Multiplexed bioluminescence imaging of cancer cell response to hypoxia and inflammation in the caudalartery injection model of bone metastasis during zoledronic acid treatment
Misa Minegishi ,Takahiro Kuchimaru,2 ,Kenji Nakagawa ,Tatsuhiro Isozaki ,Satoshi Fujimori ,Tetsuya Kadonosono ,Shinae Kizaka-Kondoh
1School of Life Science and Technology,Tokyo Institute of Technology,Kanagawa 226-8501,Japan.
2Center for Molecular Medicine,Jichi Medical University,Tochigi 329-0498,Japan.
Abstract Aim: Therapeutic agents suppressing bone remodeling have been clinically approved to delay metastatic progression and skeletal-related events in patients with bone metastasis.However,therapeutic agents including zoledronic acid (ZA) are insufficient to regress established bone metastasis.Therefore,new treatment strategies are desired,and unraveling the status of cancer cells during bone metastatic progression will help develop therapeutic strategies.Methods: We developed a unique multiplexed reporter system for bioluminescent imaging (MRS-BLI) using three luciferase reporter genes.This system allows for the noninvasive and quantitative monitoring of tumor growth and activities of nuclear factor-kappa B (NF-κB) and hypoxia-inducible factor (HIF),which are the key transcriptional factors in response to inflammation and hypoxia,respectively.PC-3/MRS-BLI,a human prostate cancer cell line that stably retains the MRS-BLI reporter genes,was applied to the caudal-artery injection model of bone metastasis to observe the status of cancer cells during bone metastasis development and ZA treatment (< 1 month).Results: MRS-BLI reveals key events during the bone metastasis development:NF-κB and HIF are activated in cancer cells after migration to the bone marrow and are transiently reduced,followed by rapid activation before proliferation begins.ZA treatment suppresses the growth of metastasized cancer cells by suppressing NF-κB and HIF activities that may be indirectly induced by osteoclast activation.Conclusion: By visualizing the NF-κB and HIF activities of PC-3/MRS-BLI in bone,MRS-BLI has enabled new discoveries regarding the regulation of bone metastases.Further analysis of the progression of bone metastases using MRS-BLI may provide important information for developing new therapeutic strategies.
Keywords: Bone metastasis,multiplexed bioluminescence imaging,zoledronic acid,nuclear factor-kappa B,hypoxia inducible factor
INTRODUCTION
The bone is frequently affected with metastases in patients with primary tumors in various tissues including the prostate,breast,lung,and kidney[1].Once bone metastasis is developed,patients suffer from pathogenic fractures,nerve compression and hypercalcemia[2].These events are caused by aberrant bone remodeling through destructive osteolytic and/or bone-forming osteoblastic lesions associated with bone metastasis.Therefore,the management of aberrant bone remodeling is a current primary strategy to improve the quality of life of patients with bone metastasis.To improve treatment strategy,it is necessary to understand the states of cancer cells during the formation and progression of bone metastases,but little research has been done on them.It has been suggested that inflammatory and hypoxic microenvironments contribute to bone metastasis development[3-5].Nuclear factor-kappa B (NF-κB) is a transcription factor that activates pro-tumor cellular programs in response to inflammation-related stimulations and plays a crucial role in the growth of bone metastasis[6].Tumor tissues often contain hypoxic microenvironments due to aberrant angiogenesis,and hypoxia-inducible transcription factors (HIFs) are activated in metastasized cancer cells,promoting adaptation and growth in the bone marrow[7-9].In addition to the pathological hypoxia,bone marrow is physiologically hypoxic[10].The pathological and physiological hypoxic microenvironments might be involved in the progression of bone metastasis.
Zoledronic acid (ZA),a third-generation aminobisphosphonate,is the gold standard as well as denosumab for the medical management of skeletal relevant events such as osteoclast activities and bone destruction in bone metastasis[11].ZA treatment significantly decreased the incidence of skeletal relevant events in patients with osteolytic bone lesions[12].In addition,ZA is recommended as an adjuvant therapy in breast cancers to prevent bone metastases,most of which are osteolytic[13].ZA further displays efficacy in early treatment of patients with prostate cancers,which are dominantly osteoblastic[14].These observations indicate that ZA is therapeutically effective in both osteolytic and osteoblastic bone metastases.However,ZA treatment is insufficient to regress established bone metastasis and improve the overall survival rate of the patients[15,16].Therefore,ZA combination treatment strategies are widely used clinically[17].Understanding the status of cancer cells in the bone marrow during the development and progression of metastasis will help develop therapeutic strategies.However,there is currently no adequate model to investigate the status of metastasized cells in the bone in real time,and therefore it is difficult to assess the efficacy of drugs in preventing metastatic progression.
Bioluminescence imaging (BLI) is a powerful technique for noninvasive real-time monitoring of various cellular states such as cell proliferation and transcriptional activities in small animals[18].Therefore,a variety of natural and artificial luciferases,and their specific substrates,are used in studies to explore the biological mechanisms,disease development and therapeutic strategies[19].Firefly luciferase (Fluc) has been primarily employed as a reporter gene,and other luciferases such as Renilla luciferase (Rluc),Vargula luciferase (Vluc),and Cypridina luciferase (Cluc) are used in combination with Fluc because of no cross-reactivity of luciferin-luciferase reactions[20,21].Previous studies described dual-luciferase reporter systems,which monitors transcription growth factor-beta (TGF-β) induced Smad transcriptional activities and metastatic cancer cell proliferation in murine bone metastatic lesions by Fluc and Rluc reporter genes[22,23].Further multiplexing of BLI would delineate informative cellular states of cancer cells during metastatic progression and may provide information to facilitate new therapeutic strategies to prevent the development and progression of bone metastasis.
Here,we developed a unique multiplexed reporter system for bioluminescent imaging (MRS-BLI) by using three luciferase reporter genes,which allows for the noninvasive and quantitative monitoring of tumor growth as well as NF-κB and HIF activities.In this work,the stable transfectant of human prostate cancer cell PC-3 of MRS-BLI (PC-3/MRS-BLI) was analyzed shortly after injection with our original caudal-artery injection model of bone metastasis,which allowed us to efficiently deliver PC-3/MRS-BLI into the bone marrow without the need for high technical proficiency[24].We demonstrated the noninvasive monitoring of MRS-BLI signals emanating from bone metastasis in mice hind limbs shortly after injection of PC-3/MRS-BLI.In addition,MRS-BLI revealed that ZA treatment suppressed NF-κB and HIF activities as well as the growth of bone metastasis.Thus,MRS-BLI successfully visualized the activities of NF-κB and HIF in PC-3/MRS-BLI in bones.Taken together,these findings suggest that MRS-BLI could be a powerful tool to develop new therapeutic strategies for bone metastasis.
METHODS
Reagents
For cell cultures,we used Roswell Park Memorial Institute (RPMI)-1640 (Nacalai tesque,Kyoto,Japan) and Dulbecco’s Modified Eagle Medium (DMEM) (Nacalai tesque,Kyoto,Japan),supplemented with fetal bovine serum (FBS) (Gibco,NY,USA),penicillin and streptomycin (Nacalai tesque,Kyoto,Japan).As selection reagents for cell cloning,we used hygromycin B (Wako,Tokyo,Japan),blasticidin S (Life Technologies,CA,USA),and G418 (Wako,Tokyo,Japan).Zoledronic acid monohydrate (Sigma-Aldrich,MO,USA) was used forin vitroandin vivostudies.The luciferases and substrates used in this study are summarized in Table 1[21,25-27].
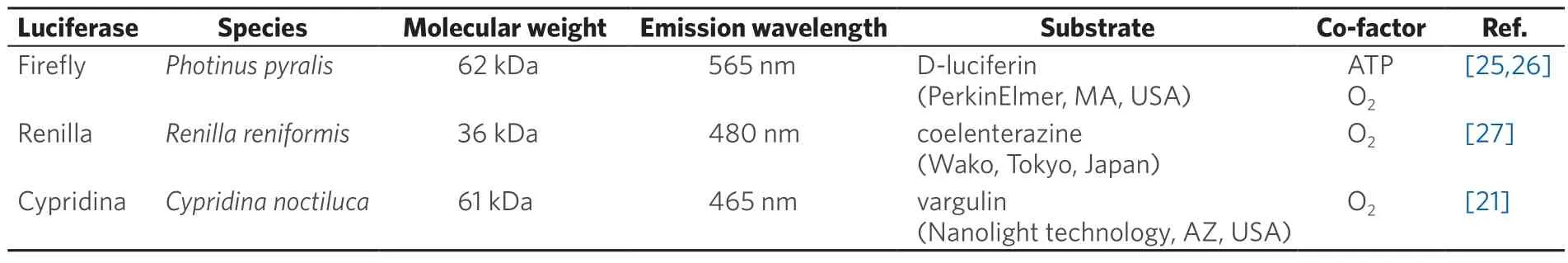
Table 1.Luciferases used in MRS-BLI
Plasmid Construction
We amplified Cluc cDNA with pCL-sv (ATTO,Tokyo,Japan) and then inserted it into the EcoRV site of pcDNA3.1/myc-HisA (Thermo Fisher Scientific,MA,USA) to obtain pcDNA3.1/CMV-Cluc.To construct the pcDNA3.1/CMV-Cluc-glycosylphosphatidylinositol (GPI) plasmid,the EGFP coding sequence of pCAG-EGFP-GPI[28]was replaced with the Cluc coding sequence,and then the Cluc-GPI fragment was amplified and inserted into the EcoRV site of pcDNA3.1/myc-HisA.We obtained pGL4.32/5HRE-CMVmp-RlucP and pGL4.32/5κB-CMVmp-FlucP plasmids by inserting 5HRE-CMVmp-RlucP and 5κB-CMVmp-FlucP between the KpnI and EcoRI recognition sites of pGL4.32 (Promega,WI,USA),respectively[29].
Cells
The human prostate cancer cell lines,PC-3 (ATCC,VA,USA) and PC-3/MRS-BLI,were cultured in an RPMI-1640 medium supplemented with penicillin (100 unit/mL)/streptomycin (100 μg/mL) and 10% FBS.The human cervical cancer HeLa cells (ATCC,VA,USA) were cultured in a DMEM medium supplemented with penicillin (100 unit/mL)/streptomycin (100 μg/mL) and 10% FBS.The cells were maintained at 37 °C,with 5% CO2,and regularly checked for mycoplasma contamination by a mycoplasma check kit.
Cluc activity measurement
PC-3 (5.0 × 104) cells were seeded onto 24-well plates with 1 mL medium and cultured overnight.The cells were transfected with pcDNA3.1/CMV-Cluc or pcDNA3.1/CMV-Cluc-GPI by polyethylenimine.After 24 h,the cultured medium was harvested and the cells were lysed with a 100 μL passive lysis buffer (Promega,WI,USA).The medium and lysed cells were centrifuged,and Cluc activity in 10 μL of the supernatant was measured after mixing with 10 μL of vargulin (0.4 μg/mL) using GL-210 luminometer (Microtech Nichion,Chiba,Japan).To measure the correlation between Cluc activity and cell number,PC-3/MRS-BLI were harvested with Accutase (Innovative Cell Technologies,Inc.,CA,USA).Cell numbers of 1.25 × 104,2.5 × 104,5.0 × 104,and 1.0 × 105were lysed with 100 μL passive lysis buffer and Cluc activity was measured as described above.
Cross-reactivity measurement of Cluc
HeLa (5.0 × 104) cells were seeded onto 24-well plates and cultured overnight.The cells were transfected with pcDNA3.1/CMV-Fluc or pcDNA3.1/CMV-Rluc or pcDNA3.1/CMV-Cluc-GPI by polyethylenimine.After 24 h,the cells were lysed with 100 μL passive lysis buffer and 10 μL of the centrifuged lysate was measured for luciferase activity after mixing with 10 μL of the following substrates:coelenterazine-h (10 μg/mL) for 5HRE-CMVmp-RlucP;luciferase assay reagent for 5κB-CMVmp-FlucP;vargulin (0.4 μg/mL) for CMV-Cluc-GPI.
Establishment of PC-3/MRS-BLI
PC-3 cells were transfected with pGL4.32/5HRE-CMVmp-RlucP plasmid by the calcium phosphate method and cultured in selection medium containing 200 μg/mL hygromycin B.To establish PC-3/5HRE-CMVmp-RlucP,colonies were selected by measuring the bioluminescence (BL) of multiple colonies cultured in 1% O2.PC-3/5HRE-CMVmp-RlucP were co-transfected with pGL4.32/5κB-CMVmp-FlucP plasmid and pEF6/Myc-HisA (Thermo Fisher Scientific,MA,USA),and were selected in a selection medium containing 6 μg/mL blasticidin S.To establish PC-3/5HRE-CMVmp-RlucP,5κB-CMVmp-FlucP,we assessed the BL of multiple colonies cultured with 5 ng/mL hTNF-α (Rocky Hill,NJ,USA).We then transfected PC-3/5HRE-CMVmp-RlucP,5κB-CMVmp-FlucP with pcDNA3.1/CMV-Cluc-GPI by ScreenFectTMA (Wako,Tokyo,Japan),followed by selection with 500 μg/mL G418.We assessed the BL of multiple antibiotic-resistant colonies to establish PC-3/MRS-BLI.
Mice
SCID mice (male) were obtained from Charles River Laboratories (Kanagawa,Japan).All mice were provided access to food and waterad libitumand were housed in animal facilities at the Tokyo Institute of Technology.All experimental procedures using mice were approved by the Animal Experiment Committee of the Tokyo Institute of Technology (authorization number 201006-3),and carried out in accordance with relevant national and international guidelines.
Bone metastasis model with ZA treatment
PC-3/MRS-BLI (1.0 × 106cells) was suspended in 100 μL PBS and was injected into the caudal artery of SCID mice under anesthesia conditions[24].A stock solution of ZA was prepared by dissolving ZA monohydrate (Sigma-Aldrich,MO,USA) in sterile ddH2O at a concentration of 10 mM,divided into small aliquots,and kept at -80 °C for long-term storage.Mice were intravenously administered with 100 μL of either 100 μg/mL ZA or PBS at 5-day intervals from day 2 to day 22.
In vivo MRS-BLI
All bioluminescence image data of tumor bearing mice were acquired with IVIS Spectrum (PerkinElmer,MA,USA).5HRE-CMVmp-RlucP images were acquired at 2 min after intravenous injection of coelenterazine-h (250 μg/kg).2 h later,CMV-Cluc-GPI images were acquired at 30 sec after intravenous injection of vargulin (2 μg/kg).After another 2 h,5κB-CMVmp-FucP images were acquired at 15 min after intraperitoneal injection of D-luciferin (50 mg/kg).For each imaging session,mice are immobilized by gas anesthesia mixing 2% isoflurane and 98% oxygen.The following conditions were used for image acquisition:open emission filter,exposure time=0.5 min for 5HRE-CMVmp-RlucP and CMVCluc-GPI,exposure time=1 min for 5κB-CMVmp-FucP,binning=medium :8,field of view=13.4 cm × 13.4 cm,and f/stop=1.The BL images were analyzed by Living Image 4.3 software (PerkinElmer,MA,USA) that is specialized for IVIS.
PC-3/MRS-BLI proliferation assay with ZA treatment
PC-3/MRS-BLI (4.0 × 103) cells were seeded onto 96-well plates,and cultured overnight.The cells were further cultured with ZA (0.05,0.5,5,50,100 μM) for 0,24 or 48 h.After the culture,cell proliferation rates were assessed with WST-1 reagents (Roche Applied Science,Penzberg,Germany) according to the manufacturer’s protocol.
MRS-BLI reporter assay with ZA treatment
PC-3/MRS-BLI (5.0 × 104) cells were seeded onto 24-well plates,and cultured overnight.The cells were further cultured with ZA (0.05,0.5,5 μM) for 16 h.The cells were lysed with a 100 μL passive lysis buffer (Promega,WI,USA) and centrifuged.Luciferase activity of 10 μL supernatant was measured using the GL-210 luminometer (Microtech Nichion,Chiba,Japan) after mixing with 10 μL of the following substrates:coelenterazine-h (10 μg/mL) for 5HRE-CMVmp-RlucP;luciferase assay reagent for 5κB-CMVmp-FlucP;vargulin (0.4 μg/mL) for CMV-Cluc-GPI.
Statistical analysis
Data are presented as means ± standard error of the mean (SEM) and were statistically analyzed with a two-side Student’st-test.P-values < 0.05 were considered statistically significant.
RESULTS
Herein,we have constructed a new bioluminescence reporter,Cluc-GPI,for use in combination with Fluc and Rluc reporters that are commonly employed in bioluminescence imaging.In reaction with vargulin,Cluc generates a stronger bioluminescence than Vluc,which was isolated from marine ostracods as Cluc was[21].However,wild-type Cluc is naturally secreted from cells and is stable in blood circulation,thus increasing background signals in mice[30].Therefore,the GPI signal sequence of CD90 was added to the C-terminus of Cluc so that the created Cluc-GPI was anchored to the cell membrane [Figure 1A][28].As we expected,secretion of Cluc-GPI into the medium was decreased 10-fold compared to wild-type Cluc,and intracellular Cluc activity was significantly increased [Figure 1B].We further confirmed that Cluc-GPI showed no cross-reactivity with Fluc and Rluc BL systems [Figure 1C].Overall,our results indicate that Cluc-GPI is a highly sensitive BLI reporter gene and can be used in combination with the Fluc and Rluc dual BL reporters.
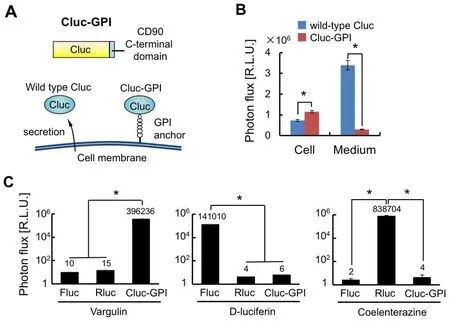
Figure 1.Construction of the Cluc-GPI reporter.A:Schematic of Cluc-GPI.Cluc is fused with the C-terminal domain of CD90;B:Intracellular (Cell) and extracellular (Medium) Cluc bioluminescence intensities were measured 24 h after transfection of the reporter gene.n=3,★P < 0.05;C:Cell lysates of HeLa transfected with Fluc,Rluc or Cluc-GPI reporter genes were measured for bioluminescence intensities after reactions with the indicated luciferase substrates.n =3,★P < 0.05.Cluc:Cypridina luciferase;Fluc:Firefly luciferase;Rluc:Renilla luciferase;GPI:glycosylphosphatidylinositol
We next established the cell line PC-3/MRS-BLI,which stably carries the multiplexed luciferase reporter system.PC-3 is a human prostate cancer cell line,often used in studies of bone metastases in small animal models[31].To establish PC-3/MRS-BLI,we transduced three reporter genes,CMV-Cluc-GPI (Cluc-GPI),5κB-CMVmp-FlucP (κB-FlucP) and 5HRE-CMVmp-RlucP (HRE-RlucP) [Figure 2A].Fluc and Rluc were fused with a PEST sequence to shorten the half-life of luciferases,thereby improving the real-time monitoring effect of reporters[32].The κB-FlucP responded as expected to inflammatory cytokine TNF-α in a dose-dependent manner [Figure 2B];TNF-α stimulation increased Fluc activity to reach peak levels in 12 h [Figure 2C];HRE-RlucP responded to hypoxia when O2concentration was less than approximately 5% [Figure 2D];while Rluc activity peaked at around 18 h under 1% O2conditions [Figure 2E].Furthermore,stable expression of CMV-Cluc-GPI in PC-3 cells displayed a linear correlation between luminescence intensity and cell number [Figure 2F].These results indicate that cellular responses to inflammation and hypoxia can be monitored by the BL of Fluc and Rluc,respectively,in addition to monitoring the cell number by Cluc BL.
PC-3/MRS-BLI cells injected through caudal artery were efficiently delivered to the hindlimb bone and developed metastases as reported previously[24].Treatment with ZA (0.4 mg/kg) was started 2 days after the caudal artery injection of PC-3/MRS-BLI cells,and ZA was intravenously injected 5 times at 5-day intervals [Figure 3A].BLI monitoring NF-κB and HIF activities and tumor mass were sequentially acquired at 2-hour intervals to minimize the influence of time lag and residual substrates [Figure 3A].The MRS-BLI was noninvasively observed from day 1.PC-3/MRS-BLI cell growth continued over time and significantly accelerated on day 17 [Figure 3B and C].ZA treatment significantly suppressed PC-3/MRS-BLI cell growth [Figure 3C].Changes in NF-κB and HIF activities per cancer cell were quantitatively analyzed by normalizing the BL intensities of Fluc and Rluc by that of Cluc (tumor mass).NF-κB and HIF increased rapidly prior to tumor growth [Figure 3B,D and E].Interestingly,ZA treatment significantly suppressed transcriptional activities by day 12,which was earlier than cell growth suppression,namely,by day 17 [Figure 3C-E].These results suggested that ZA treatment suppresses the growth of metastasized cells by reducing microenvironmental inflammation and hypoxia.

Figure 2.Establishment of human prostate cancer,PC-3/MRS-BLI,stably carrying multiplexed luciferase reporters. A:Schematic of PC-3/MRS-BLI.The 5κB motif contains five tandem repeats of the GGACTTTCC.The 5HRE motif contains five tandem repeats of the ACGTGG;B:Relative κB-FlucP bioluminescence intensity in PC-3/MRS-BLI cells treated with TNF-α (0.01,0.1,0.1,5 ng/mL) for 6 h.n =3,★P < 0.05 to the non-treated cells;C:Time-course changes of κB-FlucP bioluminescence intensity in TNF-α (5 ng/mL) treated PC-3/MRS-BLI cells.n =3,★P < 0.05 to 0 h;D:Relative HRE-RlucP bioluminescence intensity in PC-3/MRS-BLI cells at various O2 concentrations (1,3,5,10,21%) for 16 h.n =3,★P < 0.05 to the cell under 21% O2;E:Time-course changes of HRE-RlucP bioluminescence intensity in PC-3/MRS-BLI cells under 1% O2.n=3,★P < 0.05 to 0 h;F:Correlation of PC-3/MRS-BLI cell number and Cluc BL intensity.n =3 for each cell number.MRS-BLI:multiplexed reporter system for bioluminescent imaging;Cluc:Cypridina luciferase;Fluc:Firefly luciferase;Rluc:Renilla luciferase;GPI:glycosylphosphatidylinositol;TNF-α:tumor necrosis factor-alpha;NF-κB:nuclear factor-kappa B;HIF:hypoxia-inducible factor
To determine whether thesein vivoBLI results reflected the direct effects of ZA on PC-3/MRS-BLI proliferation and NF-κB and HIF activities,in vitroassays were performed.First,in vitroassessment of the ZA effect on PC-3/MRS-BLI cell proliferation reveals that ZA treatment,for up to 24 h,did not influence PC-3/MRS-BLI cell proliferation even at the highest concentration of 100 μM [Figure 4A].Prolonged ZA treatment (48 h) showed significant suppression at higher concentrations (50 and 100 μM) [Figure 4A].These concentrations are much higher than those used in thein vivotreatment (data shown in Figure 3),in which the calculated ZA concentration in metastatic lesions is less than 5 μM.Cellular BL assays of PC-3/MRS-BLI cells with D-luciferin and coelenterazine revealed that Fluc and Rluc activities corresponding to NF-κB and HIF transcriptional activities,respectively,were unaffected by ZA treatment at less than 5 μM [Figure 4B].Overall,the results suggested that the observedin vivoeffects of ZA on PC-3/MRS-BLI might be secondary effects:ZA reduced inflammation and hypoxia in the microenvironment surrounding PC-3/MRS-BLI,probably through its inhibitory effect on bone destruction [Figure 4C].

Figure 3.Non-invasive observation of bone metastasis of PC-3/MRS-BLI.A:Schematic demonstrating the administration of ZA or substrates for MRS-BLI in a murine bone metastasis model with PC-3/MRS-BLI;B:Multiplexed bioluminescent imaging of bone metastasis of PC-3/MRS-BLI treated with or without ZA;C:Relative metastasis mass of PC-3/MRS-BLI bone metastasis monitored with CMV-Cluc-GPI.n=7 (Control),n=5 (ZA),★P < 0.05;D:Relative NF-κB activity (κB-FlucP/CMV-Cluc-GPI) of PC-3/MRSBLI bone metastasis.The inset graph highlights NF-κB activity on days 1 through day 12.n =7 (Control),n=5 (ZA),★P < 0.05.Red lines indicate the statistical significance of day 1 over day 4 and day 7 in the control group;E:Relative HIF activity (HRE-RlucP/CMVCluc-GPI) of PC-3/MRS-BLI bone metastasis.The inset graph highlights HIF activity on day 1 through day 12.n=7 (Control),n=5 (ZA),★P < 0.05.Red lines indicate the statistical significance between day 1 (control group) and day 7.ZA:Zoledronic acid;MRS-BLI:multiplexed reporter system for bioluminescent imaging;Cluc:Cypridina luciferase;Fluc:Firefly luciferase;Rluc:Renilla luciferase;GPI:glycosylphosphatidylinositol;NF-κB:nuclear factor-kappa B;HIF:hypoxia-inducible factor
DISCUSSION
In this study,we developed a new multiplexed BLI system,and visualized proliferation and activities of key transcriptional factors of cancer cells,NF-κB and HIF,during metastasis development and ZA treatment in bone.Of the three reporters used here,we found the newly constructed Cluc-GPI reporter useful:its strong bioluminescence overcomes the poor tissue-penetration of visible wavelength light of Cluc (λmax=465 nm),allowing the growth of metastasized cancer cells in the mice hind limbs to be monitored from day 1 of caudal artery injection [Figure 3B].This suggests that Cluc-GPI may be a new option to combine with Fluc and Rluc reporter genes for multiplexed BLI.Multi-modal techniques combining optical imaging and positron emission tomography,magnetic resonance imaging and X-ray computed tomography have been employed,and have provided valuable information of metastatic lesions[33-35].Multiple optical imaging has also provided us a different benefit from multi-modal imaging,in that,multiple pieces of information can be easily and safely collected from the same individual at almost the same time.MRS-BLI is the first example of applying multiple luciferases to anin vivoimaging system that can visualize mass as well as dual transcriptional activities in bone metastases.
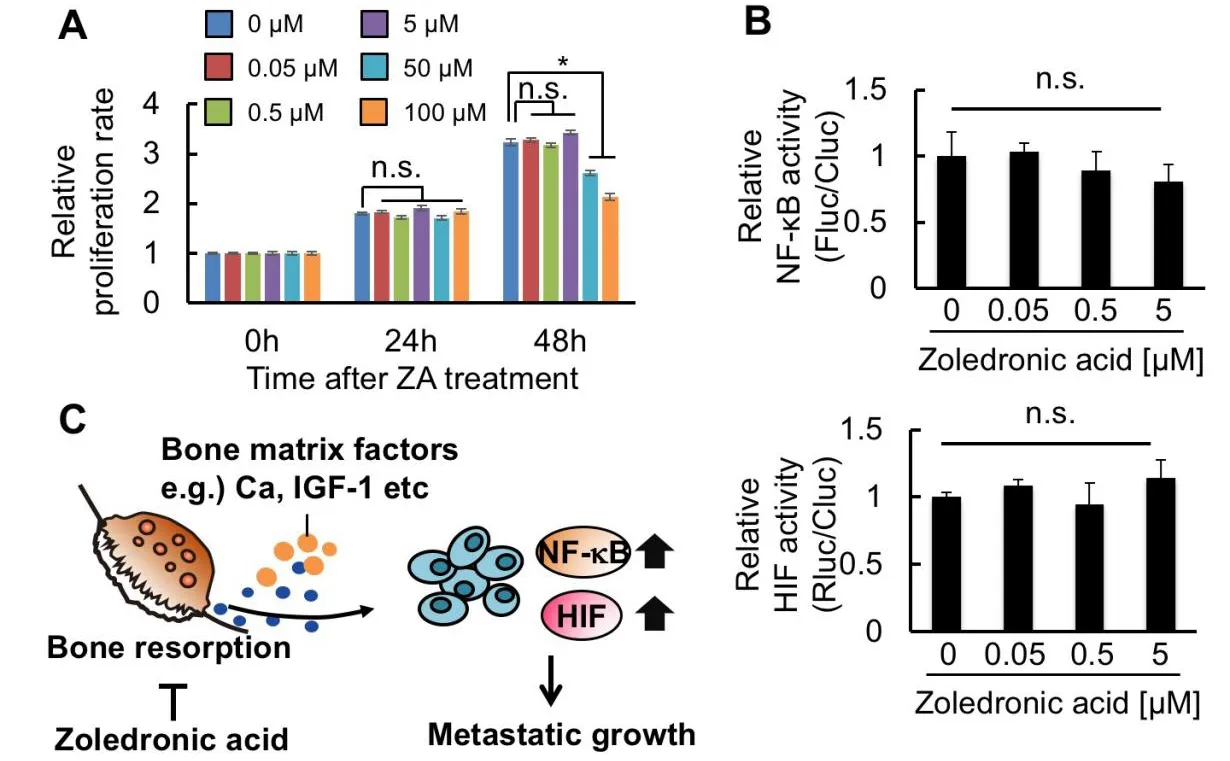
Figure 4.ZA suppresses microenvironmental factors activating NF-κB and HIF in bone metastatic lesions.A:WST-1 assay of PC-3/MRS-BLI treated with varied concentrations of ZA.n=3,★P < 0.05;B:Relative NF-κB (top) and HIF (bottom) activities of PC-3/MRSBLI treated with varied concentrations of ZA.n=3;C:Diagram of the hypothetical mechanism for NF-κB and HIF activation in bonemetastasized cancer cells,and for ZA function suppressing this activation.ZA:Zoledronic acid;NF-κB:nuclear factor-kappa B;HIF:hypoxia-inducible factor;MRS-BLI:multiplexed reporter system for bioluminescent imaging
Despite the significant growth inhibitory effect of ZA on PC3/MRS-BLI cellsin vivo[Figure 3C],treatment of cultured PC3/MRS-BLI cells with ZA at concentrations less than 5 μM did not show any inhibitory effects on the proliferation [Figure 4A].ZA treatment at higher concentrations (50 and 100 μM) resulted in significant growth inhibition of PC3/MRS-BLI cells only for 48 h.This inhibitory effect is consistent with previous studies showing that high concentrations of nitrogen-containing bisphosphonate analogues induce apoptosis in prostate cancer cell lines via inhibition of the mevalonate pathway[36,37].Pharmacokinetic study in rodents has reported a sharp decline in plasma ZA levels:the levels at 4 h became less than 1% of plasma levels at 5 min after intravenous injection[38].This indicates that the ZA concentration in bone metastatic lesions would be predominantly less than 5 μM.Therefore,the growth inhibitory effects of ZAin vivoare probably due to indirect effects on bone destruction rather than direct effects on cancer cells.
Multiplexed bioluminescent imaging of bone metastasis lesions revealed that NF-κB and HIF activities per cell were significantly high at day 1 and day 12,and continued to sharply increase thereafter in control mice [Figure 3D and E].ZA treatment significantly suppressed this increase [Figure 3D and E];the inhibitory effects of ZA on NF-κB and HIF were not direct effects on cancer cells [Figure 4B].Based on these results,we propose a mechanism for the initiation and progression of bone metastasis:Cancer cells that have migrated to the bone marrow enhance NF-κB and HIF activities in order to adapt to the physiological low partial pressure of oxygen in the bone marrow[10]and respond to immunological attacks on cancer cells[36],respectively.Cancer cells that are protected by these activities and are able to survive will start growing and activate osteoclasts,which induce bone destruction and release of factors from the bone.The released factors increase NF-κB and HIF activities in cancer cells [Figure 4C].This model is supported by previous studies that demonstrate that bone-matrix derived insulin growth factors activated NF-κB and HIF signaling in cancer cells[6,8].In addition,calcium stored in the bone matrix is a potent factor to induce accumulation of HIF[39].This may be involved in prostate cancer cells,benefiting growth from extracellular calcium in the bone marrow[40].Taken together,bone destruction may trigger activation of NF-κB and HIF signaling in cancer cells,enhancing the growth and malignant progression of cancer cells by inducing various target genes associated with NF-κB and HIF.
The multiplexed luciferase reporter system described here would be even more beneficial when combined with a clinically relevant mouse model that can reflect the aging process of the patient’s bone marrow.Accumulated evidence suggests that the aging process significantly remodels the microenvironments of mouse and human bones[41,42].Currently,relatively young-aged mice (5-10 weeks) have been used for preclinical studies of bone metastasis.It is unclear whether current murine models adequately reproduce the patient’s bone microenvironments.Further studies of the bone microenvironments are thus needed to develop clinically relevant murine models of bone metastasis that accelerate the development of effective therapeutic strategies.
DECLARATIONS
Acknowledgments
We are grateful to Shigeaki Watanabe (Summit Pharmaceuticals International Corporation,Tokyo,Japan) for discussion and technical support of IVIS.We also thank Biomaterials Analysis Division,Technical Department of Tokyo Institute of Technology for DNA sequencing analysis.
Authors’ contributions
Designed the overall project:Minegishi M,Kuchimaru T,Kizaka-Kondoh S
Performed the experiments:Minegishi M,Kuchimaru T,Nakagawa K,Isozaki T,Fujimori S
Analyzed and interpreted the data:Minegishi M,Kuchimaru T,Nakagawa K,Kadonosono T,Kizaka-Kondoh S
Wrote the manuscript:Minegishi M,Kuchimaru T,Kizaka-Kondoh S
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon request.
Financial support and sponsorship
This research was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Integrative Research on Cancer Microenvironment Networks from the Ministry of Education”,Culture,Sports,Science and Technology of Japan (S.K-K),Grant-in-Aid for Young Scientist (B) (T.Ku) and Princess Takamatsu Cancer Research Fund (T.Ku).
Conflicts of interest
The authors declare no competing financial interests.
Ethical approval and consent to participate
SCID mice (male) were obtained from Charles River Laboratory in Japan (Kanagawa,Japan).All mice were provided access to food and waterad libitumand were housed in the animal facilities at the Tokyo Institute of Technology.All experimental procedures using mice were approved by the Animal Experiment Committee of the Tokyo Institute of Technology (authorization number 201006-3),and carried out in accordance with relevant national and international guidelines.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
 Journal of Cancer Metastasis and Treatment2021年1期
Journal of Cancer Metastasis and Treatment2021年1期
- Journal of Cancer Metastasis and Treatment的其它文章
- Metabolomics in cancer and cancer-associated inflammatory cells
- Bulbine frutescens phytochemicals as novel ABCtransporter inhibitor:a molecular docking and molecular dynamics simulation study
- The use of liquid biopsy in early breast cancer:clinical evidence and future perspectives
- Epigenetic control of autophagy in women’s tumors:role of non-coding RNAs
- Autophagy and thyroid cancer
