of structure modification of PLHNs on the in vitro release of PTX
In order to elucidate the contribution of CS modification and further P-gp inhibitors incorporation on the enhanced in vivo absorption of PTX,it is highly desirable that the investigated system should have comparable drug release behavior.Therefore,in vitro release of PTX from the various PLHNs was investigated under sink condition and the release profiles were depicted in Fig.3 .Compared with the Taxol group,all the PLHNs displayed sustained drug release lasting for up to 48 h.No statistical difference in the release profiles was found between the unmodified PLHN and 50 kDa CS modified PLHN (f=83.82).Besides,compared with unmodified PLHN,the similarity factors of TPGS-PLHN,P123-PLHN and HS15-PLHN were 64.32,59.27 and 68.81 (f> 50) respectively,and when compared with 50 kDa CS modified PLHN,the similarity factors were 59.08,54.04 and 77.91 (f> 50) for TPGS-PLHN,P123-PLHN and HS15-PLHN,respectively,further demonstrating that the inclusion of P-gp inhibitors into the PLHNs had no significant influence on PTX release.
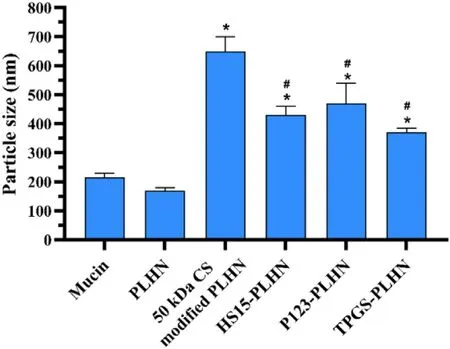
Fig.4–Mucoadhesion of various PLHNs characterized by mucin particle method.Values were presented as mean ±SD (n=3).(∗P < 0.05 compared with PLHN;# P < 0.05 compared with 50 kDa CS modified PLHN).
3.3.Influence of surface modification on the mucoadhesion of PLHNs
Influence of surface modification on the mucoadhesion of diverse PLHNs was evaluated using mucin particle method.As shown in Fig.4,no obvious change in particle size before and after mixing with unmodified PLHNs was observed,indicating its negligible bioadhesion.In contrast,all the modified PLHNs exhibited significantly increased particle size(P < 0.05) but with different extent.It was noted that 50 kDa CS modified PLHN group showed the maximum increase in particle size,implying the strongest mucoadhesion,whereas the mucoadhesion of P-gp inhibitor-added PLHNs was much weaker than that of CS modified PLHN group,and no statistical difference was found among the three P-gp inhibitors modified groups (P > 0.05).This can probably be attributed to the fact that the positive charge on the surface of PLHNs was partially covered by incorporated P-gp inhibitors (Fig.1 C),leading to weaker interaction with negatively charged mucin particles.Moreover,the P-gp inhibitors distributed on the surface of PLHNs could improve hydrophilicity as well as provide steric hindrance,further weakening the adhesion.But the mucoadhesion was still significantly higher than that of unmodified PLHN.
3.4.Influence of PLHN composition on the intestinal permeation of PTX
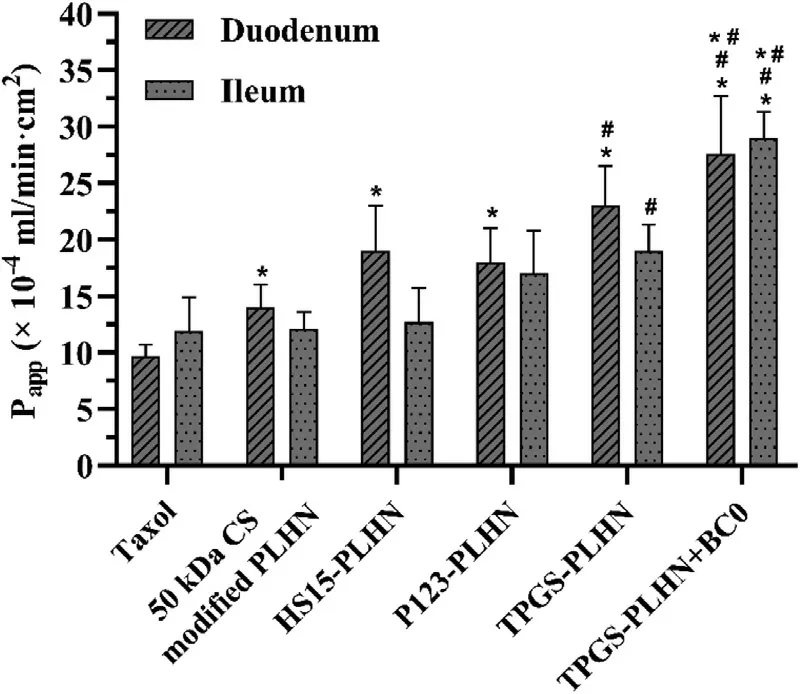
Fig.5–The apparent permeability (P app) of in situ single-pass intestinal perfusion of different formulations.Values were presented as mean ±SD (n=3).(∗P < 0.05 compared with Taxol;# P < 0.05 compared with 50 kDa CS modified PLHN;∗# P < 0.05 compared with TPGS-PLHN).
The above studies demonstrated that when PLHNs were used as the nanocarrier,irrespective of their structure,significant increase in drug solubility was achieved.Their contribution in the intestinal permeation needs further exploration.In this part,duodenum and ileum were selected as the representative of intestinal segments,and permeability of different formulations was evaluated using in situ singlepass intestinal perfusion method.As illustrated in Fig.5,in duodenum,the permeability of 50 kDa CS modified PLHN was 1.46 times higher than that of Taxol,implying PLHN itself as a nanocarrier can enhance drug absorption.Meanwhile,the enhanced mucoadhesion may also contribute to improved absorption by prolonging retention time in the intestinal tract.Similar trend was observed in the P-gp inhibitor-incorporated PLHN groups,and compared with the Taxol group,TPGSPLHN,P123-PLHN and HS15-PLHN showed a 2.46-,1.76-and 2.01-fold increase in Papp value respectively.In contrast,in ileum,when compared with 50 kDa CS modified PLHN,increased permeability was only observed in TPGS-PLHN (1.35 times) (P < 0.05),implying TPGS possessed the strongest absorption promoting effect in ileum among the three P-gp inhibitors investigated.Accordingly,TPGS-PLHN was selected for the follow-up study.Furthermore,it was worth noting that the permeability of TPGS-PLHN and HS15-PLHN exhibited obvious difference in duodenum and ileum,which might be related to the different P-gp activity and mucus thickness in the intestine.The higher P-gp expression and thicker mucus in ileum led to lower permeation efficiency compared with that in duodenum [24] .
It is known that some CYP450 enzymes in the intestine may influence drug absorption.Our previous studies demonstrated that various extracts of VBRB could exert an inhibition effect on the activity of CYP450 [10] .Herein,BC0,a natural polysaccharide extracted from VBRB,was used via coadministration with TPGS-PLHN to investigate whether it can further improve drug absorption via inhibiting the CYP450 activity.Notably,after coadministration,the apparent permeability in duodenum and ileum was increased by 1.18 and 1.77 times compared with the TPGS-PLHN group,respectively (Fig.5),implying coadministration of BC0 could further promote the absorption of PTX in the intestine,especially in ileum.
3.5.Influence of PLHN composition on the in vivo absorption of PTX
In vivo pharmacokinetic experiment further confirmed that the functional PLHNs can significantly improve the absorption efficiency of PTX.As presented in Fig.6 A and Table 2,following intravenous injection of Taxol,an extremely high C max and shorter half-life was observed,indicating rapid in vivo clearance.In contrast,as depicted in Fig.6 B,all the oral PLHN groups exhibited sustained in vivo absorption.It has been reported that when the plasma concentration of PTX is above a threshold value of 0.1 μM (equivalent to 85.3 ng/ml),it is pharmacologically active [25] .For all the PLHN groups investigated here,the plasma concentration of PTX remained above the therapeutic concentration for over 24 h.Compared with the oral Taxol group,the AUC value of the 50 kDa CS modified PLHN increased by 7.31-fold (P < 0.05),implying significant improvement in oral absorption.As for the P-gp inhibitor-incorporated PLHN groups,the AUC value was further increased compared with the 50 kDa CS modified PLHN group (P < 0.05),and the absolute bioavailability of TPGS-PLHN,P123-PLHN and HS15-PLHN was increased to 35.67%,32.84% and 33.53%,respectively.This is in good agreement with the intestinal permeation study.Moreover,it was noted that the coadministration of TPGSPLHN with BC0 achieved the best absorption among the formulations tested,with the highest bioavailability (42.60%)achieved.This prominent enhancement of PTX absorption could be explained by the combination of following effects:(1) The solubility of PTX in PLHNs was significantly increased compared to that in Taxol.Meanwhile,the GMO component in PLHNs may facilitate the uptake of PTX via Peyer’s patches into the blood through lymphatic circulation [26] ;(2) The enhanced bioadhesion can prolong the retention time in the gut,thereby increasing absorption;(3) The addition of P-gp inhibitors further promoted drug absorption by inhibiting intestinal P-gp drug efflux.Besides,polyethylene glycol (PEG)fragments in HS15 and TPGS,together with polyethylene oxide (PEO) fragments in P123 can increase hydrophilicity of the PLHNs,thus accelerating mucus layer penetration;(4) The utilization of BC0 can inhibit the activity of CYP450 enzymes,which may reduce the metabolic elimination of PTX in the gut and liver.Moreover,it was found that BC0 might have a favorable effect on inhibiting glutathione transferase activity,and glutathione transferase inhibitors have been reported as a potential adjunct to enhance the effect of anticancer drugs [27] .In addition,the time to reach maximum plasma concentration (T) and halflife (T) were extended for all the PLHNs investigated,which might be attributed to the stronger mucoadhesion and their sustained release behavior compared to Taxol group.
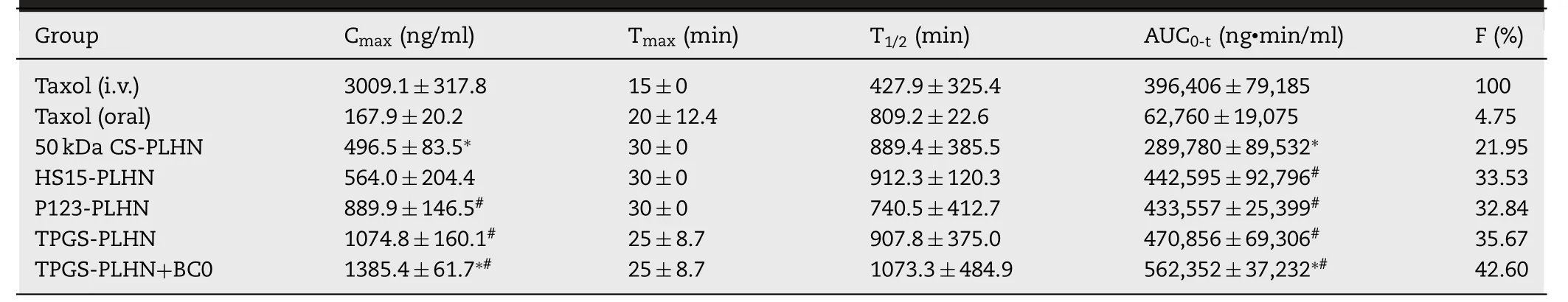
Table 2– Pharmacokinetic parameters of PTX in rats following oral administration of PLHNs and intravenous administration of Taxol.(∗P < 0.05 compared with oral Taxol;# P < 0.05 compared with 50 kDa CS modified PLHN;∗#P < 0.05 compared with TPGS-PLHN).
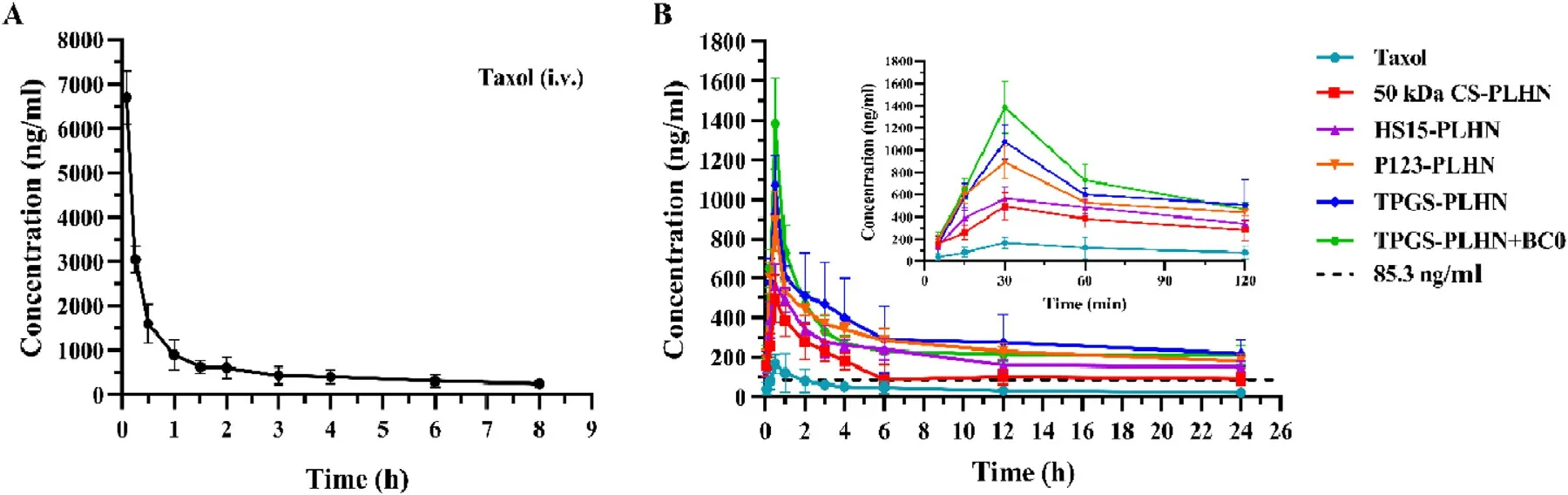
Fig.6–The plasma concentration-time profiles of PTX following (A) intravenous administration;(B) oral administration.Values were presented as mean ±SD (n=5).
Here,the contribution of different composition in this system to the enhanced in vivo absorption of PTX via oral administration was further analyzed.Taking the coadministration group of TPGS-PLHN and BC0 as an example,the oral absorption of PTX can be divided into the following four parts.Firstly,4.75% oral bioavailability was observed for the Taxol group,implying that only 4.75%of PTX could be absorbed as a free molecule.Secondly,the oral bioavailability of 50 kDa CS modified PLHN was increased to 21.95%,implying 17.2% of PTX was absorbed due to the combined contribution of PLHN system and surface modification of CS.Next,35.67% oral bioavailability was found for the TPGS-PLHN group,suggesting additional 13.72% of PTX was absorbed contributed to TPGS incorporation,a P-gp inhibitor.Moreover,TPGS-PLHN combined with BC0 group exhibited oral bioavailability of 42.6%,inferring that 6.93%of PTX was further absorbed in presence of BC0,which can inhibit the activity of CYP450 enzymes.
Among various reports on promoting oral absorption of PTX over the past few years,the nanoparticles formed by amphiphilic carboxymethyl chitosan-quercetin conjugate showed the highest oral absorption of PTX,with relative bioavailability of 718% [28] .In contrast,the TPGS-PLHN and BC0 coadministration system reported here exhibited even better performance,with relative bioavailability of 896%.In the future,influence of different types and concentrations of P-gp inhibitors and CYP450 inhibitors on oral absorption of PTX will be further investigated.
4.Conclusion
In this study,a novel PLHN system was developed from a combination of GMO and soluplus,and the potential of functionalized PLHNs for enhanced oral absorption of PTX was systemically investigated.It was proved that CS modification improved mucoadhesion and stability of the nanoparticles.P-gp inhibitors could promote PTX oral absorption effectively,with TPGS presenting the best effect.Besides P-gp inhibitors,additional CYP450 inhibitor incorporation further improved the oral absorption of PTX.And the combined use of TPGS-PLHN and BC0 displayed the highest intestinal permeability and oral absorption efficiency among all the formulations tested,with absolute oral bioavailability 42.60% achieved.In conclusion,constructing multifunctional PLHNs based on specific drug delivery barriers might be a promising approach to enhance oral absorption of BCS IV drugs.
Conflicts of interest
The author declared no conflict of interests.
Acknowledgements
This research is financially supported by the Natural Science Foundation of China (Grant No.81273446).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2021.02.004 .
 Asian Journal of Pharmacentical Sciences2021年3期
Asian Journal of Pharmacentical Sciences2021年3期
- Asian Journal of Pharmacentical Sciences的其它文章
- Advances of mRNA vaccines for COVID-19:A new prophylactic revolution begins
- Innovative color jet 3D printing of levetiracetam personalized paediatric preparations
- pH-sensitive micelles self-assembled from star-shaped TPGS copolymers with ortho ester linkages for enhanced MDR reversal and chemotherapy
- In vitro - in vivo - in silico approach in the development of inhaled drug products:Nanocrystal-based formulations with budesonide as a model drug
- Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies
- Recent advances of sorafenib nanoformulations for cancer therapy:Smart nanosystem and combination therapy
